CD28 serves as a co-stimulator for T-cell activation and survival, and is expressed on all naive T cells in newborns.1 However, as T cells experience antigen-mediated activation and differentiation, they gradually lose CD28 expression.2 The age-related increase of CD28− T cells in human peripheral blood means that immune function declines in the elderly.1 Among the populations of CD28- T cells, CD8+CD28- T cells show the greatest age-related increase.1 These cells, which were first described in aged humans, exhibit weak immune-responsiveness and shortened telomeres and are often considered to exhibit age-associated replicative senescence.3 To date, CD8+CD28- T-cell alterations have been reported in cancer, viral infections, autoimmunity and almost every chronic inflammatory disease.3,4
Recently, CD8+CD28- T cells have attracted interest because of their critical roles in immunomodulation. Suciu-Foca et al. 5 first described CD8+CD28- T cells as a subset of suppressor cells within the CD8+ T-cell population. Growing evidence suggests that CD8+CD28- T cells are a unique subpopulation of regulatory T cells (Tregs) that exert broad effects on directly regulating T cells, such as by inhibiting their activation and proliferation, decreasing the secretion of proinflammatory cytokines by activated T cells, and inducing apoptosis in activated T cells in vitro.6,7 Cell contact and soluble factors, including TGF-β, IL-7, IL-10, PDL-1 and FasL, contribute to the ability of CD8+CD28- T cells to regulate T-cell responses.6,7 Moreover, CD8+CD28- T cells may indirectly control T-cell responses, such as by up-regulating LILRB4 and LILRB2 on antigen-presenting cells (APCs), which gives rise to tolerogenic APCs that have an impaired capacity for co-stimulating T-cell activation.3,8 Tolerized APCs also interact with naive CD8+ T cells, induce their expression of Foxp3 and promote their development into CD8+CD28-Foxp3+ T cells,8 which comprise the major subset of CD8+CD28- Tregs.
Accumulating evidence indicates that CD8+CD28- T cells are associated with numerous inflammation-related disorders, regardless of age. For example, increased numbers of CD8+CD28- T cells are found in tumor microenvironments and the circulation of cancer patients, and this elevation is associated with malignancy.3 Increases of CD8+CD28- T cells are also observed in chronic viral infections, such as hepatitis C virus (HCV), human cytomegalovirus (HCMV), Epstein–Barr virus (EBV) or human immunodeficiency virus (HIV) infections.3,9 CD8+CD28- T cells also expand after bone morrow or solid organ transplantation and may serve as a mechanism of primary tolerance in transplantation, yielding better graft acceptance and stabilizing graft function, and their expansion is associated with a reduced occurrence of rejection.3,10 In autoimmune diseases, such as type 1 diabetes mellitus and multiple sclerosis,3 the CD8+CD28- T-cell population is significantly smaller than that seen in healthy controls. Subsequently, several studies have even suggested that CD8+CD28- T cells may act as mediators in inflammation-related disease. CD8(-/-)CD28(-/-)-deficient mice are susceptible to experimental autoimmune encephalomyelitis (EAE) or fully rejected allogeneic skin allografts, and the adaptive transfer of CD8+CD28- T cells has been shown to significantly suppress EAE, alleviate acute allograft rejection, ameliorate allergic diarrhea or prevent experimental inflammatory bowel disease (IBD).3,10,11 Currently, CD8+CD28- T cells contribute to the prediction of prognosis in inflammatory disease; for example, the frequency of a CD8+CD28- T-cell subset in the circulation may serve as new marker of response to antiretroviral therapy in HIV, and the CD8+CD28+/CD8+CD28- T-cell ratio can sensitively predict the outcome for IBD patients.9,12 These results support the idea that CD8+CD28- T cells may control the occurrence and progression of inflammatory disease. Thus, strategies to increase the number or immunosuppressive capacity of CD8+CD28- T cells might be an effective means of treating these diseases.
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that can be derived from various tissues, and they possess immunomodulatory capability for inhibiting the proliferation and cytotoxicity of T cells and NK cells, downregulating the maturation and function of dendritic cells, and controlling the proliferation and differentiation of B cells.13 Beginning from previous reports that showed that MSCs induce traditional CD4+CD25+Foxp3+ Tregs,13 our group demonstrated that a co-culture with MSCs increased the percentage of CD8+CD28- T cells.6 However, there were differences in the MSC co-culture-induced enhancements of these two Tregs. Previous work showed that MSCs significantly promoted the generation and expansion of CD4+CD25+Foxp3+ Tregs through TGF-β or PGE2.13 However, we were unable to generate CD8+CD28- T cells from CD8+CD28+ T cells or promote the proliferation of CD8+CD28- T cells; instead, the MSC co-culture increased the percentage of CD8+CD28- T cells in vitro by reducing apoptosis partially via IL-66 (Figure 1). Because CD8+CD28- T cells are weaker in proliferation and more prone to apoptosis compared with CD8+CD28+ T cells,3,6 we speculate that, in vivo, MSCs might indirectly induce the generation of CD8+CD28- T cells from naive CD8+ T cells. This hypothesis is supported by previous findings that showed MSCs induce tolerogenic APCs,13 which can trigger the generation of CD8+CD28- Tregs from naive CD8+ T cells 8 (Figure 1). Future studies are warranted to test this possibility.
Figure 1.
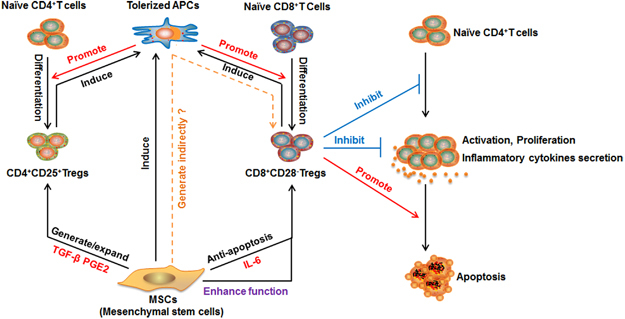
Regulatory network of CD8+CD28- Tregs. CD8+CD28- Tregs possess a pleiotropic capacity for exhibiting regulatory T-cell responses, including the abilities to inhibit naive CD4+ T-cell activation and proliferation, decrease the production of inflammatory cytokines by activated CD4+ T cells and induce apoptosis in activated CD4+T cells. MSCs enhance the regulatory function of CD8+CD28- Tregs by increasing their expression of IL-10 and FasL. Moreover, MSCs promote the survival of CD8+CD28- Tregs, thereby increasing their proportion among the T-cell population, different from the MSC-mediated increase in CD4+CD25+ Tregs, which requires the generation and expansion of these Tregs. However, MSCs might indirectly promote the generation of CD8+CD28- Tregs, such as by inducing tolerized APCs. APCs, antigen-presenting cells; MSCs, mesenchymal stem cells; Tregs, regulatory T cells.
Importantly, it has been shown that MSCs comprehensively enhance the suppressive activity of CD8+CD28- T cells by inhibiting naive CD4+ T-cell proliferation and activation, decreasing the production of IFN-γ by activated CD4+ T cells and inducing apoptosis in activated CD4+T cells 6 (Figure 1). The enhanced regulatory function of co-cultured CD8+CD28- T cells requires an MSC-induced increase in their expression of IL-10 and FasL.6 Although MSCs have been shown to trigger the generation and expansion of CD4+CD25+Foxp3+ Tregs, it is not yet clear whether MSCs impact the regulatory ability of these Tregs.13 Our studies have shown that MSCs maintain the pleiotropic effects of CD8+CD28- Tregs and can upgrade these cells to a ‘next generation’ of CD8+CD28- Tregs with a robust immunoregulatory function.6,14 These results suggest that Tregs might hold promise for future efforts aimed at treating inflammation-related diseases. However, the functional consequences of the MSC-mediated effects on CD8+CD28- T cells should be explored further.
Given the obvious MSC-mediated changes in CD8+CD28- T cells, we speculate that induced CD8+CD28- T cells might also contribute to the well-known capacity of adopted MSCs to improve inflammation-related diseases. In clinical studies of patients with chronic graft-versus-host disease (cGVHD), which is the predominant complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT), a prospective observation supports our hypothesis: CD8+CD28- T cells are elevated in patients who exhibit clinical remission of cGVHD after MSC administration, but not in those who fail to respond to MSC therapy.6,14,15 Moreover, the clinical improvement in the former group of patients was accompanied by increased expression of IL-10 and FasL on CD8+CD28- T cells,6 suggesting that MSCs might enhance immunosuppression of CD8+CD28- regulatory T cells to ameliorate cGVHD and other inflammation-related diseases. Additional studies are also needed to improve our understanding of the MSC-induced enhancement of CD8+CD28- Treg activity and its ability to modulate inflammation-related diseases.
It is tempting to believe that CD8+CD28- Tregs could be exploited as a cell therapy for inflammatory disease. A main hurdle might be the need to expand CD8+CD28- Tregs in vitro while maintaining their functionality. It could be helpful to use MSCs and dendritic cells as supporting cells. Further efforts are needed to shape CD8+CD28- T cells as robust and pleiotropic regulatory T cells.
Acknowledgements
The work is supported by grant from the National Key Research and Development Program of China, Stem Cell and Translational Research (2017YFA0103403, 2017YFA0105504); the National Natural Science Foundation of China (81425016, 81600102, 81601381, 31771616, 81730005); the Natural Science Foundation of Guangdong Province (S2013030013305, 2016A030310131); the Key Scientific and Technological Projects of Guangdong Province (2014B020226002, 2015B020228001, 2015B020229001, 2016A020214003, 2016B030229002); Key Scientific and Technological Program of Guangzhou City (201508020262, 201704020223); Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (GDUPS, 2013).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seyda M, Elkhal A, Quante M, Falk CS, Tullius SGT. Cells going innate. Trends Immunol. 2016;37:546–556. doi: 10.1016/j.it.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strioga M, Pasukoniene V, Characiejus D. CD8(+) CD28(−) and CD8(+) CD57(+) T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arosa FA, Esgalhado AJ, Padrao CA, Cardoso EM. Divide, conquer, and sense: CD8(+)CD28(−) T cells in perspective. Front Immunol. 2017;7:665. doi: 10.3389/fimmu.2016.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- T cells. Int Immunol. 1998;10:775–783. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 6.Liu QL, Zheng HQ, Chen XY, Peng YW, Huang WJ, Li XB, et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+) CD28(−) regulatory T cells. Cell Mol Immunol. 2015;12:708–718. doi: 10.1038/cmi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlad G, Cortesini R, Suciu-Foca N. License to heal: bidirectional interaction of antigen-specific regulatory T cells and tolerogenic APC. J Immunol. 2005;174:5907–5914. doi: 10.4049/jimmunol.174.10.5907. [DOI] [PubMed] [Google Scholar]
- 9.Fenoglio D, Dentone C, Signori A, Di Biagio A, Parodi A, Kalli F et al. CD8+CD28-CD127loCD39+ regulatory T-cell expansion: a new possible pathogenic mechanism for HIV infection? J Allergy Clin Immunol 2017;pii: S0091-6749(17)31474-4. doi:10.1016/j.jaci.2017.08.021. [DOI] [PubMed]
- 10.Su J, Xie Q, Xu Y, Li XC, Dai Z. Role of CD8(+) regulatory T cells in organ transplantation. Burns Trauma. 2014;2:18–23. doi: 10.4103/2321-3868.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuddamalay Y, van Meerwijk JP. CD28− and CD28lowCD8+ regulatory T cells: of mice and men. Front Immunol. 2017;8:31. doi: 10.3389/fimmu.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai SX, Gu HX, Lin QY, Huang SZ, Xing TS, Zhang QF, et al. CD8+CD28+/CD8+CD28− T cell equilibrium can predict the active stage for patients with inflammatory bowel disease. Clin Res Hepatol Gastroenterol. 2017;41:693–702. doi: 10.1016/j.clinre.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng JY, He C, Lai PL, Luo CW, Guo R, Wu SJ, et al. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther. 2012;20:2347–2354. doi: 10.1038/mt.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transpl. 2010;45:1732–1740. doi: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]


