Abstract
This systematic review with a meta-analysis aimed to summarize the current evidence of the effectiveness of mesenchymal stem cell (MSC) treatment for knee osteoarthritis (OA) and to examine whether rehabilitation is an effect modifier of the effect estimate of MSC treatment. A literature search yielded 659 studies, of which 35 studies met the inclusion criteria (n = 2385 patients; mean age: 36.0–74.5 years). The meta-analysis results suggested that MSC treatment through intra-articular injection or arthroscopic implantation significantly improved knee pain (standardized mean difference [SMD]: −1.45, 95% confidence interval [CI]: −1.94, −0.96), self-reported physical function (SMD: 1.50, 95% CI: 1.09, 1.92), and cartilage quality (SMD: −1.99; 95% CI: −3.51, −0.47). However, the MSC treatment efficacy on cartilage volume was limited (SMD: 0.49; 95% CI: −0.19, 1.16). Minor adverse events (knee pain or swelling) were reported with a wide-ranging prevalence of 2–60%; however, no severe adverse events occurred. The evidence for these outcomes was “very low” to “low” according to the Grades of Recommendation, Assessment, Development and Evaluation system because of the poor study design, high risk of bias, large heterogeneity, and wide 95% CI of the effects estimate. Performing rehabilitation was significantly associated with better SMD for self-reported physical function (regression coefficient: 0.881, 95% CI: 0.049, 1.712; P = 0.039). We suggest that more high quality randomized controlled trials with consideration of the potential rehabilitation-driven clinical benefit would be needed to facilitate the foundation of effective MSC treatment and regenerative rehabilitation for patients with knee OA.
Introduction
Osteoarthritis (OA) is the most common form of arthritis.1 OA ultimately results in cartilage degeneration, chronic knee pain, and disability. In 2010, knee OA was the 11th leading cause of disability worldwide, with increasing incidence over the last 2 decades.2 Current treatments have little impact on the progressive degeneration of articular cartilage; therefore, developing effective and financially viable disease-modifying therapies is a critical medical priority.
Mesenchymal stem cells (MSCs) have emerged as a cell type with great potential for cell-based articular cartilage repair in patients with knee OA.3 Clinical trials that investigate the effects of MSC treatments in patients with knee OA have recently begun emerging,4 and results of clinical studies are continuously reported.5,6 Several meta-analyses summarize the effects of MSC treatment in patients with knee OA;7–10 these studies contribute to the establishment of effective cell-based therapies for degenerative cartilage disease. However, some of these systematic reviews included patients with focal cartilage lesions8–10 or focused on pain and physical function as treatment outcomes,7,9,10 with a large heterogeneity and lack of evaluation of bias risk.7–9 As knee pain would be discordant with articular cartilage status, understanding the effects of MSC treatment against OA joint degeneration and exploring the mechanisms underlying symptom-modifying MSC treatment are important. In addition, confidence in the effects estimate from meta-analysis depends on the quality of the included studies and analytical process,11 as the former can be evaluated using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach.12 However, no meta-analysis has examined the effects of MSCs on knee OA considering the GRADE approach.
Physical factors such as rehabilitation programs are potential effect modifiers that were not well addressed in previous meta-analyses.7–10 Physical factors regulate MSC differentiation and tissue development, pointing to a potential therapeutic strategy for enhancing the MSCs injected or implanted into the knee joint,13,14 such as the recently proposed new field “regenerative rehabilitation”.15 Regenerative rehabilitation is defined as the integration of principles and approaches from the fields of rehabilitation science and regenerative medicine.16 The efficacy of regenerative medicine may be enhanced when coupled with mechanical input. Weight-bearing might influence the structural outcome in the postoperative phase of autologous chondrocyte implantation in adults with cartilage defects.17,18 Thus, further investigation of the effects of MSC treatment in patients with knee OA and the potential role of rehabilitation (i.e., regenerative rehabilitation) as an effect modifier would be of interest.
Potential adverse effects have a considerable impact on patient adherence to MSC treatment. To achieve a balanced perspective, a systematic review should consider the aspects of adverse events relevant to MSC treatment.19 Randomized controlled trials (RCTs) would be insufficient to provide evidence of benefits and harms; thus, non-RCT, such as prospective cohort studies with long-term follow up periods should be included.19 However, no systematic reviews have investigated adverse events after MSC treatment, even though previous systematic reviews included both RCTs and non-RCTs.7–9 Thus, the purpose of this systematic review was (i) to examine the literature on the effects of MSCs in patients with knee OA in the clinical setting and to summarize the current evidence for their potential benefits and harms, and (ii) to examine whether rehabilitation is an effect modifier of effect estimate of MSC treatment. This study would provide a framework for a future high quality study with the aim of developing effective cell-based regenerative rehabilitation in patients with knee OA.
Results
eFigure 1 shows a flow chart of the study selection. The database search yielded 659 studies, of which 31 met the eligibility criteria. With the citation index, 4 additional studies were found in accordance with the pre-specified inclusion criteria provided in eMethod 1; in total, 35 studies were used in the meta-analysis.
Study characteristics
Table 1 shows the characteristics of the included studies. Of 35 studies, 21 (60.0%)20–40 had a single-arm prospective design, 7 (20.0%)6,41–46 had a quasi-experimental design, and the remaining 7 (20.0%)5,47–52 were RCTs. From the 35 studies, 2385 patients treated with MSC therapy were included. The mean age across 35 articles was 56.7 ± 6.78 years (36.0–74.5 years). In the 30 studies that reported sex (n = 1975 patients), 1119 patients (56.7%) were female. Twenty-nine studies (82.9%)5,6,20,23–35,37–48,50 reported the radiographic severity of knee OA (i.e., Kellgren/Lawrence [K/L] grade); however, the eligibility criteria of disease severity differed between studies. The final follow-up period was 3–60 months. Fourteen studies (40.0%)5,6,20,23–27,33,38,40,42,46,47 reported funding sources (eTable 1). Of the 35 studies, 25 (73.5%)5,6,20–27,31–42,45,46,49 and 2 (5.7%)47,50 used autologous and allogeneic MSC intra-articular injection, respectively. The other studies used arthroscopic autologous MSC implantation,28–30,43,44 or a combination of these procedures with high tibial osteotomy.48,51,52 The rehabilitation program included patients’ education in the pre-MSC treatment phase, gradual increase in weight-bearing using crutches, use of physical therapy modalities, range of motion exercise, and muscle strength exercise (eTable 2). Notably, none of the included studies stratified for the presence of rehabilitation.
Table 1.
Summary of included studies
| Author | Subject population | KL grade | Treatment | Donor | Outcomes | Follow-up | Funding |
|---|---|---|---|---|---|---|---|
| Single-arm, prospective follow-up studies | |||||||
| Bui 201420 (Vietnam) | N = 21 | II–III | SVF injection + PRP | Auto | Lysholm score, VAS pain, MRI | 1, 3, 6 M | X |
| Centeno 2008a21 (Unites states) | N = 1 (age: 36 y; M) | – | BD-MSC injection (4.56 × 107 cells) | Auto | VAS pain, MRI (cartilage and meniscus volumes) | 1, 3 M | – |
| Centeno 2008b22 (Unites states) | N = 1 (46 y; M) | – | BD-MSC injection (2.24 × 107 cells) | Auto | VAS pain, functional rating index, ROM, MRI evaluation (cartilage and meniscus volumes) | 1, 3, 6 M | – |
| Davatchi 201123 (Iran) | N = 4 (age: 57.8 ± 5.0 y; 50% F) | II–III | BD-MSC injection (8–9 × 106 cells) | Auto | VAS pain, ROM | 6 M | X |
| Davatchi 201624 (Iran) | N = 4 (age: 57.8 ± 5.0 y; 50% F) | II–III | BD-MSC injection (8–9 × 106 cells) | Auto | VAS pain, ROM | 60 M | X |
| Emadedin 201225 (Iran) | N = 6 (age: 53.8 ± 8.9 y; 100% F) | IV | BD-MSC injection (2.0–2.4 × 107 cells) | Auto | VAS pain, WOMAC, ROM, MRI evaluation | 2 W; 1, 2, 6, 12 M | X |
| Emadedin 201526 (Iran) | N = 6 (age: 53.8 ± 8.9 y; 100% F) | IV | BD-MSC injection (2.0–2.4 × 107 cells) | Auto | VAS pain, WOMAC, MRI evaluation | 2, 6, 12, 30 M | X |
| Fodor 201627 (Unites states) | N = 6 patients 8 knees (age: 59.0 ± 7.3 y; 83.3% F) | I (N = 2) II (N = 2) III (N = 4) | SVF injection | Auto | VAS pain, WOMAC, ROM, TUG, MRI evaluation | 3, 12 M | X |
| Kim 2015c28 (Korea) | N = 49 patients, 55 knees (age: 58.1 ± 8.9 y; 52.7% F) | I–II | AD-MSC implantation (4.3 × 106 cells) + AD | Auto | IKDC, Tegner activity scale | 26.7 M | – |
| Kim 201629 (Korea) | N = 20 patients, 24 knees (age: 57.9 ± 5.9 y; 45.0% F) | I–II | AD-MSC implantation (4.4 × 106 cells) + AD | Auto | IKDC, Tegner activity scale, MRI evaluation (MOCART and MOAKS) | 27.9 M | – |
| Koh 201332 (Korea) | N = 18 (age: 54.6 ± 7.8 y; 66.7% F) | III–IV | AD-MSC injection (1.18 × 106 cells) + PRP | Auto | WOMAC, Lysholm score, VAS pain, MRI evaluation (WORMS) | 24.3 M | – |
| Koh 2014a30 (Korea) | N = 35 patients, 37 knees (age: 57.4 ± 5.7 y; 60.0% F) | I–II | AD-MSC implantation (3.8 × 106 cells) + AD | Auto | IKDC, Tegner activity scale, arthroscopic evaluation (ICRS grade) | 26.5 M | – |
| Koh 201531 (Korea) | N = 30 (age: 70.3 [65–80] y; 83.3% F) | II–III | AD-SVF (4.2 × 107 cells) injection + PRP + AD | Auto | Lysholm, KOOS, VAS pain, K/L grade, arthroscopic evaluation | 3, 12, 24 M | – |
| Michalek 201533 (Czech Republic) | N = 1114 (age: 62.0 [19–94] y; 47.8% F) | II–IV | AD-SVF injection (1.6 × 106 cells) + PRP | Auto | Modified KOOS, X-ray, MRI evaluation | 17.2 M | X |
| Orozco 201334 (Spain) | N = 12 (age: 49.0 ± 17.3 y; 50.0% F) | II (N = 4) III (N = 3) IV (N = 5) | BD-MSC injection (4.0 × 107 cells) | Auto | VAS pain, Lequesne index, WOMAC, PCI, SF-36 | 3, 6, 12 M | – |
| Orozco 201435 (Spain) | N = 12 (age: 49.0 ± 17.3 y: 50.0% F) | II–IV | BD-MSC injection (4.0 × 107 cells) | Auto | VAS pain score, Lequesne index, WOMAC, PCI | 3, 6, 12, 24 M | – |
| Pak 201136 (Korea) | N = 2 (age: 74.5 ± 6.4 y; 100% F) | – | AD-MSC injection + HA + PRP + CaCl2 + dexamethasone | Auto | VAS pain, ROM, MRI evaluation | 3 M | – |
| Sampson 201637 (Unites states) | N = 125 (age: 57.0 [23–79] y; 100% F) | III–IV | BMC injection + PRP | Auto | VAS, global patients satisfaction survey | 4.8 M | – |
| Soler Rich 201539 (Spain) | N = 50 (age: 57.8 ± 14.1 y; 40.0%F) | II–IV | BD-MSC injectio (4.0 × 107 cells) | Auto | VAS, Lequesne score, WOMAC, MRI evaluation T2 mapping, PCI) | 0, 6, 12 M | – |
| Soler 201638 (Spain) | N = 15 (age: 51.1 ± 10.3 y; 60.0% F) | II (N = 9) III (N = 6) | BD-MSC injection (4.1 × 107 cells) | Auto | VAS, Lequesne score, WOMAC, SF-36, MRI evaluation (T2 mapping) | 1 W; 3, 6, 12, 48 M | X |
| Trajune 201340 (Thailand) | N = 5 (age: 57.2 ± 1.92 y; 80.0% F) | II | AAPBSC injection + GFAP concentrate + HA + MCS | Auto | WOMAC, KOOS | 1, 6 M | X |
| Quasi-experimental studies | |||||||
| Centeno 201441 (Unites states) | I: N = 518 (age 54.3 ± 14.1 y) C: N = 163 (age 59.9 ± 10.3 y) | I: I (N = 223) II (N = 145) III/IV (N = 102) C: I (N = 69) II (N = 58) III/IV (N = 39) | I: BMC injection + PRP with adipose fat graft C: BMC injection + PRP | Auto | Improvement rating scale, LEFS, NPS | 1, 3, 6, 12 M | – |
| Jo 201442 (Korea) | I-a: Low dose, N = 3 (age: 63.0 ± 8.6 y; 66.7% F) I-b: Mid dose, N = 3 (age: 65.0 ± 6.6 y; 100% F) I-c: High dose, N = 12 (age: 61.0 ± 6.2 y; 83.3% F) | I-a: III (N = 2) IV (N = 1) I-b: III (N = 2) IV (N = 1) I-c: III (N = 8) IV (N = 4) | AD-MSC injection (I-a: 1.0 × 107, I-b: 5.0 × 107, I-c: 1.0 × 108 cells) | Auto | WOMAC, VAS pain, KSS, MRI evaluation (defect size and cartilage volume), arthroscopic evaluation (defect size and ICRS grade), biopsy | 1, 2, 3, 6 M | X |
| Kim 2015a43 (Korea) | I: N = 17 patients, 17 knees (age: 57.7 ± 5.8 y; 52.9% F) C: N = 37 patients, 39 knees (age: 57.5 ± 5.9 y; 62.2% F) | I–II | I: AD-MSC implantation with fibrin glue (3.9 × 106 cells) + AD C: AD-MSC implantation (3.9 × 106 cells) + AD | Auto | IKDC, Tegner activity scale, arthroscopic evaluation (ICRS grade) | 28.6 M | – |
| Kim 2015b44 (Korea) | I: N = 20 (age: 59.1 ± 3.5 y; 65.0% F) C: N = 20 (age: 59.4 ± 3.1 y; 65.0% F) | I–II | I: AD-MSC implantation (4.0 × 106 cells) + AD C: AD-MSC injection (4.0 × 106 cells) + PRP | Auto | IKDC, Tegner activity scale, arthroscopic evaluation (ICRS grade) | 28.6 M | – |
| Koh 201245 (Korea) | I: N = 25 (age: 54.2 ± 9.3 y; 68.0% F) C: N = 25 (age: 54.4 ± 11.3 y; 68.0% F) | I: 3.3 ± 0.8 C: 2.7 ± 0.7 | I: AD-MSC injection (1.89 × 106 cells) + PRP C: PRP | Auto | Lysholm, Tegner activity scale, VAS pain | 3, 16.4 M | – |
| Nguyen 201746 (Vietnam) | I: N = 15 (age: 58.6 ± 6.5 y; 80.0% F) C: N = 15 (age: 58.2 ± 5.7 y; 80.0% F) | I: II (N = 4) III/IV (N = 11) C: II (N = 5) III/IV (N = 10) | I: AD-SVF injection (1.89 × 106 cells) + AM + PRP C: AM + PRP | Auto | WOMAC, modified VAS pain, Lysholm, MRI | 1, 6, 12, 18 M | X |
| Pers 20166 (France) | I-a: Low dose, N = 6 (age: 63.2 ± 4.1 y; 50.0% F) I-b: Mid dose, N = 6 (age: 65.5 ± 8.1 y; 50.0% F) I-c: High dose, N = 6 (age: 65.2 ± 2.3 y; 66.7% F) | I-a: III (N = 2) IV (N = 41) I-b III (N = 1) IV (N = 5) I-c III (N = 0) IV (N = 6) | AD-SVF injection (I-a: 2 × 106, I-b: 10 × 106, I-c: 50 × 106 cells) | Auto | WOMAC, Global knee pain, PGA, KOOS, SAS, SF-36, MRI evaluation | 1 W; 3, 6 M | X |
| Randomized controlled trials | |||||||
| Gupta 201647 (India) | Cohort 1: I-a (Low dose): N = 10 (age: 58.1 ± 8.2 y; 70.0% F) I-b (Mid dose): N = 10 (age: 57.3 ± 9.5 y; 80.0% F) C-a: N = 10 (age: 54.9 ± 8.3 y; 100.0% F) Cohort 2: I-c (High dose): N = 10 (age: 55.0 ± 6.7 y; 80.0% F) I-d (Very high dose): N = 10 (age: 54.0 ± 6.7 y; 50.0% F) C-b: N = 10 (age: 56.7 ± 5.2 y; 70.0% F) | I-a: II (N = 4) III (N = 6) I-b: II (N = 1) III (N = 9) C-a: II (N = 3) III (N = 7) I-c: II (N = 1) III (N = 9) I-d: II (N = 3) III (N = 7) C-b: II (N = 2) III (N = 8) | I: BD-MSC injection (I-a: 25 × 106, I-b: 50 × 106, I-c: 75 × 106 cells, I-d: 150 × 106 cells) + HA C: HA | Allo | VAS, WOMAC, ICOAP, X-ray, MRI (WORMS) | 12 M | X |
| Koh 2014b48 (Korea) | I: N = 21 (age: 54.2 ± 2.9 y; 76.2% F) C: N = 23 (age: 52.3 ± 4.9 y; 73.9% F) | I: II (N = 0) III (N = 9) IV (N = 12) C: II (N = 1) III (N = 11) IV (N = 11) | I: HTO + AD-MSC implantation + PRP C: HTO + PRP | Auto | Lysholm, KOOS, VAS pain, FTA, arthroscopic evaluation (Kanamiya grade) | 24.4 M | – |
| Lamo-Espinosa 20165 (Spain) | I-a (Low dose): N = 10 (age: 65.9 [IQR: 59.5, 70.6] y; 60.0% F) I-b (High dose): N = 10 (age: 57.8 [IQR: 55.0, 60.8] y; 20.0% F) C: N = 10 (age: 60.3 [IQR: 55.1, 61.1] y; 30.0% F) | I-a: II (N = 1) III (N = 2) IV (N = 7) I-b: II (N = 3) III (N = 3) IV (N = 4) C: II (N = 4) III (N = 2) IV (N = 4) | I: BD-MSC injection (Low dose: 1 × 107 cells; High dose: 1 × 108 cells) + HA C: HA | Auto | VAS, WOMAC, ROM, X-ray, MRI (WORMS) | 3, 6, 12 M | X |
| Varma 201049 (India) | I: N = 25 (age: 50.7 ± 5.4 y) C: N = 25 (age: 48.2 ± 5.1 y) | – | I: BMC injection + AD C: AD | Auto | VAS pain, OAOS | 1, 2, 3, 6 M | – |
| Vega 201550 (Spain) | I: N = 15 (age: 56.6 ± 9.6 y; 60.0% F) C: N = 23 (age: 57.3 ± 9.4 y; 66.7% F) | I: II (N = 6) III (N = 6) IV (N = 3) C: II (N = 7) III (N = 5) IV (N = 3) | I: BD-MSC injection (4.0 × 107 cells) C: HA | Allo | VAS pain, WOMAC, Lequesne algofunctional indices, SF-12, MRI evaluation (T2 mapping, PCI) | 1 W; 3, 6, 12 M | – |
| Wakitani 200251 (Japan) | N = 24 (I: N = 12; C: N = 12) (age: 63.0 [49–70] y; 62.5% F) | – | I: HTO + BD-MSC implantation (1.0 × 107 cells) C: HTO + cell free collagen gel-sheet implantation | Auto | Hospital for special surgery knee-rating scale, arthroscopic and histological assessment | 16 M | – |
| Wong 201352 (Singapore) | I: N = 28 (age: 53.0 [36–54] y; 54.0% F) C: N = 28 (age: 49.0 [24–54] y; 50.0% F) | – | I: HTO + BD-MSC implantation (1.5 × 107 cells) C: HTO | Auto | IKDC, Lysholm, Tegner activity scale, MRI evaluation (MOCART) | 6, 12, 24 M | – |
AAPBSC autologous activated peripheral blood stem cells, AD arthroscopic debridement, AD-MSC adipose tissue derived mesenchymal stem (stromal) cells, AD-SVF adipose tissue derived stromal vascular fraction, AM arthroscopic microfracture, BD-MSC bone marrow derived mesenchymal stem (stromal) cell, BMC bone marrow concentrate, FTA femorotibial angle, GFAP growth factor addition/preservation, HA hyaluronic acid, HTO high tibial osteotomy, ICOAP intermittent and constant osteoarthritis pain, ICRS international cartilage repair society, IKDC international knee documentation committee, IQR interquartile range, K/L grade Kellgren/Lawrence grade, KOOS knee osteoarthritis outcome score, KSS knee society score, LEFS lower extremity functional questionnaire, MCS microdrilling mesenchymal cell stimulation, MOAKS MRI osteoarthritis knee score, MOCART magnetic resonance observation of cartilage repair tissue, MRI magnetic resonance image, NPS numeric pain scale, OAOS osteoarthritis outcome score, PCI poor cartilage index, PGA patient global assessment, PRP platelet-rich plasma, ROM range of motion, SAS short arthritis assessment scale, SF-12 short form-12 health survey, SF-36 short form-36 health survey, SVF stromal vascular fraction, TUG timed up and go, VAS visual analog scale, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index, WORMS whole-organ magnetic resonance imaging score. X indicates presence of funding.
Risk of bias within studies
A summary of the Downs and Black scale for assessing bias risk is shown in eTable 3. The mean score for all 35 studies was 6.1 ± 2.1 (range, 3–12); 5.5 ± 1.6 for single-arm prospective studies; 6.3 ± 1.0 for quasi-experimental studies; and 7.9 ± 3.2 for RCT. Only two studies47,50 received a score of 1, for blinding of participants and assessors who measured key outcomes and concealed randomization of patients. The main differences between RCTs and non-RCTs included the reporting of patients’ recruitment and adequate adjustment for confounders, which is important for assessing the external and internal validities of studies.
Outcome measures
Self-reported knee pain
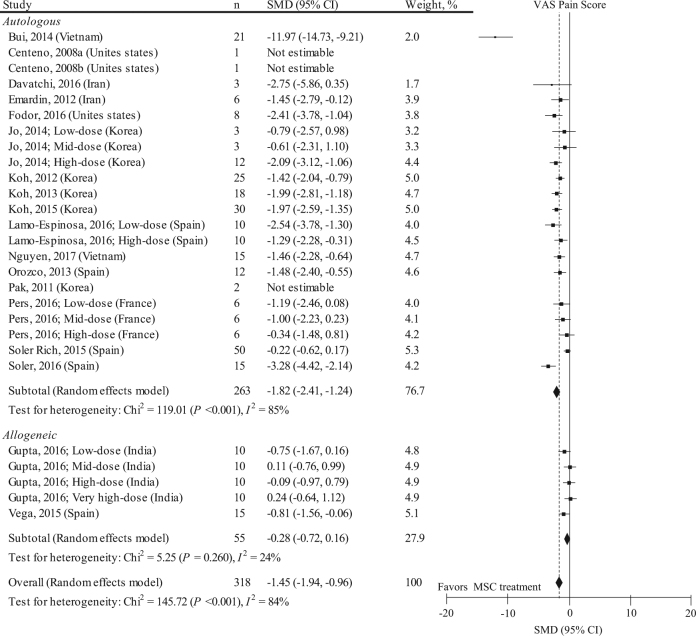
Nineteen studies with 27 data sets (n = 318) reported MSC treatment effects on knee pain by using the visual analog scale (VAS) pain score (Fig. 1). The mean follow-up period in these studies was 14.0 ± 12.9 months. The baseline VAS pain score in these studies was 60.2 ± 13.8 mm. Considering all 19 studies, the pooled standardized mean difference (SMD) on the VAS knee pain was −1.45 (95% confidence interval [CI]: −1.94, −0.96; P < 0.001). This statistical value implies a mean difference of 27.6 mm (95% CI: 13.4, 41.9 mm). However, effects estimates were highly heterogeneous among studies (I2 = 84%). Stratification for donor type (i.e., autologous vs. allogeneic) did not much improve the heterogeneity, but the pooled SMD in autologous MSC was likely to have a larger pain relief effects than those in allogeneic MSC. A meta-regression analysis indicated that a higher score of the Downs and Black scale (i.e., low risk of bias) is significantly associated with a higher (i.e., lower effect) SMD (eTable 4). Among the subitems of the Down and Black scale and SMD, clear patients’ recruitment site was significantly associated with a higher SMD (eTable 5). Rehabilitation (i.e., using physical therapy modalities, range of motion exercise, or muscle strength exercise at least one time) was not an effect modifier of SMD (regression coefficient: 0.451, 95% CI: −1.909, 2.811; P = 0.696). Small-study effects were visually observed by two independent reviewers (eFigure 2), and the Egger’s regression test was positive for significant evidence of publication bias (P = 0.016). By using the trim-and-fill method, the adjusted SMD was −0.93 (95% CI: −1.29, −0.56; P < 0.001).
Fig. 1.
SMD and 95% CI for the VAS pain score between pre and post MSC treatment at final follow-up (n = 318). The diamond represents the pooled SMD using the DerSimonian-Laird method. The vertical line at 0 represents no difference. MSC treatment was effective in improving VAS pain score (pooled SMD: −1.45, 95% CI: −1.94, −0.96; P < 0.001). SMDs were highly heterogeneous among studies (I2: 84%; P < 0.001)
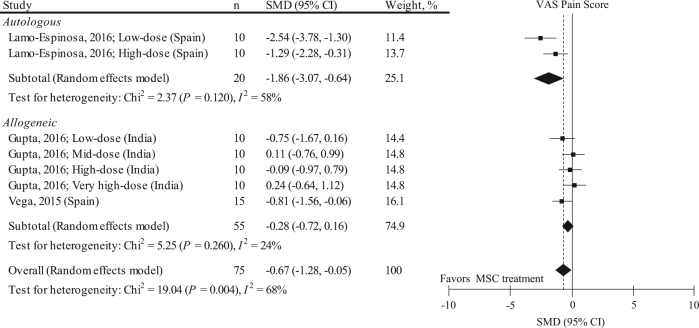
To address the possibility that effect estimates on VAS pain score and heterogeneity change if only RCTs were included in the meta-analysis, we performed a sensitivity analysis (Fig. 2). Three RCT studies with 7 data sets (n = 75) were included, and the follow-up period of all these studies was 12.0 months. The baseline VAS pain score of these studies was 60.4 ± 9.2 mm. Including only RCTs attenuated the pain relief effects (pooled SMD: −0.67, 95% CI: −1.28, −0.05; P = 0.030). This statistical value implies a mean difference of 18.1 mm (95% CI: 1.35, 34.8 mm). However, effects estimates were still highly heterogeneous among the studies (I2 = 68%). Stratification for donor type slightly improved the heterogeneity, and the pooled SMD in autologous MSC was likely to have larger pain relief effects than those in allogeneic MSC. A meta-regression analysis indicated that a higher score in the Downs and Black scale and younger age were significantly associated with higher (i.e., lower effect) SMDs (eTable 6), and blinding of participants and assessors, valid outcome measures, and concealed allocation were significantly associated with higher SMDs (eTable 7). As all the included RCTs did not report a rehabilitation program, the regression coefficient could not be calculated. No small-study effect was visually observed by two independent reviewers (eFigure 3).
Fig. 2.
Results of sensitivity analysis representing SMD and 95% CI for the VAS pain score between pre and post MSC treatment at final follow-up in 3 RCTs with 7 data sets (n = 75). The diamond represents the pooled SMD using the DerSimonian–Laird method. The vertical line at 0 represents no difference. Including only RCTs attenuates the pain relief effects (pooled SMD: −0.67, 95% CI: −1.28, −0.05; P = 0.030) compared to those shown in Fig. 1. SMDs were highly heterogeneous among studies (I2: 68%; P = 0.004)
Self-reported physical function
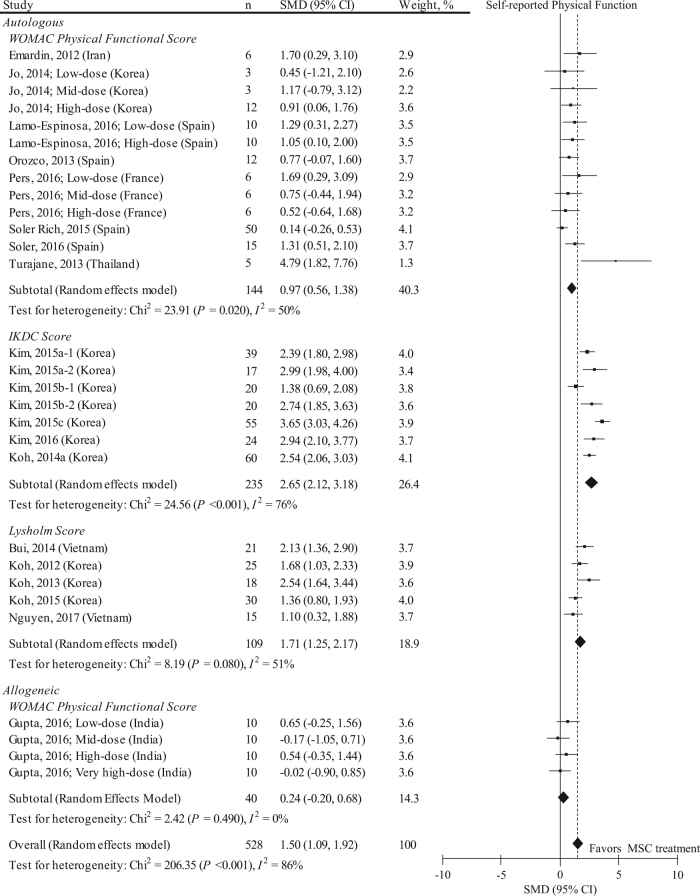
Nineteen studies with 29 data sets (n = 528) reported MSC treatment effects on self-reported physical function by using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) functional, International Knee Documentation Committee (IKDC), and Lysholm scores (Fig. 3). The mean follow-up period in these studies was 17.0 ± 10.8 months. Considering all 19 studies, the pooled SMD on the self-reported physical function was 1.50 (95% CI: 1.09, 1.92; P < 0.001). This statistical value implies a mean difference of 14.7 (95% CI: 9.39, 20.0) in the WOMAC functional outcome (0–100 points); 26.0 (95% CI: 23.1, 28.9) in the IKDC (0–100 points); and 24.1 (95% CI: 19.0, 29.2) in the Lysholm score (0–100 points). However, effects estimates were highly heterogeneous among the studies (I2 = 86%). Pooled SMD in autologous MSC was likely to have a larger functional improvement effects than those in allogeneic MSC. A meta-regression analysis indicated that implantation technique (compared to injection), lower Downs and Black scale score, presence of rehabilitation, and absence of funding source were significant factors associated with higher (i.e., higher effect) SMDs (eTable 8), and blinding of participants, unblinding of assessors, unclear patients’ recruitment site, non-randomization and non-concealed allocation were significant factors associated with higher SMDs. (eTable 9). Notably, performing rehabilitation was a significant effect modifier of SMD (regression coefficient: 0.881, 95% CI: 0.049, 1.712; P = 0.039). No small-study effect was visually observed by two independent reviewers (eFigure 4), and the Egger’s regression test was negative for significant evidence of publication bias (P = 0.516).
Fig. 3.
SMD and 95% CI for the self-reported physical functional outcome between pre and post MSC treatment at final follow-up. The diamond represents the pooled SMD using the DerSimonian-Laird method. The vertical line at 0 represents no difference. MSC treatment was effective in improving self-reported physical function (pooled SMD: 1.50, 95% CI: 1.09, 1.92; P < 0.001). SMDs were highly heterogeneous among studies (I2: 86%; P < 0.001)
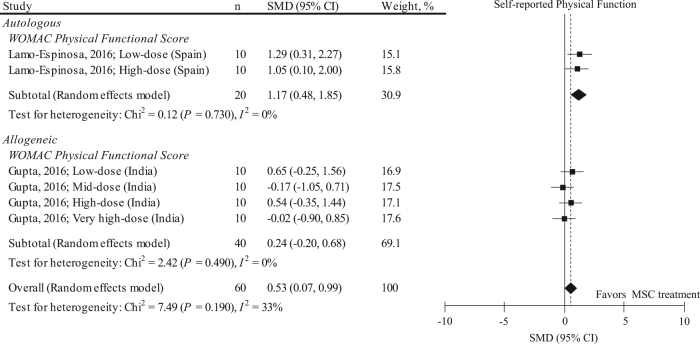
As in the VAS pain score, we performed a sensitivity analysis (Fig. 4) and included only RCTs into the meta-analysis for self-reported physical function (n = 60). We found that including only RCTs in the meta-analysis attenuated the effects of MSC in improving WOMAC functional score (pooled SMD: 0.53, 95% CI: 0.07, 0.99; P = 0.020). The follow-up period in all these studies was 12.0 months. Heterogeneity was much improved because of using a single outcome measure (I2 = 33%). Stratification for donor type improved the heterogeneity, and pooled SMD in autologous MSC was likely to have a larger functional improvement effects than those in allogeneic MSC. All the included RCTs did not perform rehabilitation. No small-study effect was visually observed by two independent reviewers (eFigure 5).
Fig. 4.
Results of sensitivity analysis representing SMD and 95% CI for the self-reported physical function (WOMAC physical functional score) between pre and post MSC treatment at final follow-up in 2 RCTs with 6 data sets (n = 60). The diamond represents the pooled SMD using the DerSimonian-Laird method. The vertical line at 0 represents no difference. Including only RCTs attenuates the effects of MSC in improving WOMAC functional score (pooled SMD: 0.53, 95% CI: 0.07, 0.99; P = 0.020) compared to those shown in Fig. 3
MRI findings in articular cartilage
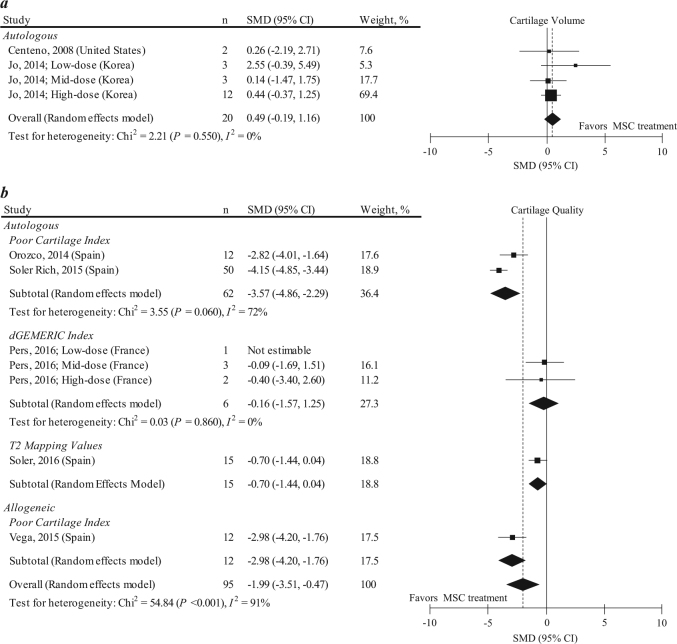
Two studies with 4 data sets (n = 20) reported the MSC treatment effect on cartilage volume, evaluated using magnetic resonance imaging (MRI; Fig. 5a). The mean follow-up period of these studies was 5.3 ± 1.5 months. In these analyses, two single case reports from the same authors21,22 were combined, as these case reports included patients with a similar clinical status. The pooled SMD on the cartilage volume was 0.49 (95% CI: −0.19, 1.16; P = 0.160), a non-significant small effect size. Excluding the combined two case reports resulted in similar results (pooled SMD: 0.51, 95% CI: −0.23, 1.26; P = 0.180).
Fig. 5.
SMD and 95% CI for cartilage volume (a) and cartilage quality (b) between pre and post MSC treatment at final follow-up. The diamond represents the pooled effect size using the DerSimonian-Laird method. The vertical line at 0 represents no difference. While MSC treatment has a non-significant tendency to improve cartilage volume (pooled SMD: 0.49, 95% CI: −0.19, 1.16; P = 0.160), MSC treatment was effective in improving cartilage quality (pooled SMD: −1.99, 95% CI: −3.51, −0.47; P < 0.001). SMDs for cartilage quality were highly heterogeneous among studies (I2: 91%; P < 0.001)
The 5 other studies with 7 data sets (n = 95) reported MSC treatment effects on cartilage quality by using the poor cartilage index (PCI), dGEMERIC index, and T2 mapping values, evaluated using MRI (Fig. 5b). The mean follow-up period in these studies was 16.3 ± 15.4 months. The pooled SMD on the cartilage quality was −1.99 (95% CI: −3.51, −0.47; P < 0.001), a significantly heightened effect size (SMD ≥ 0.8), with high heterogeneity (I2 = 91%). When the pooled SMD was evaluated in each outcome measure, it became higher in the PCI but became insignificant in the dGEMERIC index, and heterogeneity improved markedly. A meta-regression analysis indicated that the presence of funding source was a significant factor associated with a higher (i.e., lower effect) SMD (eTable 10). No small-study effect was visually observed from funnel plots by two independent reviewers (eFigures 6 and 7).
Adverse events
Of 35 studies, 17 (48.6%) reported adverse events related to MSC treatment. Adverse events included knee pain or swelling. eFigure 8 summarizes the event rates with their 95% CIs. Owing to the large clinical and statistical heterogeneity among the studies, we did not pool the adverse event rates. In 10 studies that reported timing of adverse event,5,6,31,32,34,37–39,45,50 knee pain or swelling occurred within 1 week after MSC treatment; these symptoms were treatable with pain medication.
Summary of quality of evidence
Table 2 shows a summary of evidence according to the GRADE approach.12 The effects estimate was downgraded in all outcome measures. None of these effects estimates were upgraded. Each meta-analysis scored 1 (very low) or 2 (low) with the GRADE approach, indicating very little (i.e., the true effect is likely to be substantially different from the effect estimate) or limited (i.e., the true effect may be substantially different from the effect estimate) confidences of the effects estimate.12
Table 2.
Summary of body of evidence according to the GRADE’s approach
| Outcome | SMD (95% CI) | Study design | Sample size | Downs and black scale | Heterogeneity | Effect of rehab. | Level of evidence (GRADE) |
|---|---|---|---|---|---|---|---|
| VAS pain score | −1.45 (−1.94, −0.96) | 12 × Within-subject repeated design 8 × Quasi-experimental design 7 × RCT | n = 318 | 7.2 ± 2.6 (7 [4–12]) points | I2 = 84% | Unclear | ⊕ ⊖ ⊖ ⊖ Very lowa,b,d |
| VAS pain score (Trim-and-fill) | −0.93 (−1.29, −0.56) | ⊕ ⊖ ⊖ ⊖ Very lowa,b | |||||
| VAS pain score (sensitivity analysis) | −0.67 (−1.28, −0.05) | 7 × RCT | n = 75 | 10.9 ± 2.0 (12 [8–12]) points | I2 = 68% | Unclear | ⊕ ⊖ ⊖ ⊖ Very lowb,c,d |
| Self-reported physical function | 1.50 (1.09, 1.92) | 11 × Within-subject repeated design 12 × Quasi-experimental design 6 × RCT | n = 528 | 7.2 ± 2.0 (7 [4–12]) points | I2 = 86% | Significant effect modifiere | ⊕ ⊖ ⊖ ⊖ Very lowa,b |
| Self-reported physical function (sensitivity analysis) | 0.53 (0.07, 0.99) | 6 × RCT | n = 60 | 10.7 ± 2.1 (12 [8–12]) points | I2 = 33% | Unclear | ⊕ ⊕ ⊖ ⊖ Lowc,d |
| Cartilage volume | 0.49 (−0.19, 1.16) | 1 × Within-subject repeated design 3 × Quasi-experimental design | n = 20 | 6.3 ± 1.5 (7 [4–7]) points | I2 = 0% | Unclear | ⊕ ⊖ ⊖ ⊖ Very lowa,c,d |
| Cartilage quality | −1.99 (−3.51, −0.47) | 3 × Within-subject repeated design 3 × Quasi-experimental design 1 × RCT | n = 95 | 7.4 ± 2.1 (7 [5–12]) points | I2 = 91% | Unclear | ⊕ ⊖ ⊖ ⊖ Very lowa,b,c,d |
aDowngraded for risk of bias (most of included studies scored less than 8 points on the Downs and Black scale)
bDowngraded for inconsistency (results were highly heterogeneous across included studies)
cDowngraded for imprecision (clinical action would differ if true SMD is the upper or the lower boundary of the 95% CI)
dDowngraded for publication bias (Egger’s regression test was positive or unable to determine because of a few included studies [<10 data set])
ePresence of rehabilitation (physical therapy modalities, range of motion exercise, or muscle strength exercise) is a significant effect modifier on the SMD for self-reported physical function (regression coefficient: 0.881, 95% CI: 0.049, 1.712
P = 0.039; see eTable 8 in the Supplementary Materials)
Discussion
This systematic review and meta-analysis found that MSC treatment significantly improved knee pain and self-reported physical function in patients with knee OA. While MSC treatment has an insignificant tendency to improve cartilage volume, MSC treatment significantly improved cartilage quality. However, these data should be interpreted with caution because the quality of evidence was “very low” to “low” according to the GRADE approach because of the poor study design, high risk of bias, large heterogeneity, and wide 95% CI of the pooled SMD. Sensitivity analyses showed that these GRADE ratings were comparable even if we only included RCTs in the meta-analysis; therefore, the true effect is likely to be substantially different from the effects estimate.12 Detail information about rehabilitation was lacking, but rehabilitation was a significant effect modifier of MSC treatment on self-reported physical function. We suggest that more high quality RCTs with stratification for rehabilitation are needed to facilitate a foundation of effective MSC therapy and regenerative rehabilitation.
The search strategies used in this study provide a more comprehensive assessment of relevant articles by adding new findings to the recent meta-analysis for the clinical efficacy of MSCs transplantation for knee OA and focal cartilage defect up to a maximum 24 months follow-up.10 Indeed, the current meta-analysis further added 28 non-RCTs and 4 RCTs to the previous meta-analysis,10 which enable us to examine the latest evidence of both benefits and harms of MSCs treatment on degenerative knee OA with a longer follow-up period that cannot be adequately determined by reviewing only RCTs.19
We found that the pooled effect size on the VAS pain score exceeded the effects of nonsteroidal anti-inflammatory drugs and corticosteroid injections,53,54 consistent with previous meta-analyses.7,9,10 The mean differences after intervention were ≥10% for both pain and self-reported physical function,55 exceeding the minimum for clinically important differences, and meeting the responder criteria of the Outcome Measures in Rheumatology Clinical Trials and Osteoarthritis Research Society International. However, we found a large heterogeneity among studies, which was partly explained by the level of risk of bias, cell donor type, and study design. Including only RCTs, which has a lower risk of bias than non-RCTs, in the meta-analysis attenuated the effects of MSC treatment in improving knee pain and self-reported physical function, supporting this interpretation. The observed effects from RCTs had a wide 95% CI, and clinical action would differ if the true SMD was the upper or lower boundary of the 95% CI. This suggests the need for a larger number of RCTs to elucidate whether MSC treatment can provide clinical benefit to patients with knee OA.
The strength of this meta-analysis is that we estimated pooled SMD for structural outcomes of articular cartilage evaluated by MRI. This effect estimate was based on only 2 non-RCTs with 4 data sets, raising the need for high quality RCTs for examination of the structural modifying effects of MSC treatment. We found a discrepancy between MSC efficacy on cartilage quality and MSC efficacy on cartilage quantity (volume). While MSC treatment improved cartilage quality, it did not significantly improve cartilage volume. Although these results should be interpreted cautiously because the studies that evaluated cartilage quality differed from that evaluated cartilage volume, we found that MSC treatment may have a limited therapeutic effect on cartilage volume. Three of these 4 data sets were based on data from patients with severe knee OA (K/L grade ≥3), which may cause limited efficacy in improving cartilage volume. Furthermore, the mean follow-up period in these studies was within 6 months, which might be too short to show a biological effect. One high quality study42 found that MSC injection particularly improved knee pain when a relatively large number of MSCs was used, but a significant increase in cartilage volume did not accompany this pain reduction, indicating that improved knee pain is not necessarily attributable to increased cartilage volume. Although this meta-analysis only included outcome measures for articular cartilage, some included studies found that MSC treatment improved subchondral bone edema25,26,46 and meniscus thickness,36 which are predictors of knee pain severity.56 Improved knee pain after autologous chondrocyte implantation on cartilage defects moderately correlated with bone edema, but not the cartilage structure evaluated using MRI.17 Further studies that investigate the mechanism of pain reduction after MSC treatment in patients with knee OA would be of interest.
Physical factors regulate MSC differentiation and tissue development, pointing to a potential therapeutic strategy for enhancing the MSCs injected into the knee joint.13,14 Weight-bearing might influence the structural outcome evaluated by MRI in the postoperative phase of autologous chondrocyte implantation.17,18 The mean follow-up period after MSC treatment was 3–60 months in the included studies, which includes some rehabilitation and physical activity programs in the post-MSC treatment phase. These post-MSC rehabilitations might affect the effects of cell-based therapy. Indeed, the presence of rehabilitation was a significant effect modifier of SMD on self-reported physical function. Although the presence of a rehabilitation program was not a significant effect modifier of the estimated effect on VAS pain score, rehabilitation does not necessarily have no impact; the lack of statistical power due to a small number of studies in the meta-analysis19 and the lack of details of rehabilitation program in each article may explain this absence. As physiological stimulation such as moderate level exercise,57 ultrasound irradiation,58 and mechanical loading after joint distraction59 may enhance cartilage regeneration after MSC injection in a preclinical study, applying exogenous stimulation may be one strategy for enhancing the injected MSCs. This point is particularly important because the lower boundary of the 95% CI of SMD on knee pain and physical function corresponds to the lower effect size in the meta-analysis of RCTs. As all the included RCTs did not report (perform) rehabilitation and none of the included non-RCTs stratified for rehabilitation program, investigating the effects of rehabilitation on the SMD of MSC treatment would be of interest in future studies. Rehabilitation programs was differed among the included studies; thus, this review highlights the need for a standardized rehabilitation program that encompasses at least weight-bearing schedule, range of motion exercise, and muscle strength exercise, which would influence the therapeutic effect of MSCs to facilitate further comparisons among studies. The implementation of longitudinal activity-based questionnaires might help address this question.
We observed a large heterogeneity of adverse event rates among the included studies; this observation limits our ability to summarize the adverse event rate. The causes of heterogeneity in this study are unclear. Detailed reports on adverse events are sparse, which may have contributed to the heterogeneity. Nevertheless, we found only minor adverse events (knee pain/swelling) after MSC treatment, indicating that benefits may outweigh harms of MSC treatment of knee OA. These findings can be achieved by reviewing the data from both non-RCTs and RCTs, which is the strength of the present meta-analysis. Most adverse events occurred within 1 week following MSC treatment. Conversely, pain or swelling that persists for more than 1 week should be interpreted as a rare and potentially severe adverse event that might contribute to arthrogenic muscle inhibition.60 Close attention to adverse events may be key to the clinical success in optimizing post-MSC treatment of knee OA.
Autologous MSCs are a widely selected source to minimize the immune response and an excellent therapeutic option for treating OA. Most included trials used autologous MSCs to eliminate immune rejection, while 2 of 35 articles attempted to investigate the potential application of allogeneic MSCs.47,50 No observed severe adverse event indicates the safety of allogeneic MSCs for applying knee OA. The present meta-analysis revealed that the therapeutic effects of VAS pain score and self-reported physical function were likely higher in autologous than in allogeneic MSCs. However, direct comparisons of the therapeutic effects between autologous and allogeneic MSCs are difficult because these are based on data from different studies. Moreover, two of the studies of allogeneic MSCs were RCTs, which had lower risks of bias than those of autologous MSCs, which might have contributed to the lower therapeutic effect. Thus, direct comparison between autologous and allogeneic MSCs in the same trial would be of interest.
This systematic review included patients with knee OA diagnosed either radiographically or clinically, and excluded those with a focal cartilage defect. Thus, the observed effect of MSCs on clinical outcomes may not hold true in patients with focal cartilage defects. As knees with OA have diffuse cartilage loss rather than an isolated cartilage lesion, several researchers have sought to assess the effect of inter-articular MSC injections rather than implantation to a focal lesion. Whereas MSC implantation on focal cartilage defects in both preclinical and clinical studies is effective in cartilage repair, the cartilage repair effects of intra-articular injection is controversial.61 We found that the type of treatment was a strong effect modifier of MSC treatment on physical function. It should be highlighted that 2 studies failed to detect a clear dose-response relationship between injected MSC and cartilage volume42 and cartilage quality;6 thereby no effects estimates were upgraded in the GRADE approach. Mamidi et al. recently suggested that investigating post-transplanted MSC behavior and how to enhance the potency of the transplanted MSCs are the major challenges to be directly solved in future research.4 We could not address post-injected MSC behavior in the diseased microenvironment; investigating the kinematics of injected MSCs is needed to enhance their disease-modifying effects.
The present study has some limitations. First, this meta-analysis included non-RCTs with 3 case reports. As non-RCTs would have greater bias and more confounders than RCTs, evaluating MSC efficacy using only RCTs might be preferable.19 Thus, we performed a sensitivity analysis and calculated the effect estimate based on RCTs. Meta-analyses that include non-RCTs can provide evidence of effects that are difficult to detect using a RCT, such as long-term effects and adverse events. Evaluating the beneficial and harmful effects of MSC treatment would be needed to make decisions about the clinical utility of MSC treatment. As discussed previously, as no RCTs have performed rehabilitation, the present meta-analysis, which included non-RCTs, could shed light on the importance of rehabilitation as a new strategy for enhancing functional improvement after MSC treatment and would set a basis for future high quality RCTs. Second, this meta-analysis included 35 studies, but few studies were available for use in the meta-analysis of structural outcomes. This dearth is attributable to the absence of a standard system for evaluating cartilage regeneration. Many studies that use MRI to evaluate cartilage regeneration are only qualitative;20,25–27,33,36 using validated imaging outcomes would be integral for scientifically validating cell-based therapies and precipitously advancing efficacy.62 Third, the pooled SMD included the effects of cointervention such as PRP with injected or implanted MSC. PRP improves knee pain and physical function in patients with knee OA,63 and has a similar effect to MSC injection;45 the pooled SMD might be attributed to the cointervention. Nevertheless, we confirmed that use of PRP was not a significant predictor of the pooled SMD (data not shown). Fourth, many studies included in this meta-analysis were performed by the same group of investigators.28–32,43–45,48 Thus, caution is required when interpreting the effect estimate, and further studies from different investigators are needed to elucidate the effects of MSCs on knee OA. Finally, a protocol for this systematic review has not been registered. However, protocol registration was not associated with outcome reporting bias in the meta-analysis,64 and the outcome measures were extracted according to the highest rank on the pain and functional outcome hierarchy, determined a priori.65,66
In conclusion, MSC treatment improves knee pain, physical function, and cartilage quality, without any severe adverse events. However, evidence for these outcomes that are considered critical for clinical decision making was “very low” to “low” according to the GRADE system because of the poor study design, high risk of bias, large heterogeneity, and wide 95% CI of the effects estimate. These GRADE ratings were similar even if only high quality RCTs were included in the meta-analysis. Detail information about rehabilitation is lacking; therefore, the role of rehabilitation in MSC treatment in patients with knee OA is unclear. However, rehabilitation was a significant effect modifier of better MSC treatment on self-reported physical function, supporting a concept of the newly born field, regenerative rehabilitation. Integration of rehabilitation into MSC-based therapy may be beneficial at least in improving physical function. These findings would help researchers and clinicians in designing future high quality clinical trials.
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement,67 PRISMA protocols (PRISMA-P),68 meta-analysis of observational studies in epidemiology (MOOSE) checklist,69 and Cochrane handbook for systematic reviews of interventions.19 A detailed protocol for this systematic review has not been previously published and registered.
Literature search and study selection
The electronic databases of PubMed, Physiotherapy Evidence Database (PEDro), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials were used. Searches used combined key terms, including “osteoarthritis, knee,” “transplantation,” “stem cells,” and “stromal cells,” using Medical Subject Headings terms. A database search strategy and determining inclusion are provided in the eMethods 1 and 2.
Outcome measures and data extraction
The primary outcomes in this review were (i) pain, (ii) self-reported physical function, (iii) structural outcomes of articular cartilage evaluated using MRI, and (iv) adverse events relevant to MSC treatment. Two reviewers independently extracted the data regarding authors, country, study design (single-arm, prospective follow-up studies, quasi-experimental studies, and RCTs), subject population, K/L grade, treatment, cell donor type, outcome measures, follow-up period, rehabilitation program, and funding sources using standardized data forms. When an article reported outcomes using multiple pain and functional scales, we used only the scale with the highest rank on the pain and functional outcome hierarchy, in accordance with previous recommendations65,66 and meta-analyses70 (eMethod 3).
Data analysis
Percent agreement of duplicate study removal and interrater reliability of title/abstract and full-text screening between the two reviewers were evaluated. For the meta-analysis, pooled estimates and 95% CIs for SMDs for changes in outcomes were calculated using the DerSimonian-Laird method.71 The SMD was calculated for paired samples using the within-patient change for patients treated with MSC divided by the pooled standard deviation (SD). Formulae for calculating the pooled SD and pooled SMD are shown in eMethod 5. The meta-analyses were performed using Review Manager Version 5.3 (Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). We used a forest plot to represent the meta-analysis results in accordance with a previous study.72 The size of the SMD was interpreted using Cohen’s d73 (<0.5: small effect size, 0.5–0.8: moderate effect size, and ≥0.8: large effect size). As a clinical frame of reference, a small effect is equivalent to the effect of non-steroidal anti-inflammatory drugs on knee pain in OA trials.53 A moderate effect is equivalent to the effect of corticosteroid injections on knee pain.54 When mean and SD values were not directly reported in an article, they were calculated from other available data, if possible (eMethod 6). To test for publication bias, we used a funnel plot and Egger’s test,74 where publication bias is the tendency for positive trials to be published and the tendency for negative or null trials to not be published. We interpreted P-values of <0.10 to indicate the existence of publication bias, as practiced by a previous study.74 When studies are relatively few, the power of the test is too low to distinguish chance from real asymmetry; we tested for publication bias only when least 10 studies were included in the meta-analysis,19 and if present, adjustment was planned using a trim-and-fill method.75 As SMD would be difficult to interpret in a clinical context, the mean differences in pain and functional outcomes were also calculated and compared with minimum clinically important difference (eMethod 7). Furthermore, we performed prespecified sensitivity analyses to provide pooled SMD with 95% CI by using the data from RCTs only.
Study heterogeneity was assessed using the I2 statistic and Q statistic.76 If I2 was ≥50, random effects meta-regression was performed using the certain parameters selected a priori including the presence of rehabilitation, defined when patients were treated using physical therapy modalities, range of motion exercise, or muscle strength exercise at least one time after MSC treatment (eMethod 8). Adverse events were evaluated in each study, and adverse event rates were calculated from the numbers of events and sample sizes by using the Comprehensive Meta-Analysis software (Biostat, Inc., Englewood, NJ, USA). All other statistical analyses were performed using JMP Pro 12.2 (SAS Institute, Cary, NC, USA).
Additional methods
Additional methods for assessment of risk of bias and GRADE approach are provided in eMethods in the Supplement.
Data availability
Data available on request from the authors.
Electronic supplementary material
Acknowledgements
The authors thank members of the regenerative rehabilitation team (Kyoto University, Kyoto) for their assistance and advice. This study was supported in part by a Grant-in-Aid from the Japan Society for the Promotion of Science (https://www.jsps.go.jp/) for Research Fellows to HI.
Authors contributions
All authors met following criteria: substantial contributions to the conception and design of the study, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be submitted; and accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The specific contributions of the authors are as follows: Conception and design of the study: H. I., T. I., and T. A. Analysis and interpretation of the data: H. I., T. I., H. K., and T. A. Drafting of the article: H. I., T. I., M. T., and T. A. Critical revision of the article for important intellectual content: H. I., T. I., H. K., and T. A. Final approval of the article: H. I., T. I., H. K., M. T., and T. A. Statistical expertize: H. I. and T. I. Obtaining of funding: H. I. Collection and assembly of data: H. I. and T. I.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hirotaka Iijima and Takuya Isho contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies the paper on the npj Regenerative Medicine website (10.1038/s41536-018-0041-8).
References
- 1.VanItallie TB. Gout: epitome of painful arthritis. Metabolism. 2010;59:S32–S36. doi: 10.1016/j.metabol.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pers YM, Ruiz M, Noel D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthr. Cartil. 2015;23:2027–2035. doi: 10.1016/j.joca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthr. Cartil. 2016;24:1307–1316. doi: 10.1016/j.joca.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Lamo-Espinosa JM, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II) J. Transl. Med. 2016;14:246. doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pers YM, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl. Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int. Orthop. 2015;39:2363–2372. doi: 10.1007/s00264-015-2785-8. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, et al. Effect of mesenchymal stromal cells for articular cartilage degeneration treatment: a meta-analysis. Cytotherapy. 2015;17:1342–1352. doi: 10.1016/j.jcyt.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: a meta-analysis. Exp. Ther. Med. 2016;12:3390–3400. doi: 10.3892/etm.2016.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yubo M, et al. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PloS One. 2017;12:e0175449. doi: 10.1371/journal.pone.0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murad MH, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA. 2014;312:171–179. doi: 10.1001/jama.2014.5559. [DOI] [PubMed] [Google Scholar]
- 12.Balshem H, et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Jung Y, Kim SH, Kim YH, Kim SH. The effects of dynamic and three-dimensional environments on chondrogenic differentiation of bone marrow stromal cells. Biomed. Mater. 2009;4:055009. doi: 10.1088/1748-6041/4/5/055009. [DOI] [PubMed] [Google Scholar]
- 14.Kwon HJ, Lee GS, Chun H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016;6:39302. doi: 10.1038/srep39302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosio F, Russell A. Regenerative rehabilitation: a call to action. J. Rehabil. Res. Dev. 2010;47:11–15. doi: 10.1682/JRRD.2010.03.0021. [DOI] [PubMed] [Google Scholar]
- 16.Moritz CT, Ambrosio F. Regenerative rehabilitation: combining stem cell therapies and activity-dependent stimulation. Pediatr. Phys. Ther. 2017;29:S10–S15. doi: 10.1097/PEP.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wondrasch B, Risberg MA, Zak L, Marlovits S, Aldrian S. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: a prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am. J. Sports Med. 2015;43:146–153. doi: 10.1177/0363546514554910. [DOI] [PubMed] [Google Scholar]
- 18.Edwards PK, Ackland TR, Ebert JR. Accelerated weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint: early clinical and radiological outcomes. Am. J. Sports Med. 2013;41:2314–2324. doi: 10.1177/0363546513495637. [DOI] [PubMed] [Google Scholar]
- 19.Higgins, J. P. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions. (John Wiley & Sons, 2011).
- 20.Bui KHT, et al. Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomed. Res. Ther. 2014;1:02–08. doi: 10.15419/bmrat.v1i01.11. [DOI] [Google Scholar]
- 21.Centeno CJ, et al. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med. Hypotheses. 2008;71:900–908. doi: 10.1016/j.mehy.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Centeno CJ, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 23.Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011;14:211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 24.Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2016;19:219–225. doi: 10.1111/1756-185X.12670. [DOI] [PubMed] [Google Scholar]
- 25.Emadedin M, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch. Iran. Med. 2012;15:422–428. [PubMed] [Google Scholar]
- 26.Emadedin M, et al. Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch. Iran Med. 2015;18:336–344. [PubMed] [Google Scholar]
- 27.Fodor PB, Paulseth SG. Adipose derived stromal cell (adsc) injections for pain management of osteoarthritis in the human knee joint. Aesthet. Surg. J. 2016;36:229–236. doi: 10.1093/asj/sjv135. [DOI] [PubMed] [Google Scholar]
- 28.Kim YS, Choi YJ, Koh YG. Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes. Am. J. Sports Med. 2015;43:2293–2301. doi: 10.1177/0363546515588317. [DOI] [PubMed] [Google Scholar]
- 29.Kim YS, et al. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthr. Cartil. 2016;24:237–245. doi: 10.1016/j.joca.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Koh YG, Choi YJ, Kwon OR, Kim YS. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am. J. Sports Med. 2014;42:1628–1637. doi: 10.1177/0363546514529641. [DOI] [PubMed] [Google Scholar]
- 31.Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:1308–1316. doi: 10.1007/s00167-013-2807-2. [DOI] [PubMed] [Google Scholar]
- 32.Koh YG, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Michalek, J., et al. WITHDRAWN: autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transpl. (2015). [DOI] [PubMed]
- 34.Orozco L, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95:1535–1541. doi: 10.1097/TP.0b013e318291a2da. [DOI] [PubMed] [Google Scholar]
- 35.Orozco L, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Transplantation. 2014;97:e66–e68. doi: 10.1097/TP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 36.Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J. Med. Case Rep. 2011;5:296. doi: 10.1186/1752-1947-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampson S, et al. Intra-articular bone marrow concentrate injection protocol: short-term efficacy in osteoarthritis. Regen. Med. 2016;11:511–520. doi: 10.2217/rme-2016-0081. [DOI] [PubMed] [Google Scholar]
- 38.Soler R, et al. Final results of a phase I-II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23:647–654. doi: 10.1016/j.knee.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Soler Rich R, et al. Treatment of knee osteoarthritis with autologous expanded bone marrow mesenchymal stem cells: 50 cases clinical and MRI results at one year follow-up. J. Stem Cell Res. Ther. 2015;5:1–7. [Google Scholar]
- 40.Turajane T, et al. Combination of intra-articular autologous activated peripheral blood stem cells with growth factor addition/ preservation and hyaluronic acid in conjunction with arthroscopic microdrilling mesenchymal cell stimulation Improves quality of life and regenerates articular cartilage in early osteoarthritic knee disease. J. Med. Assoc. Thail. 2013;96:580–588. [PubMed] [Google Scholar]
- 41.Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed. Res. Int. 2014;2014:370621. doi: 10.1155/2014/370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jo CH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 43.Kim YS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am. J. Sports Med. 2015;43:176–185. doi: 10.1177/0363546514554190. [DOI] [PubMed] [Google Scholar]
- 44.Kim YS, et al. Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am. J. Sports Med. 2015;43:2738–2746. doi: 10.1177/0363546515599632. [DOI] [PubMed] [Google Scholar]
- 45.Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19:902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen PD, et al. Comparative clinical observation of arthroscopic microfracture in the presence and absence of a stromal vascular fraction injection for osteoarthritis. Stem Cells Transl. Med. 2017;6:187–195. doi: 10.5966/sctm.2016-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta PK, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel(R)): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016;18:301. doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koh YG, Kwon OR, Kim YS, Choi YJ. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30:1453–1460. doi: 10.1016/j.arthro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 49.Varma HS, Dadarya B, Vidyarthi A. The new avenues in the management of osteo-arthritis of knee–stem cells. J. Indian Med. Assoc. 2010;108:583–585. [PubMed] [Google Scholar]
- 50.Vega A, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99:1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 51.Wakitani S, et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 52.Wong KL, et al. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29:2020–2028. doi: 10.1016/j.arthro.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 53.Biswal S, Medhi B, Pandhi P. Longterm efficacy of topical nonsteroidal antiinflammatory drugs in knee osteoarthritis: metaanalysis of randomized placebo controlled clinical trials. J. Rheumatol. 2006;33:1841–1844. [PubMed] [Google Scholar]
- 54.Zhang W, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Pham T, et al. OMERACT-OARSI initiative: osteoarthritis research society international set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr. Cartil. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Torres L, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthr. Cartil. 2006;14:1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi S, et al. The effect of exercise on the early stages of mesenchymal stromal cell-induced cartilage repair in a rat osteochondral defect model. PLoS One. 2016;11:e0151580. doi: 10.1371/journal.pone.0151580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi S, et al. Effect of low-intensity pulsed ultrasound after mesenchymal stromal cell injection to treat osteochondral defects: an in vivo study. Ultrasound Med. Biol. 2016;42:2903–2913. doi: 10.1016/j.ultrasmedbio.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 59.Harada Y, et al. Combination therapy with intra-articular injection of mesenchymal stem cells and articulated joint distraction for repair of a chronic osteochondral defect in the rabbit. J. Orthop. Res. 2015;33:1466–1473. doi: 10.1002/jor.22922. [DOI] [PubMed] [Google Scholar]
- 60.Rice DA, McNair PJ, Lewis GN, Dalbeth N. Quadriceps arthrogenic muscle inhibition: the effects of experimental knee joint effusion on motor cortex excitability. Arthritis Res. Ther. 2014;16:502. doi: 10.1186/s13075-014-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freitag J, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet. Disord. 2016;17:230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tucker JD, Ericksen JJ, Goetz LL, Elmore LW. Should clinical studies involving “regenerative injection therapy,” strive to incorporate a triad of outcome measures instead of only including clinical outcome measures? Osteoarthr. Cartil. 2014;22:715–717. doi: 10.1016/j.joca.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Chang KV, et al. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014;95:562–575. doi: 10.1016/j.apmr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Tsujimoto Y, et al. Protocol registration of systematic reviews published in high-impact factor journals: a meta-epidemiological study. J. Clin. Epidemiol. 2017;84:54–60. doi: 10.1016/j.jclinepi.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 65.McAlindon TE, et al. OARSI clinical trials recommendations: design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthr. Cartil. 2015;23:747–760. doi: 10.1016/j.joca.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res. 2011;63:S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 68.Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 69.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 70.Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115–3121. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 71.Deeks, J. J. & Higgins, J. P. Statistical algorithms in review manager 5. Statistical Methods Group of The Cochrane Collaboration. 1–11 (2010).
- 72.Anzures-Cabrera J, Higgins JP. Graphical displays for meta-analysis: an overview with suggestions for practice. Res. Synth. Methods. 2010;1:66–80. doi: 10.1002/jrsm.6. [DOI] [PubMed] [Google Scholar]
- 73.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 74.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 76.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors.