Introduction
Granulomatous dermatitis (GD) describes disorders in which mixed inflammatory infiltrates composed primarily of histiocytes invade the skin. The pathogenesis of GD is unknown; however, GD has been noted to occur in areas previously affected by trauma, sun damage, or infection.1 When GD presents at the same site of a healed, unrelated skin disease, it falls within the category of a Wolf's isotopic response.2 The regional restriction of a Wolf's isotopic response is proposed to occur due to an area of localized immunocompromise known as an immunocompromised district.3 This immunocompromised district is believed to result from various types of cutaneous damage that hinder lymph circulation, like chronic regional lymphedema or prior herpes virus infection (eg, varicella zoster virus [VZV] and herpes simplex virus [HSV]).3 Postherpetic isotopic response (PHIR) is the most commonly reported isotopic response, and more cases of PHIR-GD have been reported than any other type of isotopic response.3 It can occur within the same dermatomal distribution either immediately after primary lesion resolution (VZV>HSV) or many years later.3 Persistent VZV DNA has been detected in PHIR lesions within 4 weeks after an acute episode4, 5 but not after 7 weeks.6, 7 The presence of viral DNA in some lesions has led to the proposal that VZV glycoproteins (gpI/II) may still be expressed at a sufficient level to initiate granuloma formation.8
Since the first report of granuloma annulare (GA) as an isotopic response,9 38% of the 32 cases have been reported in the setting of immunocompromise.10 We now add 5 unreported cases and 16 literature cases (not reviewed in prior meta-analyses) of immunocompromised PHIR-GD. Our review of the literature has also added 23 cases of nonimmunocompromised PHIR-GD. Our analysis of 33 immunocompromised and 43 immunocompetent cases highlights PHIR-GD associations with immunocompromise, chronic lymphocytic leukemia (CLL), and male sex.
Methods
We conducted a retrospective study of 5 immunocompromised PHIR-GD patients at Barnes Jewish Hospital in St Louis, Missouri between 2008 and 2015. Through a literature search of PubMed, we reviewed all previous cases of PHIR-GD. Search terms included Wolf's isotopic response, postherpetic isotopic response, granulomatous dermatitis, granuloma annulare, and perturbations of these terms. References from the identified literature were used to expand our search.
Results
Case 1
A 53-year-old white woman with systemic lupus erythematosus (SLE), Sjögren syndrome (SS), and IgM deficiency on methotrexate, Plaquenil, prednisone, and sulfasalazine presented with right V1 dermatome VZV reactivation. Treatment included valacyclovir, tobramycin ophthalmic ointment, and gabapentin. Less than a month after the lesions resolved, an erythematous, alopecic plaque appeared in the same dermatome (Fig 1; SLE+SS) complicated by severe postherpetic neuralgia (PHN) and trigeminal trophic syndrome with ulceration and superinfection (methicillin-resistant Staphylococcus aureus, Candida keratitis). Biopsy found no viral cytopathic changes (Fig 1; SLE+SS). HSV/VZV assays were negative. She received valacyclovir, corticosteroids (topical, intralesional, oral), and calcineurin inhibitors (tacrolimus, pimecrolimus) for her PHIR-GD. Her PHN required oral gabapentin, pregabalin, duloxetine, hydroxyzine, and topical lidocaine. Autoimmune treatments were replaced with abatacept 6 months after PHIR-GD onset. Over 19.5 months, her cutaneous disease and PHN significantly improved, but her PHN never resolved completely.
Fig 1.
Clinical and histologic images. Four of the immunocompromised patients with granulomatous PHIR from this case series are shown with their clinical photographs above and the corresponding H&E pathology immediately below. Inset images show magnified areas of rash/histology. Immunocompromise etiology and microscopic magnifications are listed. No clinical/histologic images were available for the heart transplant subject in our case series.
Case 2
A 56-year-old white man with a history of acute myelogenous leukemia (AML) treated with chemotherapy and unrelated donor stem cell transplant (SCT) later complicated by chronic graft-versus-host disease presented for suture removal after Mohs micrographic surgery for squamous cell carcinoma of the left side of the forehead. He was found to have VZV reactivation of the left V1 dermatome. He received intravenous acyclovir, oral valacyclovir, and gabapentin for PHN. Less than a month later, an erythematous, sclerotic plaque developed in the same dermatome consistent with GD on biopsy (Fig 1; AML+SCT). He was treated topically (desonide, pimecrolimus) and orally (valacyclovir, prednisone, minocycline, dicloxacillin). PHIR-GD skin lesions and PHN persisted despite treatment at 1-year follow-up.
Case 3
A 57-year-old African-American woman with a history of multiple myeloma (MM) treated with chemotherapy followed by autologous SCT presented with right upper extremity (dermatomes C5-C6) VZV reactivation. She was treated with high-dose acyclovir. Two weeks later, flat-topped, violaceous, polygonal papules appeared among resolving VZV lesions (Fig 1; MM+SCT). Biopsy found poorly formed epithelioid granulomas in the superficial dermis with extension to the dermoepidermal junction and no lichenoid infiltrate or viral cytopathic change (Fig 1; MM+SCT). Grocott methenamine silver stain, and acid-fast bacilli stains were negative. PHIR-GD treatment (hydroxyzine, high potency topical steroids, intralesional Kenalog) resulted in significant improvement by 5 months. Complete resolution of cutaneous findings was noted at 2-year follow-up, although PHN persisted requiring gabapentin, topical lidocaine, and epidural steroid injections.
Case 4
A 73-year-old white man with a history of heart transplant in 1985 presented with disseminated VZV reactivation (right V3 and C5 dermatomes). At presentation, his immunosuppressive regimen included cyclosporine, azathioprine, and prednisone. VZV treatment included valacyclovir and gabapentin for PHN. Within 3 weeks, erythematous papules developed interspersed within his healing VZV lesions (right C5 dermatome) with significant PHN. VZV polymerase chain reaction was negative for both lesions. Biopsy found a prominent interstitial granulomatous process with necrobiosis and no viral cytopathic changes. Gram and Fite stains were negative. PHIR-GD was treated with clobetasol.
Case 5
A 71-year-old African-American woman with CLL complicated by immune thrombocytopenic purpura presented with VZV reactivation (left C5-T1 dermatomes) and significant PHN 1 month after rituximab treatment and ibrutinib initiation. She initially received valacyclovir, gabapentin, and acetaminophen-hydrocodone. Once the lesions resolved, she was treated with acyclovir prophylaxis. Eight months later, firm, erythematous papules in an annular pattern developed in a left-sided C5-8 distribution consistent with PHIR-GD (Fig 1; CLL). Biopsy found superficial and deep perivascular loose granulomas and lymphocytes (Fig 1; CLL). Gram, Grocott methenamine silver stain, and Fite stains were negative. Clobetasol cream improved her PHIR-GD by her follow-up at 4 months.
Summary of reported cases
These 5 cases were integrated into a review of all published immunocompromised PHIR-GD clinical and histologic data (Supplemental Figs 1 and 2). For comparison, PHIR granulomatous vasculitis and folliculitis cases were summarized separately (Supplemental Fig 3, Supplemental Fig 4). The average age of PHIR-GD presentation was similar in immunocompromised patients (65 ± 11 years) and immunocompetent patients (59 ± 18 years). Although most PHIR-GD cases were initiated by VZV (>96%), HSV was identified in 9% of immunocompromised PHIR-GD cases. Similar to prior literature, herpes virus infection occurred on average 4.2 months before PHIR (range, 0.1 to 36), and most cases resolved within 1 to 2 years with conservative GD management, although our 5 new cases all were complicated by severe PHN.
Supplemental Fig 1.
Clinical summary of immunosuppressed PHIR-GD cases. Clinical data including patient characteristics, immunosuppression, virus, and treatment for all reported cases of PHIR-GD and the 5 cases reported in this manuscript are summarized.
Supplemental Fig 2.
Histologic summary of immunosuppressed PHIR-GD cases. Patient characteristics (age, sex, immunosuppression) are listed next to available histologic data for all reported cases of PHIR-GD and the 5 cases reported in this manuscript.
Supplemental Fig 3.
Clinical summary of immunosuppressed PHIR-GV/GF cases. Clinical data including patient characteristics, immunosuppression, virus, and treatment for all reported cases of PHIR-GV/GF (granulomatous vasculitis/folliculitis) are summarized.
Supplemental Fig 4.
Histologic summary of immunosuppressed PHIR-GF/GV cases. Patient characteristics (age, sex, immunosuppression) are listed next to available histologic data for all reported cases of PHIR-GF/GV.
Immunocompromise analysis
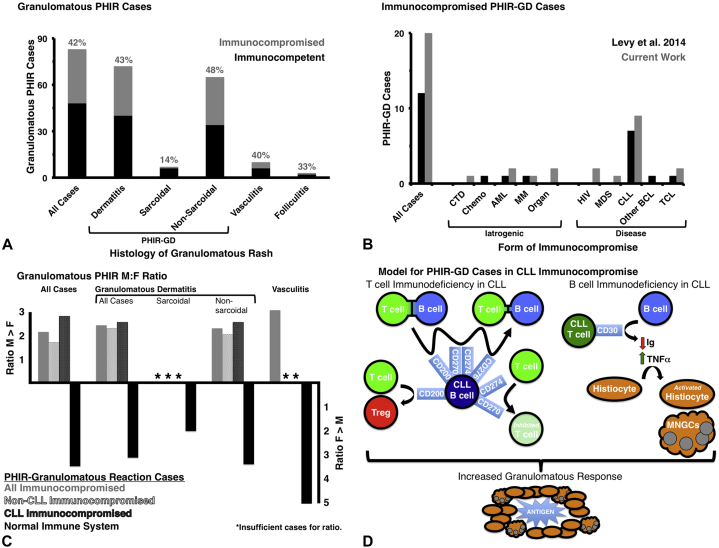
Immunocompromise in PHIR-GD appears more commonly (43%) than previously reported (38%) (Fig 2, A). We have expanded the previously identified immunocompromised context for PHIR-GD (chemotherapy or hematopoietic malignancy) to include HIV, myelodysplastic syndrome, solid organ transplant, and connective tissue disease (Fig 2, B). CLL remained the most common cause of immunocompromise in granulomatous PHIR patients (44% all cases/48% GD). Interestingly, our work suggests that sex may play a role in PHIR-GD, as immunocompromised men appeared particularly susceptible to granulomatous PHIR independent of their higher incidence of CLL (Fig 2, C). Conversely, granulomatous PHIR in immunocompetent patients is far more frequent in women (Fig 2, C) paralleling the increased occurrence of GA in women.1
Fig 2.
Immunocompromise and granulomatous PHIR. A, The presence or absence of immunocompromise in all published cases of granulomatous PHIR are summarized and separated by type of inflammation. PHIR-GD granulomatous dermatitis encompasses sarcoidal and nonsarcoidal (ie, GA and GA variants). B, Cases of PHIR-GD from the most recent meta-analysis before this publication are compared with the cases added by this work. The types of immunocompromise are listed and highlight the predominance of CLL in both studies. C, The male/female ratio for each type of granulomatous PHIR was calculated as a function of immunocompromise. Granulomatous PHIR folliculitis is not included, as there were too few cases to include in this analysis. D, Model of how CLL immunocompromise could lead to increased granulomatous response through both the T- and B-cell axes is shown. The upregulated CLL B-cell factors impair immunologic synapse formation, promote Treg expansion, and impair T cell activation/proliferation. Upregulated CLL T-cell CD30 impairs B-cell isotype switching, increases B-cell sensitivity to FasL-mediated apoptosis, and increases tumor necrosis factor-α production.
Discussion
Nearly half of PHIR-GD cases (33 of 76; 43%) have been reported in immunocompromised patients. Immunocompromise likely worsens the regional neuroimmune axis imbalance caused by herpetic nerve injury and thereby increases the likelihood of PHIR-GD.11 This hypothesis is supported by recent PHIR-GD work demonstrating perineurovascular lymphohistiocytic infiltrates.12 Further, the severe PHN of patients both in our study and prior publications may reflect this proposed neuroimmune imbalance. We recommend that practitioners aggressively manage these symptoms particularly in immunocompromised PHIR-GD patients. Beyond local neuroimmune effects, the overrepresentation of CLL in our PHIR-GD patients (16 of 33; 48%) compared with baseline CLL incidence (0.5%) may help illuminate what humoral and cell-mediated impairments led to granulomatous PHIR. The upregulation of specific cell surface proteins on adaptive immune cells (Fig 2, D),13 immune cell-mediated hampered lymph drainage,13 and increased tumor necrosis factor-α in CLL may help drive granuloma formation (Fig 2, D). Future studies of this phenomenon are needed to determine which facet(s) of CLL immunocompromise favors PHIR-GD, and we believe these studies will shed light on both PHIR (GD and other responses) and CLL. Although CLL association with PHIR-GD had been previously noted, male predominance in immunocompromised PHIR-GD has not been previously identified. It is particularly striking because of the reported approximately 33% increased incidence of VZV in women over men14 and the increased incidence of granuloma annulare in women over men (2.5:1),1 which we also observed in our review of immunocompetent patients with PHIR-GD (2.9:1 overall, 2.6:1 for PHIR-GD). Although the male/female ratio in CLL (1.5:1) may account for part of the male predominance in PHIR-GD (2.8:1), it cannot account entirely for this observation. Although we do not yet understand the role of sex in PHIR-GD, we recommend screening men with PHIR-GD for immunocompromise (particularly CLL) and aggressively treating PHN in these patients to improve clinical outcomes. We hope that these cases and our literature review will spark future investigations to improve our understanding of PHIR, granulomatous reactions, and CLL.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Anadkat has received honoraria as a speaker and/or consultant from Aspire Bariatrics, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb, Eisai, ImClone, and Therakos. He has also served as an investigator for Biogen, Hana Biosciences, Vanda Pharmaceuticals, and Xoma. Elaine Otchere and Drs McCoy and Musiek have no conflicts to disclose.
Appendix
References
- 1.Muhlbauer J.E. Granuloma annulare. J Am Acad Dermatol. 1980;3(3):217–230. doi: 10.1016/s0190-9622(80)80181-2. [DOI] [PubMed] [Google Scholar]
- 2.Wolf R., Brenner S., Ruocco V., Filioli F.G. Isotopic response. Int J Dermatol. 1995;34(5):341–348. doi: 10.1111/j.1365-4362.1995.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 3.Ruocco V., Brunetti G., Puca R.V., Ruocco E. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23(12):1364–1373. doi: 10.1111/j.1468-3083.2009.03345.x. [DOI] [PubMed] [Google Scholar]
- 4.Serfling U., Penneys N.S., Zhu W.Y., Sisto M., Leonardi C. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. A study by the polymerase chain reaction. J Cutan Pathol. 1993;20(1):28–33. doi: 10.1111/j.1600-0560.1993.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 5.Gibney M.D., Nahass G.T., Leonardi C.L. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134(3):504–509. [PubMed] [Google Scholar]
- 6.Langenberg A., Yen T.S., LeBoit P.E. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24(3):429–433. doi: 10.1016/0190-9622(91)70066-b. [DOI] [PubMed] [Google Scholar]
- 7.Requena K., Escalonilla O., Schaller R. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138(1):161–168. doi: 10.1046/j.1365-2133.1998.02045.x. [DOI] [PubMed] [Google Scholar]
- 8.Nikkels A., Sadzot-Delvaux C., Cloes J.-M., Rentier B., Pierard G. Granulomatous reactions following herpes-zoster contain varicella-zoster glycoprotein GPI. J Invest Dermatol. 1992;98(4) http://orbi.ulg.ac.be/handle/2268/62490 Available from: [Google Scholar]
- 9.Guill M.A., Goette D.K. Granuloma annulare at sites of healing herpes zoster. Arch Dermatol. 1978;114(9):1383. doi: 10.1001/archderm.1978.01640210074023. [DOI] [PubMed] [Google Scholar]
- 10.Levy J., Barber D., Robertson L. Granuloma annulare as an isotopic response to herpes zoster. J Cutan Med Surg. 2014;18(6):413–419. doi: 10.2310/7750.2014.14019. [DOI] [PubMed] [Google Scholar]
- 11.Piccolo V., Russo T., Bove D., Baroni A. Segmental immune disorders resulting from neurologic injuries. Clin Dermatol. 2014;32(5):628–632. doi: 10.1016/j.clindermatol.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor R., Piris A., Saavedra A.P., Duncan L.M., Nazarian R.M. Wolf isotopic response manifesting as postherpetic granuloma annulare: a case series. Arch Pathol Lab Med. 2013;137(2):255–258. doi: 10.5858/arpa.2011-0643-OA. [DOI] [PubMed] [Google Scholar]
- 13.Riches J.C., Gribben J.G. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27(2):207–235. doi: 10.1016/j.hoc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Insinga R.P., Itzler R.F., Pellissier J.M., Saddier P., Nikas A.A. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20(8):748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]