Abstract
Low grade serous ovarian cancer (LGSOC) is a slowly growing, relatively chemoresistant neoplasm that is associated with a more favorable prognosis, especially compared to the disease's high-grade serous counterpart. We recount a case involving a 47-year-old, heavily pretreated LGSOC patient who presented with an elevated CA-125 of 1047 U/mL during her recent course of pemetrexed therapy. Thereafter, she underwent molecular profiling, which revealed a BRAF V600E mutation; accordingly, the patient was administered dabrafenib and trametinib combination therapy, a regimen that resulted in a precipitous decline of her CA-125 to 35 U/mL following the 6th cycle. The patient's favorable response to BRAF and MEK 1/2 inhibitor therapy underscores the significance of molecular profile testing and the use of targeted therapy regardless of tissue origin, especially in cases for whom standard management is limited or ineffective.
Keywords: Low grade serous ovarian cancer, BRAF mutation, Dabrafenib, Trametinib
Highlights
-
•
Low grade serous ovarian cancers are typically chemoresistant and difficult to manage.
-
•
We report on a low grade serous ovarian cancer patient diagnosed with a BRAF mutation.
-
•
The patient's symptoms and CA-125 normalized after dabrafenib and trametinib therapy.
1. Introduction
Ovarian cancer is the fifth most frequent cause of cancer-related mortality among women in the United States, resulting in 14,070 deaths annually (Siegel et al., 2018). High grade serous carcinoma is the most commonly encountered subtype whereas low grade serous ovarian adenocarcinoma comprises only 10% of cases (Seidman et al., 2004). Additionally, high grade serous ovarian cancers are extremely aggressive, whereas the low grade classification is more quiescent but less chemo-sensitive and relatively intractable (Grisham et al., 2013).
Ovarian serous borderline tumors are often a precursor of low-grade serous carcinoma and morphologically typified by noninvasive growth and low-grade cytology (Grisham et al., 2013). Invasive low-grade serous carcinomas and serous borderline line tumors feature BRAF and KRAS mutations in approximately 68% of cases, but rarely p53 mutations (Sieben et al., 2004). Alternatively, high-grade serous carcinomas frequently contain p53 mutations but uncommonly express BRAF and KRAS mutations (Leitao et al., 2004; Wong et al., 2010).
Low grade serous ovarian cancer features a high mutation rate in the MAP-kinase pathway, which is mediated by KRAS and BRAF mutations; and approximately 35% of these patients have a V600E BRAF mutation, for which targeted medication may confer an enhanced benefit (Grisham et al., 2013; Combe et al., 2015). Similar to melanoma, low grade serous ovarian cancer patients with a V600E BRAF mutation have responded favorably to molecular targeted medications, namely BRAF and MEK 1/2 inhibitor therapy (e.g., cobimetinib, vemurafenib) (Combe et al., 2015; Hyman et al., 2015). In the current report, we describe a heavily pretreated, low grade serous ovarian cancer patient with a BRAF V600E mutation who was successfully treated with dabrafenib and trametinib combination therapy.
2. Case report
A 47-year-old (gravida 3, para 3) woman with an unremarkable medical history originally presented to our gynecologic oncology service with a complex, fixed pelvic mass and a CA-125 of 183 U/mL in September 2010. She underwent an exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, resection of the posterior cul-de-sac peritoneum, omentectomy and tumor debulking. At the surgery's conclusion, all gross tumor was resected; following pathologic evaluation, the patient was diagnosed with a Stage IIIB, low grade serous ovarian carcinoma.
The patient initiated 6 cycles of paclitaxel (175 mg/m2) and carboplatin (AUC 6) chemotherapy in October 2010 and continued with paclitaxel maintenance chemotherapy (135 mg/m2), which ultimately induced a decline in her CA-125 to 40 U/mL. However, in August 2014, the patient's CA-125 was elevated at 191 U/mL and a subsequent CT/PET scan was positive for an abdominopelvic recurrence. Thereafter, she was treated with six cycles of paclitaxel (175 mg/m2) and carboplatin (AUC 6) chemotherapy, which resulted in a decline in her CA-125 to 72 U/mL.
The patient's disease remained stable until March 2015, whereupon a laparoscopic evaluation revealed miliary disease on the vesicouterine peritoneum and also on the hemidiaphragm, bilaterally; she was administered 4 cycles of topotecan (2 mg/m2) and at the conclusion of her treatment, the CA-125 remained essentially constant at 77 U/mL. Liposomal doxorubicin (40 mg/m2) and bevacizumab (10 mg/kg) combination therapy was completed in October 2015, but alas, the patient's CA-125 was persistently elevated at 130 U/mL. Gemcitabine (900 mg/m2) and etoposide (50 mg/m2) were subsequently administered as contiguous regimens from October 2015 until April 2016, however, her CA-125 levels increased to 909 U/mL.
In May 2016, pemetrexed (500 mg/m2) was administered and following cycle 14 in February 2017, the patient's CA-125 declined to 166 U/mL; but after cycle 15 in May 2017, the CA-125 had ascended to 203 U/mL. Nevertheless, the cancer antigen values continually fluctuated and hence, the regimen was maintained until September of 2017, whereupon the CA-125 increased to 334 U/mL. Since the patient's original cancer tissue was obtained nearly seven years ago and tumor heterogeneity was a concern (Gui et al., 2015), a laparoscopic biopsy was performed in October 2017 to acquire tissue for a Caris assay (Caris MI Profile, Caris Life Sciences, Phoenix, AZ); the results suggested that carboplatin was potentially beneficial but following completion of two cycles, the CA-125 ascended to 1047 U/mL, indicative of progressive disease. Additionally, the molecular profiling disclosed a BRAF V600E mutation, an ATM mutation and overexpression of TUBB3 and TOP2A. Clinically, the patient began experiencing respiratory difficulties and was referred for radionuclide angiography, which revealed normal cardiac wall motion and mild tachycardia but no evidence of pulmonary embolism.
In response to the BRAF mutation, the patient was treated with dabrafenib (150 mg) and trametinib (2 mg) combination therapy in January 2018; after cycle 4 in April 2018, the CA-125 declined from 1186 to 102 U/mL. A CT scan of the chest, abdomen and pelvis revealed a 5 × 2.3 cm lesion within the mid aspect of the pelvis, suggestive of recurrent tumor (Fig. 1). She continued on therapy and reported only mild fatigue as a side effect; following the conclusion of cycle 6 in June 2018, the patient's CA-125 measurement further decreased to 35 U/mL (Fig. 2). A subsequent CT scan of the chest, abdomen and pelvis indicated an absence of peritoneal nodules or abdominal pelvic lesions (see Fig. 3); she currently remains on dabrafenib and trametinib therapy.
Fig. 1.
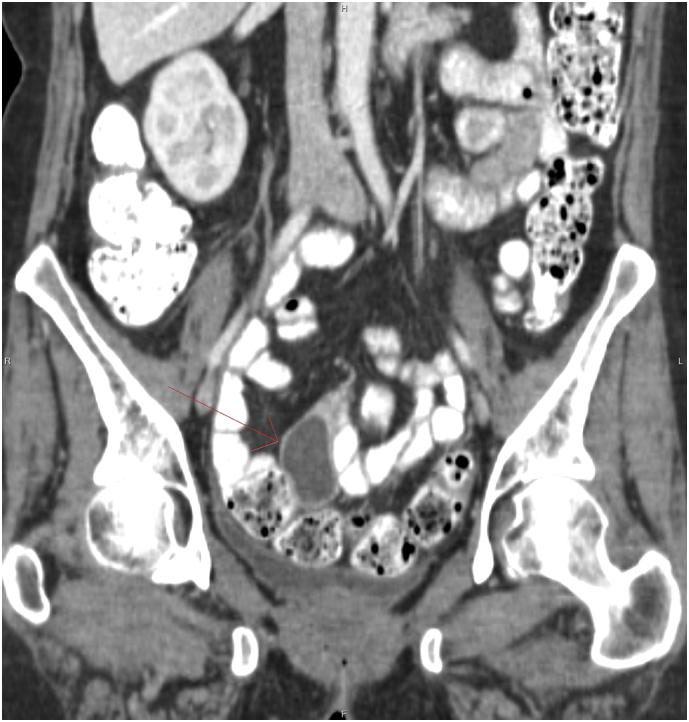
Contrast enhanced coronal projection of the pelvis demonstrating an encapsulated 4.5 cm cystic lesion with enhancing wall in the mesentery adjacent to normal small bowel opacified with oral contrast.
Fig. 2.
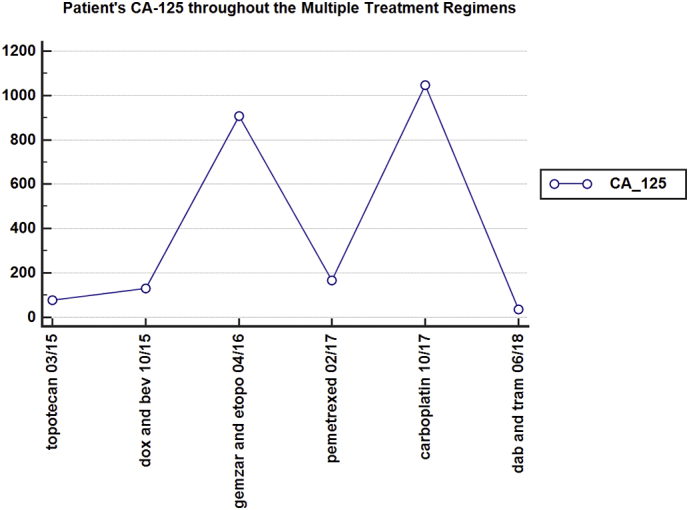
The patient's fluctuating CA-125 levels throughout her multiply treatment regimens (liposomal doxorubicin and bevacizumab (dox and bev), gemcitabine and etoposide (gemzar and etopo), dabrafenib and trametinib (dab and tram)).
Fig. 3.
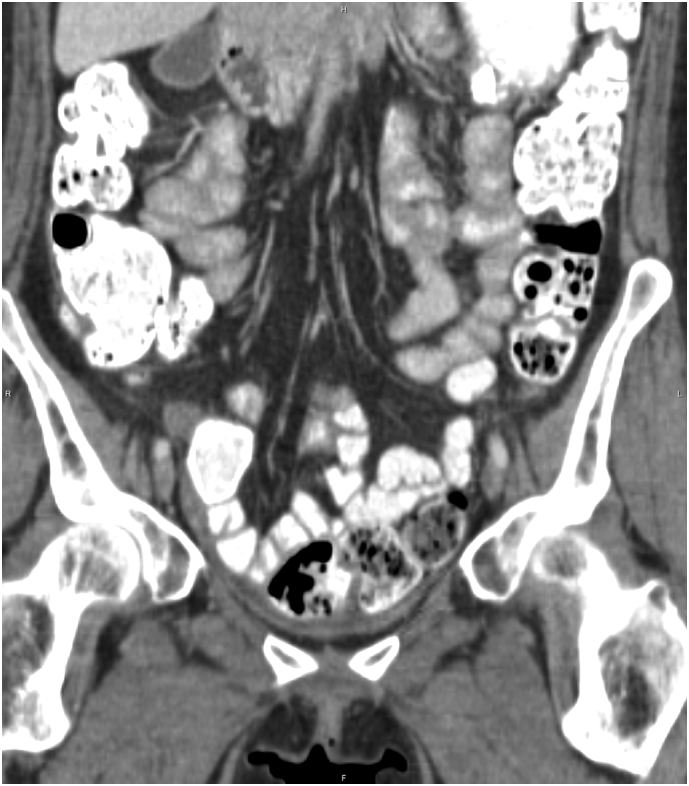
Contrast enhanced coronal projection of the pelvis performed 2 months later demonstrated resolution of the cystic lesion.
3. Conclusions
In cases of heavily pretreated ovarian cancer, viable chemotherapy options are limited. Moreover, once a patient has failed multiple treatments, the successive therapies are characterized by significantly reduced disease-free intervals (Corrado et al., 2017). Therefore, molecular pathway evaluations have been studied to ascertain targeted medications that may further enhance survival (Kaldawy et al., 2016).
The MAPK pathway is intriguing insofar as BRAF mutations seemingly impede disease progression, especially in serous borderline and low grade serous neoplasms (Wong et al., 2010; Zeppernick et al., 2014). MAPK expression has been identified in approximately 80% of low grade serous carcinomas and 78% of serous borderline tumors (Hsu et al., 2004); however, the incidence of BRAF mutations is significantly lower with low grade serous ovarian cancer (Grisham et al., 2013; Wong et al., 2010). When considering the presence of a BRAF mutation, these two ovarian cancer subtypes ostensibly have a reduced risk of disease progression and improved outcomes (Wong et al., 2010; Kaldawy et al., 2016) but do not respond uniformly to specific, targeted therapy (Kaldawy et al., 2016).
In the current study, we review the history of a low grade serous ovarian cancer patient who failed multiple lines of therapy and accordingly, her CA-125 remained significantly elevated. Henceforth, the fortuitous identification of a V600E BRAF mutation alerted us to the prospect of employing a BRAF inhibitor and MEK 1/2 inhibitor. Since the initiation of dabrafenib and trametinib therapy, the patient's CA-125 declined significantly and is now within normal limits; additionally, her clinical symptoms have dramatically improved.
Combe et al. described their experience with a low grade serous ovarian cancer patient who underwent several lines of chemotherapy in the management of her disease, none of which were definitively successful in achieving a sustained, clinical response (Combe et al., 2015). Ultimately, a V600E BRAF mutation was detected, for which she was treated with the BRAF inhibitor, vemurafenib (960 mg bid). Initially, the medication was discontinued because of dose limiting toxicity, but was eventually reinstated at a lower dose (240 mg/day). The patient's CA-125 levels decreased from 1600 to 265 U/mL and her clinical response was maintained for more than 21 months. Additionally, Hyman et al. conducted a basket study involving vemurafenib for the treatment of numerous malignancies with an attendant BRAF V600 mutation; in particular, they reported an anecdotally, improved response for a low grade serous ovarian cancer patient (Hyman et al., 2015).
Dabrafenib and trametinib appear to confer enhanced patient survival in the treatment of both BRAF V600E and V600 melanoma (Long et al., 2017), non-small cell lung cancer (Khunger et al., 2018) and high-grade primary brain tumors (Schreck et al., 2018). Single agent vemurafenib has also been reportedly effective at improving the overall survival of BRAF V600 mutated melanoma patients, especially compared to single agent dacarbazine (13.6 months vs. 9.7 months) (Chapman et al., 2017). Conversely, pembrolizumab was not associated with improved survival in ipilimumab-refractory, BRAF V600 mutant-positive melanoma patients who were treated with BRAF and/or MEK inhibitor therapy (Hamid et al., 2017). The combination of dabrafenib and trametinib is reasonably tolerable although dabrafenib is associated with cutaneous toxicities (e.g., rash, hyperkeratosis and keratoacanthoma), whereas acneiform rash, diarrhea, and peripheral edema coincide with trametinib (Knispel et al., 2018).
Low grade serous ovarian cancer patients are relatively unresponsive to initial taxane/platinum therapy and thereafter, the majority of available chemotherapy regimens have similarly, limited efficacy in treating this disease. Consequently, in accordance with the NCCN guidelines that recommend genetic testing of all ovarian cancer tumors at the time of recurrence (Daly et al., 2017), routine testing for BRAF and KRAS mutations in low grade serous ovarian cancer may identify patients who are amenable to targeted medications that will theoretically benefit their prognosis.
Conflict of interest
All authors deny any conflict of interest associated with this manuscript.
Author Contributions
AM contributed significantly to the study's initiation, development and manuscript revision.
BG substantially contributed to the study's development and manuscript revision.
KB conducted the review of the patient's diagnosis and treatment history and also substantially contributed to the study's development and manuscript revision.
PT was instrumental in reviewing the patient's chart, manuscript revision and conducting the radiologic evaluation.
RB reviewed the patient's chart, analyzed the chemotherapy treatment lines and corresponding outcomes, and significantly assisted with the manuscript development and revision.
Funding
This study was supported by the Women's Cancer Research Foundation.
References
- Chapman P.B., Robert C., Larkin J., Haanen J.B., Ribas A., Hogg D. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann. Oncol. 2017;28:2581–2587. doi: 10.1093/annonc/mdx339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe P., Chauvenet L., Lefrère-Belda M.A., Blons H., Rousseau C., Oudard S. Sustained response to vemurafenib in a low grade serous ovarian cancer with a BRAF V600E mutation. Investig. New Drugs. 2015;33:1267–1270. doi: 10.1007/s10637-015-0297-4. [DOI] [PubMed] [Google Scholar]
- Corrado G., Salutari V., Palluzzi E., Distefano M.G., Scambia G., Ferrandina G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert. Rev. Anticancer. Ther. 2017;17:1147–1158. doi: 10.1080/14737140.2017.1398088. [DOI] [PubMed] [Google Scholar]
- Daly M.B., Pilarski R., Berry M., Buys S.S., Farmer M., Friedman S. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J. Natl. Compr. Cancer Netw. 2017;15:9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- Grisham R.N., Iyer G., Garg K., Delair D., Hyman D.M., Zhou Q. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119:548–554. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui T., Cao D., Yang J., Shen K. Tumor heterogeneity has important consequences for personalized medicine in ovarian cancer. Histol. Histopathol. 2015;30:173–181. doi: 10.14670/HH-30.173. [DOI] [PubMed] [Google Scholar]
- Hamid O., Puzanov I., Dummer R., Schachter J., Daud A., Schadendorf D. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur. J. Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Hsu C.Y., Bristow R., Cha M.S., Wang B.G., Ho C.L., Kurman R.J. Characterization of active mitogen-activated protein kinase in ovarian serous carcinomas. Clin. Cancer Res. 2004;10:6432–6436. doi: 10.1158/1078-0432.CCR-04-0893. [DOI] [PubMed] [Google Scholar]
- Hyman D.M., Puzanov I., Subbiah V., Faris J.E., Chau I., Blay J.Y. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldawy A., Segev Y., Lavie O., Auslender R., Sopik V., Narod S.A. Low-grade serous ovarian cancer: a review. Gynecol. Oncol. 2016;143:433–438. doi: 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- Khunger A., Khunger M., Velcheti V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: clinical evidence and experience. Ther. Adv. Respir. Dis. 2018;12 doi: 10.1177/1753466618767611. (1753466618767611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knispel S., Zimmer L., Kanaki T., Ugurel S., Schadendorf D., Livingstone E. The safety and efficacy of dabrafenib and trametinib for the treatment of melanoma. Expert Opin. Drug Saf. 2018;17:73–87. doi: 10.1080/14740338.2018.1390562. [DOI] [PubMed] [Google Scholar]
- Leitao M.M., Soslow R.A., Baergen R.N. Mutation and expression of the TP53 gene in early stage epithelial ovarian carcinoma. Gynecol. Oncol. 2004;93:301–306. doi: 10.1016/j.ygyno.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Long G.V., Flaherty K.T., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017;28:1631–1639. doi: 10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck K.C., Guajardo A., Lin D.D.M., Eberhart C.G., Grossman S.A. Concurrent BRAF/MEK inhibitors in BRAF V600-mutant high-grade primary brain tumors. J. Natl. Compr. Cancer Netw. 2018;16:343–347. doi: 10.6004/jnccn.2017.7052. [DOI] [PubMed] [Google Scholar]
- Seidman J.D., Horkayne-Szakaly I., Haiba M. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int. J. Gynecol. Pathol. 2004;23:41–44. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- Sieben N.L.G., Macropoulos P., Roemen G. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumors. J. Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Wong K.K., Tsang Y.T., Deavers M.T., Mok S.C., Zu Z., Sun C. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am. J. Pathol. 2010;177:1611–1617. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppernick F., Ardighieri L., Hannibal C.G., Vang R., Junge J., Kjaer S.K. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am. J. Surg. Pathol. 2014;38:1603–1611. doi: 10.1097/PAS.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]


