Introduction
Pityriasis rubra pilaris (PRP) is a rare condition that has been classified into 6 subtypes dependent on morphologic features, prognosis, age of onset, and HIV status.1, 2 Classic adult subtype I accounts for approximately 55% of cases. It is clinically characterized by diffuse follicular plugging and perifollicular erythema coalescing to form orange-red scaly plaques with follicular plugging extending caudally to form generalized erythema with characteristic small islands of normal-appearing skin. This appearance is typically associated with palmoplantar keratoderma, nail thickening and subungual hyperkeratosis, and erythema with micaceous scale of the face and scalp.1
Histopathologic findings include irregular broad acanthosis with short rete ridges and thick suprapapillary plates, focal or confluent hypergranulosis, diffuse orthokeratosis with spotted parakeratosis in both horizontal and vertical dimensions, follicular plugging with a collar of perifollicular parakeratosis, and a superficial perivascular and perifollicular lymphocytic infiltrate in the dermis.3
We present a case of classic adult subtype I PRP unresponsive to therapy with acitretin, methotrexate, and etanercept but with a marked rapid and sustained response to the interleukin (IL)-17A inhibitor, ixekizumab.
Case report
A 63-year-old white man was admitted to a local burn unit for a progressive eruption of 4 weeks' duration. It began on the face and upper torso with redness and scaling, progressing caudally rapidly over the next several weeks. The eruption was relatively asymptomatic, but he did note pain on ambulation.
Initial physical examination found a widespread eruption with erythema and micaceous scale of the scalp, face, and neck; sharply demarcated erythema of the trunk and extremities with follicular prominence of scale and distinct islands of sparing; thick scale of the hands and feet; thickened nails; and subungual hyperkeratosis. No ectropion or oral mucosal lesions were noted. Laboratory data were unremarkable, including previous HIV testing, and other than mild hypertension treated with lisinopril and hydrochlorothiazide, the patient was in good health.
Based on the clinical presentation, a diagnosis of PRP was made, and a skin biopsy was obtained. This biopsy showed broad acanthosis, compact hyperkeratosis with follicular plugging, irregular parakeratosis, and a superficial dermal perivascular lymphocytic infiltrate consistent with PRP. He was placed on acitretin at the dose of 50 mg daily and discharged from the hospital.
Some initial improvement was followed by gradual exacerbation. Five months after initiation, much of the body surface was involved, and methotrexate at the dose of 10 mg weekly was added, with the continuation of acitretin (Figs 1 and 2). There was no subsequent clinical improvement, and etanercept (50 mg twice weekly) was added with the continuation of methotrexate and acitretin. The patient discontinued etanercept after 1 month of therapy because of perceived exacerbation. Ixekizumab was subsequently initiated (160 mg initial dose, 80 mg every 2 weeks for 3 months, 80 mg monthly thereafter) with the continuation of acitretin and the discontinuation of methotrexate. There was significant clinical improvement by week 4, and 8 weeks after initiation of ixekizumab, acitretin was discontinued (Figs 3 and 4). Long-term remission continued through 12 months of therapy with ixekizumab as the sole therapeutic agent with no adverse events.
Fig 1.

Pityriasis rubra pilaris before treatment with ixekizumab.
Fig 2.
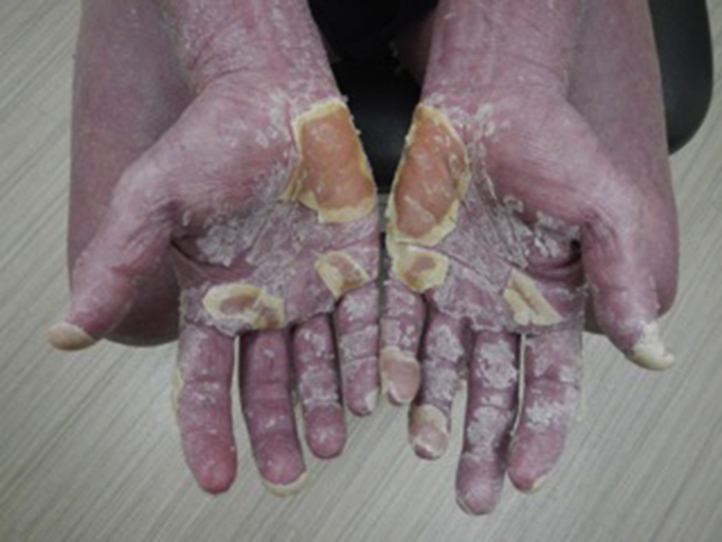
Pityriasis rubra pilaris before treatment with ixekizumab.
Fig 3.

Pityriasis rubra pilaris 8 weeks after treatment with ixekizumab.
Fig 4.

Pityriasis rubra pilaris 8 weeks after treatment with ixekizumab.
Discussion
Although PRP is a rare condition, diagnosed in approximately 1 in 5,000 presenting patients, it frequently progresses to general erythroderma, and the morbidity of the condition is significant.1 Although classic adult type I PRP is severe and frequently debilitating, it is reported that 80% of cases spontaneously resolve within 3 years.4 Historically, because of similarities clinically and histopathologically with psoriasis, PRP has been treated with oral retinoids as first-line therapy with methotrexate as an alternative, and more recently the use of biologic agents has been reported.5, 6, 7 However, the assumptions regarding the natural course of PRP and its response to treatment with traditional therapies for psoriasis have been questioned. In a retrospective case study of 100 patients with PRP, Ross et al8 found that only 26% of patients were correctly diagnosed at the time of presentation, diagnosis was delayed by 29 months on average, and that 72% had persistent clinical findings, with persistence of 58 months on average and up to 300 months. Only 59% of patients treated with oral retinoids, 52% treated with methotrexate, and 40% treated with TNF inhibitors felt treatment was helpful. In addition, in a retrospective analysis of 7 patients, Maloney et al9 found only partial (<75% response) or no response in 7 of 9 biologic regimens with adalimumab, etanercept, and ustekinumab. As such, the effectiveness of traditional therapies and biologic agents in the treatment of PRP has been questioned, and the possibility of overestimation of benefit caused by selective case reporting of positive outcomes has been noted. Clearly, a reasoned approach to treatment based on the pathophysiology of PRP is desirable.
Feldmeyer et al10 found the upregulation of helper T cell 17 (TH17) cytokines IL-17A, IL-17F, and IL-22 in lesional PRP skin samples in a patient comparable with that found in psoriasis and that a decrease in levels of expression of these TH17 cytokines paralleled improvement in histopathologic findings, suggesting a common inflammatory pathway in psoriasis and PRP.
Ixekizumab (Taltz, Eli Lilly, Indianapolis, Indiana), approved for the treatment of adult patients with moderate-to-severe plaque psoriasis in March, 2016, is a humanized IgG4 monoclonal antibody that selectively binds with IL-17A and inhibits its interaction with the IL-17A receptor and has been found to be well tolerated and efficacious.11 Previously, case reports have shown that secukinumab, another monoclonal antibody directed at IL-17A, has shown effectiveness in the treatment of PRP.12, 13 Although further investigation is needed, the demonstration of TH17 cytokines paralleling disease clinically, and case reports showing rapid response to therapy with IL-17 inhibition, offer intriguing therapeutic options for the treatment of refractory PRP.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Griffiths W.A.D. Pityriasis rubra pilaris*. Clin Exp Dermatol. 1980;5(1):105–112. doi: 10.1111/j.1365-2230.1980.tb01676.x. [DOI] [PubMed] [Google Scholar]
- 2.Miralles E.S., Núñez M., De Las Heras M.E., Pérez B., Moreno R., Ledo A. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;122:990–993. doi: 10.1111/j.1365-2133.1995.tb06939.x. [DOI] [PubMed] [Google Scholar]
- 3.Soeprono F.F. Histologic criteria for diagnosis of pityriasis rubra pilaris. Am J Dermatopath. 1986;8:277–283. doi: 10.1097/00000372-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths A. Pityriasis rubra pilaris. etiologic considerations. J Am Acad Dermatol. 1984;10(6):1086–1088. doi: 10.1016/s0190-9622(84)80365-5. [DOI] [PubMed] [Google Scholar]
- 5.Klein A., Landthaler M., Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. (report) Am J Clin Dermatol. 2010;11(3):157–170. doi: 10.2165/11530070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Wang D., Chong V.C., Chong W., Oon H.H. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018 doi: 10.1007/s40257-017-0338-1. [DOI] [PubMed] [Google Scholar]
- 7.Moretta G., De L.E., Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451–457. doi: 10.2147/CCID.S124351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross N.A., Chung H., Li Q., Andrews J.K., Keller M.S., Vitto J. Epidemiologic, clinicopathologic diagnostic and management challenges of pityriasis rubra pilaris: a case series of 103 patients. JAMA Dermatol. 2016;152(6):670–675. doi: 10.1001/jamadermatol.2016.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maloney N.J., Hisaw L.D., Worswick S. Refractory pityriasis rubra pilaris treated with etanercept, adalimumab, or ustekinumab: a retrospective investigation. Dermatol Ther. 2017 doi: 10.1111/dth.12559. [DOI] [PubMed] [Google Scholar]
- 10.Feldmeyer L., Mylonas A., Demaria O. Interleukin 23-helper T cell 17 axis as a treatment target for pityriasis rubra pilaris. JAMA Dermatol. 2017;153(4):304–308. doi: 10.1001/jamadermatol.2016.5384. [DOI] [PubMed] [Google Scholar]
- 11.Blauvelt A., Gooderham M., Iversen L. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3) J Am Acad Dermatol. 2017;77(5):855–862. doi: 10.1016/j.jaad.2017.06.153. [DOI] [PubMed] [Google Scholar]
- 12.Gauci M., Jachiet M., Gottlieb J. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2(6):462–464. doi: 10.1016/j.jdcr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster D., Pfister-Wartha A., Bruckner-Tuderman L., Schempp C.M. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152(11):1278–1280. doi: 10.1001/jamadermatol.2016.3885. [DOI] [PubMed] [Google Scholar]


