ABSTRACT
Although major progress in our understanding of the genes and mechanisms that regulate lymphatic vasculature development has been made, we still do not know how lumen formation and maintenance occurs. Here, we identify the Ras-interacting protein Rasip1 as a key player in this process. We show that lymphatic endothelial cell-specific Rasip1-deficient mouse embryos exhibit enlarged and blood-filled lymphatics at embryonic day 14.5. These vessels have patent lumens with disorganized junctions. Later on, as those vessels become fragmented and lumens collapse, cell junctions become irregular. In addition, Rasip1 deletion at later stages impairs lymphatic valve formation. We determined that Rasip1 is essential for lymphatic lumen maintenance during embryonic development by regulating junction integrity, as Rasip1 loss results in reduced levels of junction molecules and defective cytoskeleton organization in vitro and in vivo. We determined that Rasip1 regulates Cdc42 activity, as deletion of Cdc42 results in similar phenotypes to those seen following the loss of Rasip1. Furthermore, ectopic Cdc42 expression rescues the phenotypes in Rasip1-deficient lymphatic endothelial cells, supporting the suggestion that Rasip1 regulates Cdc42 activity to regulate cell junctions and cytoskeleton organization, which are both activities required for lymphatic lumen maintenance.
KEY WORDS: Rasip1, Lymphatic endothelial cells, Endothelial cell junctions, Lumen maintenance, Lymphatic valves, Mouse
Summary: The first description of genes (for example, Rasip1) and mechanisms participating in lumen maintenance and valve formation in the lymphatic vasculature.
INTRODUCTION
The lymphatic vasculature consists of two morphologically different types of vessels, with distinct functions. Blind-ended initial lymphatics are interconnected by discontinuous button-like junctions. Larger collecting lymphatics transport lymph to lymph nodes, in which immune response occurs, and eventually bring lymph back to the blood circulation at the junction with the subclavian veins (Oliver, 2004). Collecting lymphatics are connected by continuous zipper-like junctions and are covered by a basement membrane and smooth muscle cells. They also contain lymphatic valves to prevent lymph backflow. As part of the circulatory system, the lymphatic vasculature is essential for maintaining fluid homeostasis, immune surveillance and fat uptake from the intestinal tract (Schulte-Merker et al., 2011). Malfunction of the lymphatic vasculature leads to congenital or inherited disorders, including lymphedema. The importance of the lymphatic vasculature was not appreciated in full detail until recently, as emerging studies unraveled additional and important functional roles for this vascular network in diseases such as cancer, obesity, atherosclerosis and hypertension (Schulte-Merker et al., 2011; Alitalo, 2011). Recent work also indicates that defects of the lymphatic vasculature could be responsible for some diseases that have not previously been linked to lymphatic malfunction. For example, our previous studies revealed that subtle defects in the lymphatic vasculature (i.e. leakage), leads to obesity in mice heterozygous for the gene encoding the transcription factor Prox1, the activity of which is necessary and sufficient for the specification of lymphatic endothelial cell (LEC) fate (Harvey et al., 2005). Furthermore, recent studies have demonstrated that cardiac lymphatic vessels have repaired heart function in response to cardiac injury (Klotz et al., 2015; Henri et al., 2016). Delivery of vascular endothelial growth factor (VEGF) C stimulates new coronary lymphatic vessel formation, reduces fibrosis and promotes cardiac repair in mice after postcardiac injury (Klotz et al., 2015; Henri et al., 2016; Chen et al., 2016). In addition, two types of recently identified lymphatic vessels, meningeal lymphatics (Louveau et al., 2015) and the Schlemm's canal (Aspelund et al., 2014; Thomson et al., 2014; Kizhatil et al., 2014), are responsible for maintaining fluid homeostasis in the central nervous system and the eye, respectively.
In the last few years, our knowledge about the genes and mechanisms regulating LEC fate specification and lymphatic vasculature development has improved substantially (Yang and Oliver, 2014; Semo et al., 2016; Ma and Oliver, 2017). Crucial for proper lymphatic vasculature function is the presence of a lumen that allows the constant flow of fluids along this vascular network. Surprisingly, we still do not know about the morphological and molecular events that participate in this important process. In the blood vasculature, Ras family members and their regulators are major players in the process of lumen formation (Tan et al., 2008), endothelial cell migration (Tan et al., 2008; Sosnowski et al., 1993), capillary tube assembly (Connolly et al., 2002), blood vessel homeostasis (Komatsu and Ruoslahti, 2005), angiogenesis (Serban et al., 2008; Aitsebaomo et al., 2004) and vascular permeability (Serban et al., 2008). Among the Ras family, Rasip1 is a recently identified Ras-interacting protein characterized as an endomembrane Ras effector (Mitin et al., 2004). In Xenopus and in mice, Rasip1 expression is restricted to endothelial cells (ECs) (Xu et al., 2009). In humans, the most significant single-nucleotide polymorphism associated with defects in retinal venular caliber was identified in the RASIP1 gene (Ikram et al., 2010). Studies in mouse and zebrafish revealed that Rasip1 is essential for blood vasculature lumen formation and/or its maintenance (Xu et al., 2011; Wilson et al., 2013; Koo et al., 2016). Functional inactivation of Rasip1 in mice leads to embryonic lethality at embryonic day (E) 10.5, with homozygous null embryos failing to form obvious lumens in blood vessels (Xu et al., 2011). This same group has also reported that loss of Rasip1 increases RhoA/ROCK/myosin signaling and dramatically reduces Cdc42/Rac1 signaling (Xu et al., 2011). Rasip1 temporally controls different types of GTPases, which in turn regulate different pools of non-muscle myosin II (NMII) to coordinate junction clearance (remodeling) and actomyosin contractility during vascular tubulogenesis (Barry et al., 2016). As a consequence, Rasip1-deficient ECs display defective cell polarity, with mis-localization of junction proteins along the apical/luminal surface, and they fail to adhere to the surrounding extracellular matrix (ECM) (Xu et al., 2011). Thus, according to these reports, Rasip1 acts as an endothelial-specific regulator of GTPase signaling, and controls blood vessel tubulogenesis by regulating EC-EC junctions and integrin-mediated EC-ECM junctions (Xu et al., 2011). However, work by another group argued that blood vessel lumen formation is not affected in Rasip1-deficient embryos (Wilson et al., 2013); instead they show that, in these embryos, lumens form but eventually collapse, leading to hemorrhage and embryonic lethality (Wilson et al., 2013), a result suggesting that Rasip1 activity is instead required for lumen maintenance. Mechanistically, they showed that Rasip1 binds RAP1 to regulate endothelial junction stability (Wilson et al., 2013).
Whether similar players and molecular processes regulate lumen formation and maintenance in lymphatic vessels is not yet known; therefore, we decided to evaluate whether Rasip1 function could also be required to regulate certain aspects of lymphatic vasculature morphogenesis. We show that LEC-specific Rasip1 deletion in the mouse leads to embryos with severe edema and dilated lymphatic vessels with defective cell junctions. Initially, mutant lymphatic vessels form lumens; however, later on their lumens collapse, and these vessels become discontinuous and fragmented, and the number of mesenteric lymphatic valves is dramatically reduced. Using cultured LECs, we determined that depletion of Rasip1 in LECs abolishes Cdc42 activity, which in turn disrupts LEC junctions, leading to fragmented vessels. Furthermore, we also show that Cdc42-deficient embryos largely phenocopy the defective lymphatic junctions and lymphatic valve formation phenotypes observed in Rasip1-deficient embryos. These data identify Rasip1 as a key player in the process of lymphatic vasculature morphogenesis.
RESULTS
Rasip1 is expressed in lymphatic endothelial cells
In the mouse, lymphatic vasculature formation starts at ∼E9.5, when a subpopulation of blood endothelial cells in the cardinal vein (CV) expresses the transcription factor Prox1 (Wigle and Oliver, 1999). These Prox1+ lymphatic progenitor cells bud off from the CV and migrate in a dorsal-lateral manner to form lymph sacs, from which, eventually, most of the mammalian lymphatic vasculature will be derived. To determine whether Rasip1 is expressed in LECs, we performed immunostaining at different embryonic stages. As seen in Fig. 1A, at E11.5 Rasip1 is expressed in LEC progenitor cells in the CV and in migrating LECs. At E14.5, Rasip1 expression was detected in Prox1+ LECs in the jugular lymph sacs (Fig. 1B) and in dermal lymphatic capillaries (Fig. 1C). At E18.5, mesenteric collecting lymphatics and their valves start to become obvious. Rasip1 expression is detected in those collecting lymphatic vessels and it is highly expressed in the valve-forming cells (Fig. 1D). As has been previously shown for blood endothelial cells (BECs) (Wilson et al., 2013), Rasip1 expression also shows a junctional localization in LECs (Fig. 1C,D). Together, these data identify Rasip1 as a novel endothelial cell marker expressed in both BECs and LECs.
Fig. 1.
Rasip1 is expressed in LEC progenitors and lymphatic valve-forming cells. (A) Immunostaining of E11.5 transverse sections. Yellow arrows indicate Rasip1 expression in LEC progenitors in the CV; white arrows indicate Rasip1 expression in initial migrating LECs. n=3. DA, dorsal aorta; CV, cardinal vein. (B) Immunostaining of E14.5 sagittal sections. White arrows indicate Rasip1 expression in lymph sacs. n=3 embryos. LS, lymph sac. (C) Whole-mount immunostaining of E14.5 dermal lymphatic capillaries. White arrows indicate lymphatic capillaries. n=3. (D) Whole-mount immunostaining of E18.5 mesentery collecting lymphatic vessels. Note that Rasip1 expression shows a junctional localization in C and D. n=3 embryos. Arrow indicates developing lymphatic valves. Scale bars: 100 µm in A,B; 50 µm in C,D.
Abnormal lymphatic morphogenesis in LEC-specific Rasip1-deficient embryos
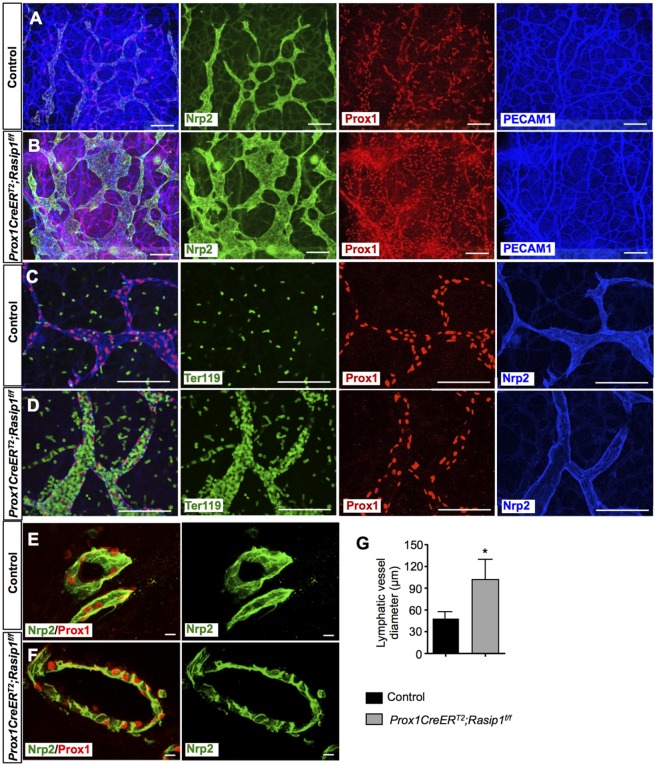
Next, we evaluated whether Rasip1 also has a functional role during lymphatic development. Rasip1 standard null embryos die at ∼E10 because of impaired blood vessel lumen formation (Xu et al., 2011). Accordingly, to evaluate Rasip1 lymphatic function, we generated LEC-specific Rasip1 conditional mutants by crossing Rasip1-floxed mice (Xu et al., 2011) with Lyve1eGFPCre mice (Pham et al., 2010). Unfortunately, those conditional null embryos (Lyve1eGFPCre;Rasip1f/f) also died at ∼E10, presumably because of yolk sac blood vessel defects, as Lyve1 is expressed in those vessels (Gordon et al., 2008). To overcome this issue, we crossed Rasip1f/+ mice with Prox1CreERT2 mice (Srinivasan et al., 2007). Cre activity was induced by tamoxifen (TM) injections at E9.5 and E10.5, and embryos were isolated at E14.5. Initial analysis of Prox1CreERT2;Rasip1f/f (Rasip1 conditional null) embryos at E14.5 showed that they exhibited severe edema and/or hemorrhage, whereas Prox1CreERT2;Rasipf/+ embryos showed no obvious phenotype and were used as controls in all experiments (Fig. S1A). As seen in Fig. S1B-D, Rasip1 deletion in LECs was very efficient, whereas Rasip1 expression in blood vessels was not affected. Next, the skin of E14.5 Rasip1 conditional null embryos and control littermates was dissected and the wholemount was stained with antibodies against the blood and lymphatic endothelial cell markers PECAM1, Prox1 and Nrp2 (Fig. 2A-D). Different from the collapsed blood vessel phenotypes that have been reported (Xu et al., 2011; Barry et al., 2015), dermal lymphatic vessels were abnormally dilated in Rasip1 conditional null embryos (Fig. 2A,B) (PECAM1-stained blood vessels appeared to be normal). As indicated by Ter119 staining, we also observed some blood filled lymphatic vessels in Rasip1 conditional null embryos at this stage (Fig. 2C,D). To investigate whether a lack of Rasip1 affects lymphatic lumen development, thick vibratome sections of control and Rasip1 conditional null embryos were collected and stained for Nrp2 and Prox1. Different from Rasip1 function in blood vessels, the lymphatic vessels of E14.5 Rasip1 conditional null embryos had a lumen that appeared to be abnormally enlarged (Fig. 2E-G). To investigate the possible causes of this phenotype, we analyzed changes in LEC proliferation and apoptosis rates at E14.5. Ki67 immunostaining revealed that the proliferation rate was significantly increased in LECs in Rasip1 conditional null embryos (Fig. S2A-C). LEC apoptosis, however, showed no differences between control and Rasip1 conditional nulls (Fig. S2D-F). These data indicate that LEC-specific loss of Rasip1 increases LEC proliferation, resulting in abnormally dilated lymphatics with enlarged lumens at E14.5.
Fig. 2.
Enlarged and blood-filled dermal lymphatic capillaries in E14.5 Prox1CreERT2;Rasip1f/f embryos. (A,B) Whole-mount immunostaining of E14.5 control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) skin showing severely dilated capillary lymphatics in the mutant embryos. (C,D) Whole-mount immunostaining of E14.5 control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) skin showing blood-filled lymphatics in the mutant embryos. (E,F) Immunostaining of thicker vibratome sections of control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) embryos showing that lumen formation takes place in Rasip1 conditional null embryos. However, the diameter of those lumens is enlarged compared with those of control embryos, likely reflecting the enlarged lymphatic capillary. (G) Quantification of lumen diameters in dermal lymphatics of control and Prox1CreERT2;Rasip1f/f embryos. Data are derived from six randomly selected fields per genotype. Data are mean±s.e.m. *P<0.05 (Student's t-test, versus control). TM was induced at E9.5 and E10.5. Scale bars: 100 µm in A,B; 50 µm in C,D; 25 µm in E,F.
Defective lymphatic endothelial junctions in LEC-specific Rasip1-deficient embryos
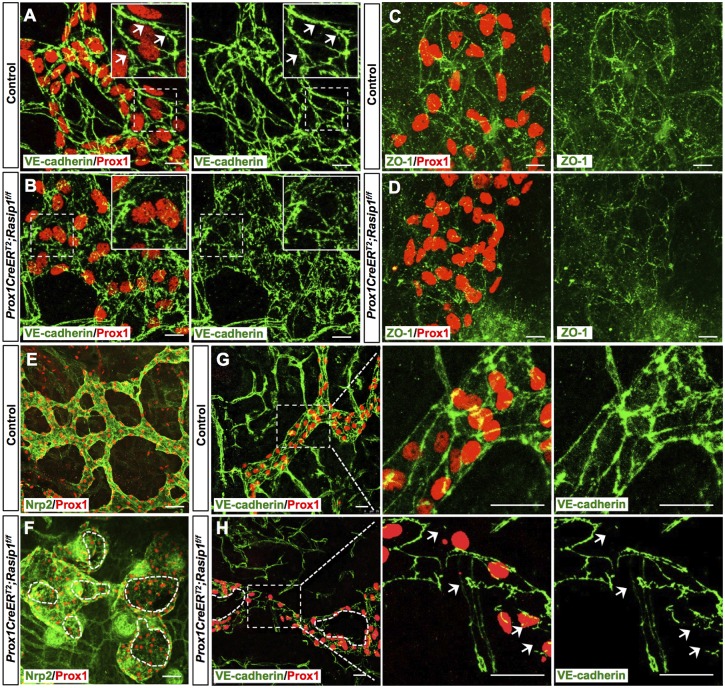
Loss of Rasip1 in blood vessels leads to mis-localization of adhesion junctions during blood vessel tubulogenesis (Xu et al., 2011). Therefore, we evaluated whether similar defects were present in Rasip1 conditional null embryos. Consistent with previous reports (Yao et al., 2012; Zheng et al., 2014), initial lymphatics exhibit continuous zipper-like junctions in control embryos, as visualized by VE-cadherin (also known as cadherin 5) immunostaining (Fig. 3A). However, Rasip1 conditional null embryos exhibited discontinuous and abnormal cell junctions (Fig. 3B). Further characterization showed that tight junctions were also significantly impaired in Rasip1 conditional null embryos, as revealed by immunofluorescence against ZO-1 (also known as Tjp1) (Fig. 3C,D). This finding was further validated by western blot analysis showing that both VE-cadherin and ZO-1 levels were decreased in dermal LECs, which were isolated from E14.5 Rasip1 conditional null embryos (Fig. S3A,B). To determine whether this was a general phenotype in developing lymphatics, we analyzed some additional organs and, indeed, similar alterations were also seen, for example, in lung and mesentery-associated lymphatics (Fig. S3C-F).
Fig. 3.
Disorganized cell junctions in dermal lymphatics of E14.5 Prox1CreERT2;Rasip1f/f embryos. (A,B) Whole-mount immunostaining of dermal lymphatic capillaries from E14.5 control (n=4) and Prox1CreERT2;Rasip1f/f (n=3) embryos. Insets are higher magnifications of selected regions (dashed boxes). Control lymphatics show zipper-like junctions as indicated by white arrows. Instead, obvious cell junctions are difficult to detect in Rasip1 conditional null littermates, as cell borders appear to be disorganized and diffuse. (C,D) Similar skin whole-mount immunostaining was performed in control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) embryos but using antibodies against the tight junction ZO-1 and Prox1. In this case, junctions were also diffuse and defective, and levels of ZO-1 appeared to be reduced. (E-H) Whole-mount immunostaining of dermal lymphatic capillaries from E16 control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) embryos. Dashed lines indicate open and broken lumens in F and H. Right panels show higher magnifications of the boxed regions in G and H. Arrows indicate the broken junctions in Rasip1 conditional null embryos. Scale bars: 10 μm in A-D; 50 µm in E,F; 25 µm in G,H.
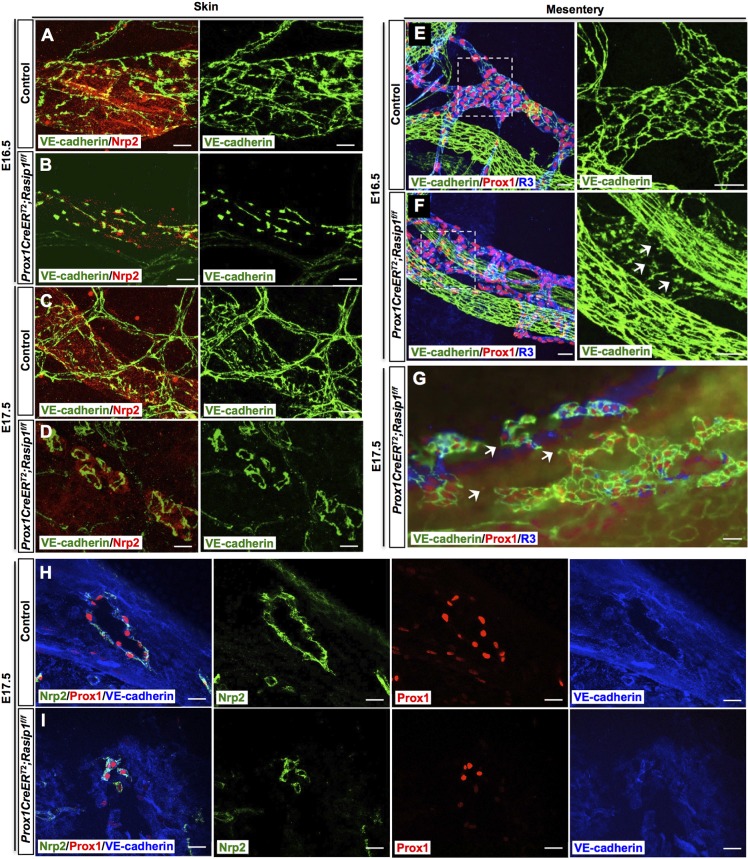
Because of the severe defects in cell-cell junctions, we next evaluated lymphatic vessel morphogenesis at later stages. At E15.5, dermal lymphatics of Rasip1 conditional null embryos were still significantly dilated compared with control littermates (Fig. S4). Surprisingly, at ∼E16, these dilated lymphatic vessels in Rasip1 conditional null embryos still retain a lumen, but start to get fragmented (Fig. 3E,F). Lymphatic endothelial junctions appeared to be discontinued and broken, as revealed by VE-cadherin immunostaining (Fig. 3G,H). Furthermore, lymphatic vessels were narrow and severely disorganized in Rasip1 conditional null embryos at E16.5 (Fig. 4A,B) and, one day later, those lymphatic vessels appeared to be fragmented and broken (Fig. 4C,D). This phenotype can also be detected in E16.5 collecting lymphatic vessels, in which endothelial cell junctions also appeared to be disorganized (Fig. 4E,F). Eventually, at E17.5 most of the collecting lymphatic vessels were discontinuous and fragmented (Fig. 4G) with narrower lumens and compromised integrity (Fig. 4H,I). These data demonstrated that Rasip1 is required for proper cell junction organization and, therefore, lymphatic lumen maintenance. To determine whether the broken and collapsed vessel phenotype was associated with LEC apoptosis, we performed active caspase 3 staining. We found that the percentage of apoptotic LECs in dermal lymphatics was comparable among control and Rasip1 conditional null embryos at E16.0, suggesting that the fragmented vessel phenotype is likely a direct consequence of defective lymphatic endothelial junctions, rather than increased apoptosis (Fig. S5). Therefore, we started to evaluate the timing and origin of the cell junction defects observed in the mutant embryos by analyzing earlier (E11.5) embryonic stages, before lymphatic vessel formation. At this early stage, an apparently normal number of Prox1-expressing LEC progenitors was seen inside the CV and budding off into the surrounding mesenchyme in the mutant embryos (Fig. S6A,B). These data suggested that loss of Rasip1 did not affect LEC differentiation and migration at this early stage. However, analysis of LEC junctions in these embryos revealed that VE-cadherin and ZO-1 expression levels were already dramatically reduced at this early stage (Fig. S6). We investigated whether this dramatic reduction in junction molecules could be secondary to changes in cytoskeleton organization or signaling through cell-matrix adhesion. Phalloidin labeling indicated that actin filament organization was apparently normal in Rasip1 conditional null embryos (Fig. S7A,B). Similarly, no obvious differences in cell-matrix adhesion were detected when using p-Paxillin staining, a downstream signaling molecule of focal adhesion kinases (Fig. S7C-E). Together, these results argue that the defective junction integrity observed in Rasip1 conditional null embryos is likely to be the primary defect caused by the loss of Rasip1 function.
Fig. 4.
Impaired lymphatic integrity in Prox1CreERT2;Rasip1f/f embryos at later embryonic stages. (A-D) Whole-mount immunostaining of E16.5 (A,B) and E17.5 (C,D) dermal lymphatics in control and Prox1CreERT2;Rasip1f/f embryos against VE-cadherin and the lymphatic marker Nrp2. Normal morphology and cell junctions are seen in control embryos at those stages, whereas lymphatic vessel integrity appears to be compromised in the mutant embryos, dermal vessels get fragmented and cell junction borders appear to be diffuse and abnormal. (E,F) Whole-mount staining of E16.5 mesentery lymphatics in control (E) and Prox1CreERT2;Rasip1f/f (F) embryos. Right panels show higher magnifications of the boxed regions in E and F. Similar to the dermal lymphatics, VE-cadherin junctions appear to be disorganized in Prox1CreERT2;Rasip1f/f embryos (arrows). (G) At E17.5, the mesenteric lymphatics are severely fragmented, collapsing and losing VE-cadherin expression (arrows) in the mutant embryos. n=3 embryos per genotype per stage. TM was induced at E9.5 and E10.5. (H,I) Immunostaining of thicker vibratome sections of control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) embryos, showing narrow and broken lumens in Rasip1 conditional null embryos at E17.5, reflecting the fragmented vessels at this stage. Scale bars: 10 µm in A-D; 25 µm in E-I.
Rasip1 is required for lymphatic valve formation
As Rasip1 is highly colocalized with Prox1 in lymphatic valve-forming cells (Fig. 1D), we evaluated its functional role in those cells. Rasip1 conditional null embryos injected with TM at E9.5 and E10.5 die at ∼E16.5, and in addition to the fragmented collecting lymphatic vessel defects, lymphatic valve formation was also severely impaired or missing (Fig. 4F,G). To investigate whether this phenotype was a consequence of a direct role of Rasip1 in lymphatic valve development, or whether it was secondary to the collapsing lymphatics, we injected TM at E14.5, a stage when lymphatic valve formation is starting, and analyzed the mesenteric vessels at E17.5. Whole-mount staining with antibodies against PECAM1, Prox1 and VEGFR3 revealed that V-shaped lymphatic valves were well established in control embryos at this stage (Fig. 5A,B). However, Rasip1 conditional null embryos showed dilated collecting lymphatic vessels without, or with only a few, lymphatic valves (Fig. 5C-E). This phenotype was further confirmed by whole-mount staining against integrin α9. Integrin α9 has been reported to play an important role in the LEC-ECM signaling required for lymphatic valve formation (Bazigou et al., 2009). Integrin α9 is highly expressed in lymphatic valve regions, but expressed at lower levels in the intermediate lymphangion areas in littermate controls (Fig. 5F). However, in Rasip1 conditional null embryos, integrin α9 levels were uniformly low along the collecting lymphatic vessels without any indication of valve formation (Fig. 5G). The dramatic reduction of integrin α9 levels suggested that LEC-ECM interactions in Rasip1 conditional null embryos are impaired.
Fig. 5.
Impaired valve formation in E17.5 Prox1CreERT2;Rasip1f/f mesenteric lymphatics. (A-D) Whole-mount immunostaining of E17.5 mesentery lymphatic vessels in control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) embryos. B and D are higher magnifications of the boxed regions in A and C, respectively. Valve formation is obvious in control embryos at this stage (arrows). This process appears to be arrested in mutant littermates, as no indication of valve forming regions are detected. (E) Quantification of lymphatic valve numbers in mesenteric lymphatics of control and Prox1CreERT2;Rasip1f/f embryos. Data are derived from six randomly selected fields per genotype. Data are mean±s.e.m. *P<0.05 (Student's t-test, versus control). (F,G) Whole-mount immunostaining of E17.5 control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) mesenteric lymphatics with antibodies against integrin α9, Prox1 and PECAM1 confirms the lack of valves in mutant vessels. Scale bars: 50 µm in A-D; 25 µm in F,G.
Using the same experimental setting (TM injection at E14.5), we also examined lymphatic vessel junctions in E17.5 collecting lymphatics (Fig. S8A,B) and capillaries (Fig. S8C,D). Similar to the phenotype observed when TM was injected at E9.5 and E10.5 (Fig. 3), collecting lymphatics and lymphatic capillaries were also abnormally dilated with disorganized junctions and reduced VE-cadherin expression levels. These data suggest that Rasip1 is required for lymphatic valve development and lymphatic cell junction maintenance.
Rasip1 activity is not required in postnatal lymphatics
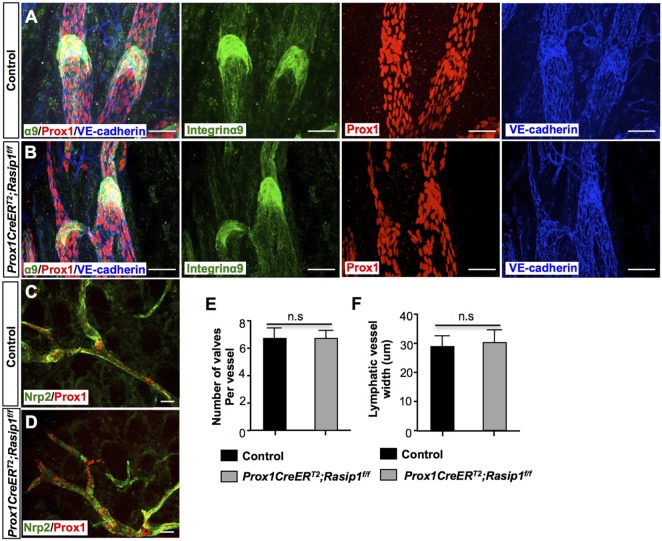
Finally, we examined whether Rasip1 was also required for lymphatic lumen and lymphatic valve maintenance. To do this, we induced Rasip1 deletion at postnatal stages, from postnatal day (P) 0 to P4, and Rasip1 deletion was examined at P6 (Fig. S9). No obvious lymphatic phenotypes were observed in these conditional mutant pups, as both lymphatic valves (Fig. 6A,B) and lymphatic capillaries (Fig. 6C,D) appeared to be normal. Furthermore, the total number of mature lymphatic valves and the lymphatic vessel caliber in dermal capillaries were comparable with those of control embryos (Fig. 6E,F). These data indicated that Rasip1 is indispensable for early lymphatic morphogenesis, but it is not required for lymphatic valves or lymphatic lumen maintenance during postnatal stages.
Fig. 6.
Rasip1 is not required for lymphatic vessel and lymphatic valve maintenance at later developmental stages. (A,B) Whole-mount staining of P6 mesenteric lymphatic vessels in control (n=3) and Prox1CreERT2; Rasip1f/f (n=3) embryos with antibodies against integrin α9, Prox1 and VE-cadherin. TM was induced from P0 to P4. Valve formation appears to be normal in the mutant embryos at this stage. (C,D) Whole-mount staining of P6 dermal lymphatic vessels in control (n=3) and Prox1CreERT2;Rasip1f/f (n=3) embryos with antibodies against Nrp2 and Prox1. TM was induced from P0 to P4. No obvious changes in lymphatic vessel morphology were observed in the mutant embryos. (E) Quantification of the number of integrin α9+ lymphatic valves in control and Prox1CreERT2;Rasip1f/f pups. Data are derived from five randomly selected fields per genotype. (F) Quantification of lymphatic vessel diameter in control and Prox1CreERT2;Rasip1f/f pups. Data are derived from five randomly selected fields per genotype. Data are mean±s.e.m. Student's t-test. n.s., not significant. Scale bars: 50 µm.
Rasip1 regulates LEC junction stability and EC-ECM adhesion by regulating Cdc42 activity
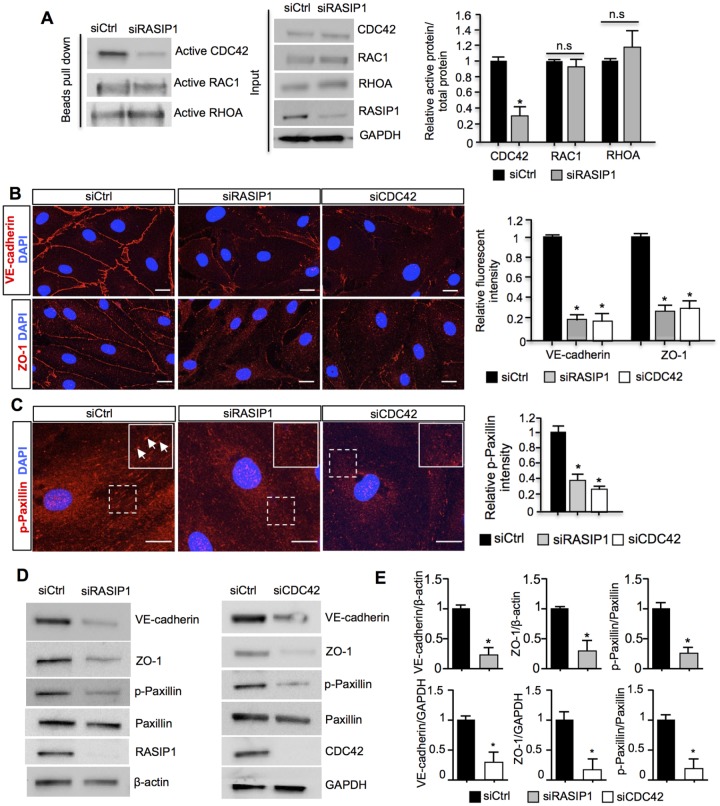
Previous studies demonstrated that, in blood vessels, Rasip1 regulation of VE-cadherin-mediated EC-EC junctions and integrin-mediated EC-ECM junctions is crucial for blood vessel tubulogenesis and blood endothelial junctions (Xu et al., 2011). In these cells, Rasip1 inhibits RhoA, and in turns promotes the activity of Cdc42 and Rac1, to allow controlled expansion of vessel lumens during embryonic growth (Xu et al., 2011; Barry et al., 2016). To evaluate these regulatory pathways in LECs, we carried out an in vitro culture approach to remove RASIP1 function and found a significant reduction in CDC42 activity in siRNA-treated human dermal LECs, compared with controls (Fig. 7A). In contrast, the activity of RAC1 and RHOA were not significantly affected (Fig. 7A). These data suggested that Cdc42 is a potential target of Rasip1 in LECs during lymphatic morphogenesis. In fact, previous studies demonstrated that Cdc42 is essential for maintaining blood vessel stability, by regulating cell-cell junctions and focal adhesions in blood endothelial cells (Barry et al., 2015). Therefore, we next tested whether RASIP1 regulates LEC cell junctions and focal adhesion activities by modulating CDC42 activity. Human dermal LECs were treated with control siRNA (siCtrl), RASIP1 siRNA (siRasip1) or CDC42 siRNA (siCdc42). Compared with the control group, LECs treated with siRASIP1 or siCDC42 displayed highly disorganized and reduced adherens junctions (VE-cadherin) and tight junctions (ZO-1) (Fig. 7B). Previous reports indicated an important role of Cdc42 in regulating the actin cytoskeleton in ECs, and deletion of Cdc42 in ECs leads to disruptive F-actin organization and EC adhesions (Barry et al., 2015). Therefore, we investigated whether actin organization was impaired in Rasip1 conditional null embryos. Phalloidin labeling revealed that mesentery lymphatics displayed abnormal punctuated and scattered actin filaments in Rasip1 conditional null embryos (Fig. S10A,B). Moreover, the intensity of the Phalloidin labeling was reduced overall (Fig. S10C) in Rasip1 conditional mutants. These data suggested an additional potential regulatory role of Rasip1 in lymphatic actin organization via its regulation of Cdc42 activity in LECs. We therefore tested actin organization in siRASIP1 or siCDC42 treated human dermal LECs in vitro. Similar to the in vivo situation, siRASIP1- or siCDC42-treated LECs exhibited highly reduced punctuated and abnormal F-actin organization compared with control siRNA-treated groups, as shown by Phalloidin labeling (Fig. S10D,E). Furthermore, cultured human dermal LECs treated with siRASIP1 or siCDC42 exhibited impaired LEC-ECM adhesions, as indicated by reduced p-Paxillin staining (a marker for immature focal adhesion complexes) (Fig. 7C) and active integrin β1 staining (a marker for mature focal adhesions) (Fig. S11). This result suggested a reduction in focal adhesion activities and in LEC-ECM interactions upon Rasip1 inactivation, further supporting the proposal that Rasip1 regulates LEC-ECM interactions by regulating Cdc42 activity. Similarly, western blot analysis revealed an overall reduction of VE-cadherin, ZO-1 and p-Paxillin levels following siRASIP1 or siCDC42 treatment (Fig. 7D,E). Together, these data indicate that Rasip1 regulates both LEC junction integrity and LEC-ECM adhesion by regulating Cdc42 activity.
Fig. 7.
Rasip1 regulates cell-cell junctions and LEC-ECM junctions by regulating Cdc42 activity in LECs. (A) Western blot analysis of active Rho GTPases (CDC42, RAC1 and RHOA) in siCtrl and siRASIP1-treated human dermal LECs. As seen in the left panel, the level of active CDC42 is severely downregulated in siRASIP1-treated cells. No obvious changes in activity are seen for active RAC1 or RHOA. The total levels of CDC42, RAC1, RHOA, RASIP1 and GAPDH are shown in the middle panel. Quantification of the ratio of active versus total CDC42, RAC1 and RHOA in siCtrl and siRASIP1-treated LECs is shown on the right. Data are mean±s.e.m. of triplicate experiments. *P<0.05 (Student's t-test, versus siCtrl). (B) Immunostaining of siCtrl, siRASIP1- or siCDC42-treated LECs with antibodies against VE-cadherin (upper panel) and ZO-1 (lower panel). The fluorescent intensity of VE-cadherin and ZO-1 from each group were quantified and are shown in the right panel. The levels of VE-cadherin and ZO-1 are drastically reduced in the siRASIP1- or siCDC42-treated LECs. Data are mean±s.e.m. of triplicate experiments. *P<0.05 (Student's t-test, versus siCtrl). (C) Immunostaining of siCtrl, siRASIP1- or siCDC42-treated LECs with antibodies against p-Paxillin. siRASIP1- and siCDC42-treated LECs showed reduced p-Paxillin levels. Insets in red channels show higher magnification of the boxed regions. White arrows indicate p-Paxillin+ regions. Quantifications of p-Paxillin levels in siCtrl, siRASIP1- and siCDC42-treated cells are shown on the right. Data are mean±s.e.m. of triplicate experiments. *P<0.05 (Student's t-test, versus siCtrl). (D) Western blot analysis of protein levels in siCtrl, siRASIP1- or siCDC42-treated LECs with antibodies against VE-cadherin, ZO-1, p-Paxillin, Paxillin, RASIP1 and β-actin or GAPDH. (E) Quantification of the western blot analysis shown in D. Data are normalized to the intensity of β-actin or GAPDH in each group. Data are mean±s.e.m. of triplicate experiments. *P<0.05 (Student's t-test, versus siCtrl). Scale bars: 10 µm.
Cdc42-deficient embryos phenocopy the lymphatic defects of LEC-specific Rasip1-deficient embryos
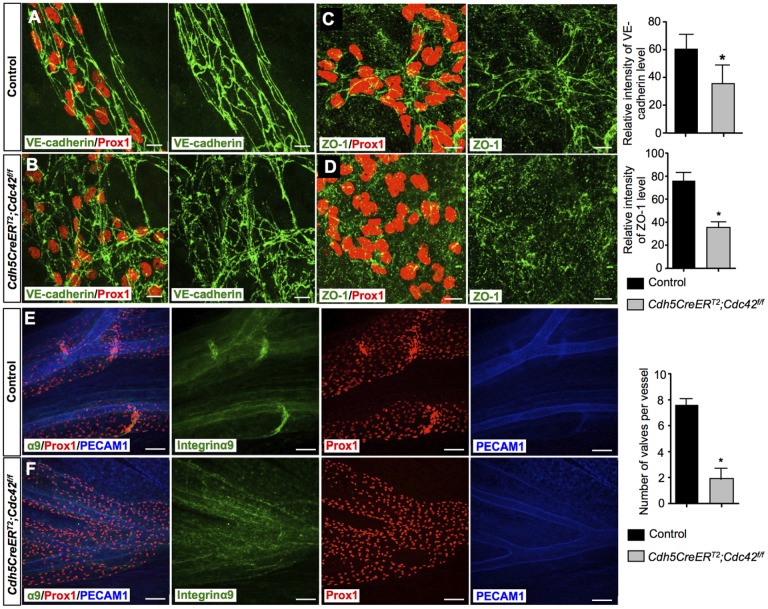
To further validate the proposal that Rasip1 regulation of Cdc42 activity is responsible for the lymphatic defects identified in Rasip1 conditional null embryos, we analyzed Cdc42-deficient embryos. To do this, we crossed Cdc42-floxed mice (Chen et al., 2006) with Cdh5CreERT2 mice (Wang et al., 2010). Previous work showed that deletion of Cdc42 with Tie2Cre resulted in embryonic lethality between E9.5 and E10.5, because of blood vessels defects; later deletion using Cdh5CreERT2 induced at E11.5 led to 100% lethality at E14.5 (Barry et al., 2015). To bypass those blood vessel defects, Cre activity was induced at E12.5 and E13.5. In agreement with that previous report (Barry et al., 2015), Cdh5CreERT2; Cdc42f/f embryos showed widespread hemorrhage and milder edema (Fig. S12A). To examine lymphatic vessel morphogenesis, skin was used for whole-mount immunostaining using antibodies against Nrp2, Prox1 and PECAM1. Similar to Rasip1 conditional null embryos, E14.5 Cdc42-deficient embryos showed dramatically enlarged lymphatic vessels compared with littermate controls (Fig. S12B). Importantly, lymphatic endothelial cell junctions were also very disorganized and abnormal, and the levels of VE-cadherin and ZO-1 expression were severely downregulated (Fig. 8A-D). These data indicated that Cdc42 activity is important for the regulation of lymphatic vessel morphogenesis. Furthermore, to investigate whether Cdc42 activity is also required for lymphatic valve formation, we induced Cdc42 deletion at E14.5 and E15.5. Similar to Rasip1 conditional null embryos, Cdc42-deficient embryos failed to form lymphatic valves in E17.5 lymphatic mesenteries (Fig. 8E,F). Thus, Cdc42-deficient embryos phenocopy those lymphatic defects that are observed in Rasip1 conditional null embryos.
Fig. 8.
Abnormal lymphatic endothelial junctions and impaired collecting lymphatic valve formation in Cdh5CreERT2;Cdc42f/f embryos. (A-D) Whole-mount staining of E14.5 dermal lymphatics of control (n=3) and Cdh5CreERT2;Cdc42f/f (n=3) embryos with antibodies against VE-cadherin and Prox1 (A,B) or ZO-1 and Prox1 (C,D). Cell junctions are diffuse and abnormal in the mutant embryos and levels of VE-cadherin and ZO-1 are downregulated. TM was induced at E12.5 and E13.5. Quantifications of VE-cadherin and ZO-1 levels in both genotypes are shown in the right panel. Data are derived from six randomly selected fields per genotype per experiment. Data are mean±s.e.m. *P<0.05 (Student's t-test, versus control). (E,F) Whole-mount staining of E17.5 mesenteric lymphatics of control (n=5) and Cdh5CreERT2;Cdc42f/f (n=5) embryos with antibodies against integrin α9, Prox1 and PECAM1. Valve formation is obvious in the control embryos but is missing in the mutant littermates. TM was induced at E14.5 and E15.5. Quantification of number of lymphatic valves in both genotypes is shown on the right. Data are derived from six randomly selected fields per genotype. Data are mean±s.e.m. *P<0.05 (Student's t-test, versus control). Scale bars: 10 µm in A-D; 50 µm in E,F
To further support our proposal that Rasip1 regulates Cdc42 activity in LECs, human dermal LECs were treated with control shRNA (shCtrl) or RASIP1 shRNA (shRASIP1). We then transfected these cells with CDC42 adenovirus (Cherry-CDC42) or Cherry control adenovirus (Cherry-Ctrl). After 48 h post-transfection, we found that Cherry-CDC42-infected shRASIP1-treated LECs restored their VE-cadherin expression levels and organization (Fig. 9A-C) and exhibited a well-organized cytoskeleton, as visualized by Phalloidin labeling (Fig. 9D-F). These data further support our proposal that, in LECs, Rasip1 regulation of Cdc42 activity is required to maintain proper cell junctions and cytoskeleton organization.
Fig. 9.
Ectopic Cdc42 expression restores reduced adhesion junctions and abnormal cytoskeleton organization in Rasip1-deficient LECs. (A-C) Human dermal LECs were transfected with shCtrl or shRasip1. After 24 h post-transfection, these cells were further transfected with control adenovirus (Cherry-Ctrl) or Cdc42 adenovirus (Cherry-Cdc42) for two more days. Rasip1-deficient LECs (shRasip1-treated LECs) showed impaired adhesion junctions with decreased VE-cadherin levels (B). Ectopic Cdc42 expression restored VE-cadherin levels in Rasip1-deficient LECs (C). (D-F) Rasip1-deficient LECs (shRasip1 treated LECs) showed decreased F-actin fibers (E). Ectopic Cdc42 expression restored F-actin levels in Rasip1-deficient LECs (F). Scale bars: 10 µm.
DISCUSSION
Rasip1 conditional null embryos form a lumen
In this study we identified Rasip1 as a key player in lymphatic vasculature lumen formation and maintenance. We show that LEC-specific deletion of Rasip1 initially leads to dilated lymphatic vessels with enlarged lumens at E14.5. However, these defective lymphatics become disorganized and fragmented at later stages, a phenotype likely because of the dramatic reduction and disorganization of their LEC junctions. Similar to the in vivo situation, a reduction of VE-cadherin and ZO-1 levels was observed in cultured LECs upon RASIP1 inactivation.
Previous work has reported that Rasip1 is essential for blood vasculature lumen formation and/or for its maintenance (Xu et al., 2011; Wilson et al., 2013). One study reported that functional inactivation of Rasip1 in mice results in null embryos failing to form lumens in blood vessels (Xu et al., 2011). Work by another group argued that blood vessel lumen formation was not affected in Rasip1-deficient embryos (Wilson et al., 2013); instead they show that lumens form, but eventually collapse, suggesting that Rasip1 activity is required for lumen maintenance. Interestingly, our results differ from those that have been reported in blood vessels in various aspects, as Rasip1 conditional null embryos instead showed dilated lymphatic vessels with enlarged lumens and increased LEC cell numbers at E14.5. We showed that this dilation is because of increased LEC proliferation that is concomitant with a reduction in the level of VE-cadherin expression. Previous studies showed that blocking VE-cadherin activity increases proliferation in ECs (Nelson and Chen, 2003). Therefore, the observed increase in LEC proliferation could be due to the dramatic reduction in the level of VE-cadherin expression in Rasip1 conditional null embryos.
Rasip1 regulation of lymphatic endothelial cell junction integrity is required for lymphatic lumen maintenance
Mature lymphatic capillaries have been described as having button-like, loose overlapping cell-cell junctions that facilitate the uptake of interstitial fluid into the lymphatic system. However, during early embryonic development, lymphatic capillaries exhibit non-permeable zipper-like junctions that eventually become permeable button-like junctions at later embryonic stages (Yao et al., 2012; Zheng et al., 2014). Failure in this transition, at least in part, contributes to edema and abnormal fluid drainage capacity (Zheng et al., 2014). Interestingly, dermal and mesenteric lymphatics of Rasip1 conditional null embryos were dramatically disorganized at E16.5 and, one day later, they appeared to be fragmented and collapsed. Importantly, LEC junctions were also very disorganized and abnormal, and their levels of VE-cadherin and ZO-1 expression were severely downregulated. These results indicated that Rasip1 is required for proper cell junction organization and, therefore, for lymphatic lumen maintenance. In addition, we found that LECs in these vessels showed disorganized subcellular F-actin localization. Accordingly, it is likely that the discontinued and broken vessels seen at later stages are a consequence of the impaired junction organization that has already occurred by E14.5.
Rasip1 activity is required for lymphatic valve formation
One of the major characteristics of collecting lymphatic vessels is the presence of lymphatic valves, which prevent retrograde lymph flow and ensure a unidirectional lymph flow back to the blood circulation. Recent studies have identified key genes and the mechanical flow that regulate lymphatic valve formation (Koltowska et al., 2013). Deletion of Rasip1 in LECs at early embryonic stages results in fragmented and discontinued collecting lymphatic vessels, and these vessels fail to form lymphatic valves. Deletion of Rasip1 at E14.5, a stage when lymphatic valve formation starts, resulted in impaired lymphatic valve formation, which suggested that Rasip1 regulates this process. More importantly, we found that the level of integrin α9 was dramatically decreased in collecting lymphatic vessels of Rasip1 conditional null embryos. Interaction of integrin α9 with the ECM is required for proper lymphatic valve formation (Bazigou et al., 2009).
Thus, failure in lymphatic valve formation in Rasip1 conditional null embryos is likely because of impaired interactions of LECs with the ECM. This finding is consistent with previous studies showing that Rasip1 regulates EC integrin-dependent adhesion to surrounding ECM during blood vessel lumen formation (Xu et al., 2011).
Cdc42 is a likely direct mediator of Rasip1 function in LECs
We also found that loss of Rasip1 dramatically abolished Cdc42 activity in vitro and in vivo, suggesting that Cdc42 is the potential downstream effector of Rasip1 function. In addition, Rasip1 deletion also led to reduced and abnormal organized F-actin in vivo and in vitro. This phenotype is different from that reported in BECs, showing that F-actin (RhoA dependent) was significantly increased in Rasip1-deficient cells (Xu et al., 2011). This discrepancy is presumably due to the fact that RhoA activity is dramatically increased by Rasip1 loss in BECs; however, we did not observe significant changes in RhoA activity, but a dramatic reduction in Cdc42 activity in LECs. Therefore, these data suggested an additional potential regulatory role of Rasip1 in lymphatic actin organization, via its regulation of Cdc42 activity in LECs. Conversely, our data agreed with previous work showing that Cdc42 regulates F-actin organization and EC adhesions (Barry et al., 2015). Similarly, we found that siRNA knockdown of Rasip1 or Cdc42 affected the LEC cytoskeleton and lymphatic endothelial cell adhesions. Focal adhesion kinases have been established as a key component of the signal transduction pathways triggered by integrins (Guan, 1997). We determined that siRNA knockdown of Rasip1 or Cdc42 significantly reduced the focal adhesion markers p-Paxillin and active integrin β1 in LECs. Moreover, ectopic Cdc42 expression greatly restored F-actin organization and focal adhesions in Rasip1-deficient LECs, further supporting the proposal that Cdc42 activity is regulated by Rasip1.
More importantly, we showed that Cdc42-deficient embryos exhibited enlarged lymphatic vessels with disorganized lymphatic endothelial junctions at E14.5. These embryos also failed to form collecting lymphatic valves. Together, these defects largely phenocopied those observed in Rasip1 conditional null embryos. Accordingly, based on our in vivo and in vitro data, we argue that Rasip1 regulation of Cdc42 activity is required for cytoskeleton organization and regulation of LEC junctions and LEC-ECM interactions during lymphatic vessel development (Fig. S13). In blood vessel lumen formation, Rasip1 controls a pool of GTPases, such that Rasip1 and its binding partner, the GTPase-activating protein Arhgap29, promotes the activation of Pak4 by Cdc42, which in turn activates NMII (Barry et al., 2016). In the case of lymphatics, we still do not know about the downstream signaling pathways which are regulated by Cdc42 that are required to maintain cell junctions and, therefore, lymphatic vessel and lymphatic lumen integrity.
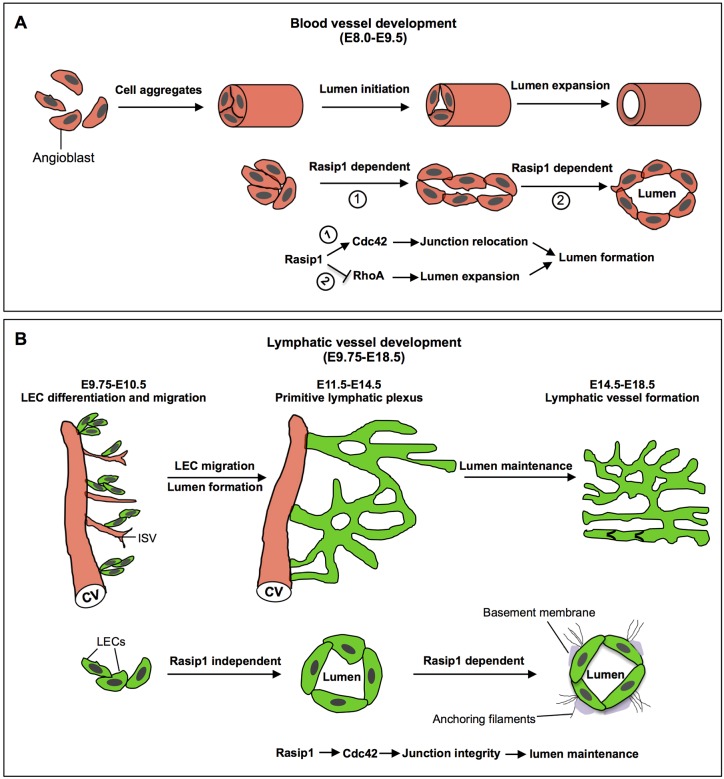
Taken together, our data support a somewhat different role for Rasip1 during lymphatic vessel development than the one which has been reported during blood vessel formation (Xu et al., 2011). We predict that Rasip1 plays different roles in these two different types of vessels, because of the important differences in the timing and mechanisms controlling lumen formation in these two vasculature networks. Vasculogenesis occurs at early embryonic stages (E8-E9 in the mouse) and single angioblasts aggregate to form continuous cords. The cord-to-tube transition involves a series of cellular changes that are required for lumen initiation and lumen expansion, including junction molecule re-localization and distribution. Rasip1 is required in these processes by temporally regulating different pools of Rho GTPases to clear the adhesions from the apical membrane to form a single, continuous lumen (Xu et al., 2011; Barry et al., 2016) (Fig. 10A). In contrast, the majority of mammalian LECs originate from the CV and intersomitic vessels (ISVs) at ∼E9.75. As these LECs bud off from the CV, they remain attached together by high levels of VE-cadherin. These LECs migrate away from the CV in clusters attached by multiple junctions containing narrow lumens (Fig. 10B). The presence and expansion of these lumens could be due to the fluid flowing through the remaining connections between budding LECs and the CV, such that anchoring filaments eventually sense changes in fluid pressure and/or other yet unknown physiological causes. Blood vessel lumen formation is a stepwise process controlled by the temporal regulation of Rasip1, whereas lymphatic vessel lumen formation is rather a passive process, taking place concomitant with the process of early LEC migration and proliferation. Accordingly, Rasip1 is not required for lymphatic lumen formation, but is necessary for lymphatic vessel lumen maintenance during embryonic stages.
Fig. 10.
Schematic view of the role of Rasip1 in lumen formation and maintenance in blood and lymphatic vessels. (A) Schematic view of blood vessel vasculogenesis at early embryonic stages in mice. Single angioblasts aggregate to form linear cords. Cord-to-tube transition involves a series of cellular changes from lumen initiation to lumen expansion, including junction molecule re-localization and distribution. Rasip1 is required in these processes to clear the adhesions from the apical membrane, to form a single continuous lumen, by temporally regulating different pools of Rho GTPases. (B) Schematic view of lymphatic vessel development during embryonic stages in mice. LECs bud off from the CV as LEC aggregates that already have a lumen; these eventually separate from the venous system and form a mature lymphatic network. Blood vessel lumen formation is a stepwise process and Rasip1 activity is required for lumen formation and maintenance. Instead, lymphatic vessel lumen formation is a rather passive process that occurs as LECs migrate and form the lymphatic network. Therefore, in lymphatics, Rasip1 is not required for lymphatic vessel lumen formation, but it is necessary for lumen maintenance during embryonic developmental stages.
MATERIALS AND METHODS
Mice handling and inducible deletion of Rasip1 and Cdc42
Rasip1-floxed mice (Xu et al., 2011) were generated by Ondine Cleaver's group (UT Southwestern, Dallas, TX, USA); Lyve1eGFPCre mice (Pham et al., 2010) were purchased from the Jackson Laboratory; Cdh5CreERT2 mice were obtained from Ralf H. Adams's group (Wang et al., 2010) (Max Planck Institute for Molecular Biomedicine, Münster, Germany); Prox1CreERT2 mice have previously been described (Srinivasan et al., 2007). Rasip1 conditional null embryos were generated by crossing Rasip1-floxed mice with Prox1CreERT2 mice. In general, 2-month- to 8-month-old male and female mice were used for mating and breeding. Mice were backcrossed into the NMRI background for five generations. For deletion of Rasip1 in LECs, pregnant mothers were injected intraperitoneally with 5 mg TM per 40 g mouse at E9.5 and E10.5, to examine lymphatic defects at E14.5, E15.5, E16.5 and E17.5. To study the role of Rasip1 in lymphatic valve development, pregnant mothers were injected intraperitoneally with 5 mg TM per 40 g mouse at E14.5. To study the role of Rasip1 in postnatal lymphatic development, control and Rasip1 conditional null neonates received 20 μg of TM per day from P0 to P4 by oral delivery. Cdc42 conditional null embryos were generated by crossing Cdc42-floxed mice (Chen et al., 2006) with Cdh5CreERT2 mice (Wang et al., 2010) in a CD1 background. To delete Cdc42, pregnant mothers were gavaged with 3 mg TM per 40 g mouse either at E12.5 and E13.5 for embryos harvested at E14.5, or at E14.5 and E15.5 for embryos harvested at E17.5. At least three litters per stage were used to assess each time point. All animal husbandry was performed in accordance with protocols approved by Northwestern University and UT Southwestern Medical Center Institutional Animal Care and Use Committee.
Immunofluorescent staining
Cryosections
Embryos were washed with ice-cold PBS and fixed in 4% PFA in the cold room at 4°C overnight and then dehydrated in 30% sucrose. Embryos were embedded in Tissue-Tek O.C.T (Sakura) and 10 μm thick cryosections were used for immunostaining. Sections were washed with PBS and blocked for 1 h at room temperature. Primary antibodies were incubated at 4°C overnight. Secondary antibodies were incubated for 2 h at room temperature. Slides were washed with TBST (Tris-based saline+1% Tween 20) and mounted using mounting medium containing DAPI.
Vibratome sections
Immunostaining was performed as has previously been described (Yang et al., 2012). Briefly, fixed embryos were embedded in 7% low melting point agarose and 100 µm sections were collected for immunostaining. Immunostaining was performed as described above.
Whole-mount staining
Skin and mesentery were isolated at the stages described in the results section and fixed with 4% paraformaldehyde (PFA) overnight or 30 min at room temperature, respectively. Fixed tissues were washed with PBS and blocked at 4°C overnight. Primary antibodies were incubated at 4°C overnight and then tissues were washed with PBST (phosphate-buffered saline+0.1% Triton). Secondary antibodies were incubated for 2 h at room temperature. Tissues were washed with PBST and then mounted with mounting medium containing DAPI.
Cell culture
Cells were washed with PBS and fixed with 4% PFA for 30 min. Immunostaining was performed as described above.
Antibodies
The following primary antibodies were used: rabbit anti-Prox1 (11002, 1:500, AngioBio); goat anti-Prox1 (AF2727, 1:200, R&D Systems); rat anti-PECAM1 (553370, 1:100, BD Pharmingen); goat anti-Lyve1 (AF2125, 1:200, R&D Systems); rat anti-VE-cadherin (clone: 11D4.1, 555289, 1:200, BD Pharmingen); rat anti-VE-cadherin (clone: BV13A, 1:200, ImClone Systems); rabbit anti-VE-cadherin (ab33168, 1:500, Abcam); goat anti-integrin α9 (AF3827, 1:100, R&D Systems); goat anti-VEGFR3 (AF743, 1:100, R&D Systems); goat anti-Nrp2 (AF567, 1:500, R&D Systems); rabbit anti-ZO-1 (40-2200, 1:200, Invitrogen); rabbit anti-Ki67 (clone: SP6, MA5-14520, 1:200, Invitrogen); rabbit anti-active caspase 3 (clone: C92-605, 559565, 1:200, BD Pharmingen); mouse anti-paxillin (clone: 349, 610051, 1:200, BD Transduction Laboratories); rat anti-integrin β1 (clone: 9EG7, 550531, 1:200, BD Biosciences); rabbit-anti p-paxillin (2541, 1:500, Cell Signaling Technology); goat anti-Rasip1 (ab21018, 1:500, Abcam); mouse anti-Rac1 (ARC03, 1:100, Cytoskeleton); Cdc42 (ACD03,1:100, Cytoskeleton); RhoA (ARH04, 1:100, Cytoskeleton); rabbit anti-Gapdh (clone: 6C5, sc-32233, 1:1000, Santa Cruz Biotechnology). The following secondary antibodies were used: Alexa 488-conjugated donkey anti-rabbit (A-21206, 1:300, Invitrogen); Alexa 488-conjugated donkey anti-goat (A-11055, 1:300, Invitrogen); Alexa 488-conjugated donkey anti-rat (A-21208, 1:300, Invitrogen); Cy3-conjugated donkey anti-rabbit (711-165-152, 1:300, Jackson ImmunoResearch); Cy3-conjugated donkey anti-mouse (715-165-151, 1:300, Jackson ImmunoResearch); Cy3-conjugated donkey anti-goat (705-165-147, 1:300, Jackson ImmunoResearch); Cy5-conjugated donkey anti-goat (705-495-147, 1:300, Jackson ImmunoResearch) and Cy5-conjugated donkey anti-rat (712-175-150, 1:300, Jackson ImmunoResearch).
siRNA transfection
Human dermal LECs were purchased from PromoCell and cultured in endothelial basal medium (EBM) complemented with supplement mix (C-39225, PromoCell) according to the supplier's instructions. Human RASIP1 siRNA or CDC42 siRNAs were obtained from GE Dharmacon and were transfected into cultured human dermal LECs using Lipofectamine 2000 (Thermo Fisher Scientific) as has previously been described (Srinivasan et al., 2014). After 48 h post-transfection, cells were either harvested for western blot or fixed with 4% PFA for immunostaining.
Virus transfection
RASIP1 shRNA lentivirus and control shRNA lentivirus were purchased from OriGene. Cherry-CDC42 adenovirus and Cherry-Control adenovirus were obtained from Dr Gorge Davis's laboratory (University of Missouri School of Medicine, Columbia, USA). RASIP1 transfection was performed according to the manufacturer's instructions. Briefly, P4 human dermal LECs were cultured to 30% confluence and either RASIP1 shRNA lentivirus or control lentivirus was added into culture medium containing 8 µg/ml Polybrene (Sigma-Aldrich). After 24 h post-transfection, cells were changed into EBM basal medium and CDC42 adenovirus transfection was performed as has previously been described (Norden et al., 2016). Two days after infection, cells were fixed with 4% PFA for immunostaining.
In vitro GTPase pull-down assays
Rho GTPase activity was detected by using the RHOA/RAC1/CDC42 Activation Assay Combo Biochem Kit (Cytoskeleton). Briefly, cultured control siRNA- and RASIP1 siRNA-treated human dermal LECs were lysed using ice-cold cell lysis buffer supplemented with 1× protease inhibitor cocktail (Roche) on ice for 30 min according to the manufacturer's instruction (Cytoskeleton). Cell lysates from control siRNA and RASIP1 siRNA treated LECs were incubated with rhotekin-RBD (for RHOA activation assay) or PAK- PBD beads (for RAC1 and CDC42 activation assays) for 1 hour at 4°C. Beads were centrifuged and washed four times with washing buffer and eluted with 2× Laemmli sample buffer. Bound active CDC42/RAC1 or RHOA proteins were detected by western blot. In separate experiments, 20-50 μg of cell lysates from each sample were used to determine total RHOA, RAC1 or CDC42 by western blot.
Image analysis
Whole-mount samples and vibratome tissue sections were immunostained and then visualized with a confocal laser-scanning microscope (Zeiss LSM510). For images to be compared quantitatively, the same laser intensity settings were used and images were acquired on the same day. Quantification of the fluorescence intensity was performed using ImageJ. The identity of LECs was defined by Prox1 staining and the LEC boundaries were outlined using ImageJ. Images were transformed to 8-bit grayscale. Regions of interest were selected manually by using the section tools, and the mean fluorescent intensity of the selected areas was measured after background subtraction. For quantification, at least three regions of interest were selected and mean values were quantified for each image. The numbers of samples in each group and the statistical analysis, are listed in the individual figure legends.
Supplementary Material
Acknowledgements
We thank Ralf H. Adams for the Cdh5CreERT2 mice. We thank all the members in G.O.'s and Beatriz Sosa-Pineda's laboratories for invaluable discussions. We thank Benjamin R. Thomson and Isabel A. Carota for skilled technical help. We thank Donald McDonald and Peter Baluk for valuable comments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: X.L., G.O.; Methodology: X.L., M.O., H.J.G.; Validation: X.L., H.J.G.; Formal analysis: X.L.; Investigation: X.L., W.M., M.O.; Resources: X.G., W.M., G.E.D., O.C., G.O.; Writing - original draft: X.L., G.O.; Supervision: G.O.; Funding acquisition: G.O.
Funding
This work was supported by the National Institutes of Health (grants RO1HL073402-16 to G.O., RO1HL126518 to O.C. and the National Research Service Award T32 training grant 1T32HL134633-01A1 to X.L.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.165092.supplemental
References
- Aitsebaomo J., Wennerberg K., Der C. J., Zhang C., Kedar V., Moser M., Kingsley-Kallesen M. L., Zeng G. Q. and Patterson C. (2004). p68RacGAP is a novel GTPase-activating protein that interacts with vascular endothelial zinc finger-1 and modulates endothelial cell capillary formation. J. Biol. Chem. 279, 17963-17972. 10.1074/jbc.M311721200 [DOI] [PubMed] [Google Scholar]
- Alitalo K. (2011). The lymphatic vasculature in disease. Nat. Med. 17, 1371-1380. 10.1038/nm.2545 [DOI] [PubMed] [Google Scholar]
- Aspelund A., Tammela T., Antila S., Nurmi H., Leppänen V. M., Zarkada G., Stanczuk L., Francois M., Mäkinen T., Saharinen P. et al. (2014). The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Invest. 124, 3975-3986. 10.1172/JCI75395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry D. M., Xu K., Meadows S. M., Zheng Y., Norden P. R., Davis G. E. and Cleaver O. (2015). Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development 142, 3058-3070. 10.1242/dev.125260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry D. M., Koo Y., Norden P. R., Wylie L. A., Xu K., Wichaidit C., Azizoglu D. B., Zheng Y., Cobb M. H., Davis G. E. et al. (2016). Rasip1-mediated Rho GTPase signaling regulates blood vessel tubulogenesis via nonmuscle myosin II. Circ. Res. 119, 810-826. 10.1161/CIRCRESAHA.116.309094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E., Xie S., Chen C., Weston A., Miura N., Sorokin L., Adams R., Muro A. F., Sheppard D. and Makinen T. (2009). Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell. 17, 175-186. 10.1016/j.devcel.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liao G., Yang L., Campbell K., Nakafuku M., Kuan C.-Y. and Zheng Y. (2006). Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc. Natl. Acad. Sci. USA 103, 16520-16525. 10.1073/pnas.0603533103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-G., Lv Y.-X., Zhao D., Zhang L., Zheng F., Yang J.-Y., Li X.-L., Wang L., Guo L.-Y., Pan Y. et al. (2016). Vascular endothelial growth factor-C protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Mol. Cell. Biochem. 413, 9-23. 10.1007/s11010-015-2622-9 [DOI] [PubMed] [Google Scholar]
- Connolly J. O., Simpson N., Hewlett L. and Hall A. (2002). Rac regulates endothelial morphogenesis and capillary assembly. Mol. Biol. Cell. 13, 2474-2485. 10.1091/mbc.e02-01-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. J., Gale N. W. and Harvey N. L. (2008). Expression of the hyaluronan receptor LYVE-1 is not restricted to the lymphatic vasculature; LYVE-1 is also expressed on embryonic blood vessels. Dev. Dyn. 237, 1901-1909. 10.1002/dvdy.21605 [DOI] [PubMed] [Google Scholar]
- Guan J.-L. (1997). Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell Biol. 29, 1085-1096. 10.1016/S1357-2725(97)00051-4 [DOI] [PubMed] [Google Scholar]
- Harvey N. L., Srinivasan R. S., Dillard M. E., Johnson N. C., Witte M. H., Boyd K., Sleeman M. W. and Oliver G. (2005). Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37, 1072-1081. 10.1038/ng1642 [DOI] [PubMed] [Google Scholar]
- Henri O., Pouehe C., Houssari M., Galas L., Nicol L., Edwards-Lévy F., Henry J.-P., Dumesnil A., Boukhalfa I., Banquet S. et al. (2016). Selective Stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial InfarctionCLINICAL PERSPECTIVE. Circulation 133, 1484-1497. 10.1161/CIRCULATIONAHA.115.020143 [DOI] [PubMed] [Google Scholar]
- Ikram M. K., Sim X., Xueling S., Jensen R. A., Cotch M. F., Hewitt A. W., Ikram M. A., Wang J. J., Klein R., Klein B. E. K. et al. (2010). Four novel Loci (19q13, 6q24, 12q24, and 5q14) influence the microcirculation in vivo. PLoS Genet. 6, e1001184 10.1371/journal.pgen.1001184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K., Ryan M., Marchant J. K., Henrich S. and John S. W. M. (2014). Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 12, e1001912 10.1371/journal.pbio.1001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L., Norman S., Vieira J. M., Masters M., Rohling M., Dubé K. N., Bollini S., Matsuzaki F., Carr C. A. and Riley P. R. (2015). Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522, 62-67. 10.1038/nature14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltowska K., Betterman K. L., Harvey N. L. and Hogan B. M. (2013). Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development 140, 1857-1870. 10.1242/dev.089565 [DOI] [PubMed] [Google Scholar]
- Komatsu M. and Ruoslahti E. (2005). R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat. Med. 11, 1346-1350. 10.1038/nm1324 [DOI] [PubMed] [Google Scholar]
- Koo Y., Barry D. M., Xu K., Tanigaki K., Davis G. E., Mineo C. and Cleaver O. (2016). Rasip1 is essential to blood vessel stability and angiogenic blood vessel growth. Angiogenesis 19, 173-190. 10.1007/s10456-016-9498-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A., Smirnov I., Keyes T. J., Eccles J. D., Rouhani S. J., Peske J. D., Derecki N. C., Castle D., Mandell J. W., Lee K. S. et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337-341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W. and Oliver G. (2017). Lymphatic endothelial cell plasticity in development and disease. Physiology 32, 444-452. 10.1152/physiol.00015.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitin N. Y., Ramocki M. B., Zullo A. J., Der C. J., Konieczny S. F. and Taparowsky E. J. (2004). Identification and characterization of rain, a novel Ras-interacting protein with a unique subcellular localization. J. Biol. Chem. 279, 22353-22361. 10.1074/jbc.M312867200 [DOI] [PubMed] [Google Scholar]
- Nelson C. M. and Chen C. S. (2003). VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J. Cell Sci. 116, 3571-3581. 10.1242/jcs.00680 [DOI] [PubMed] [Google Scholar]
- Norden P. R., Kim D. J., Barry D. M., Cleaver O. B. and Davis G. E. (2016). Cdc42 and k-Ras control endothelial tubulogenesis through apical membrane and cytoskeletal polarization: novel stimulatory roles for GTPase effectors, the small GTPases, Rac2 and Rap1b, and inhibitory influence of Arhgap31 and Rasa1. PLoS ONE 11, e0147758 10.1371/journal.pone.0147758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G. (2004). Lymphatic vasculature development. Nat. Rev. Immunol. 4, 35-45. 10.1038/nri1258 [DOI] [PubMed] [Google Scholar]
- Pham T. H. M., Baluk P., Xu Y., Grigorova I., Bankovich A. J., Pappu R., Coughlin S. R., McDonald D. M., Schwab S. R. and Cyster J. G. (2010). Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207, 17-27. 10.1084/jem.20091619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S., Sabine A. and Petrova T. V. (2011). Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607-618. 10.1083/jcb.201012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo J., Nicenboim J. and Yaniv K. (2016). Development of the lymphatic system: new questions and paradigms. Development 143, 924-935. 10.1242/dev.132431 [DOI] [PubMed] [Google Scholar]
- Serban D., Leng J. and Cheresh D. (2008). H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ. Res. 102, 1350-1358. 10.1161/CIRCRESAHA.107.169664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowski R. G., Feldman S. and Feramisco J. R. (1993). Interference with endogenous ras function inhibits cellular responses to wounding. J. Cell Biol. 121, 113-120. 10.1083/jcb.121.1.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R. S., Dillard M. E., Lagutin O. V., Lin F.-J., Tsai S., Tsai M.-J., Samokhvalov I. M. and Oliver G. (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 21, 2422-2432. 10.1101/gad.1588407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R. S., Escobedo N., Yang Y., Interiano A., Dillard M. E., Finkelstein D., Mukatira S., Gil H. J., Nurmi H., Alitalo K. et al. (2014). The Prox1–Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 28, 2175-2187. 10.1101/gad.216226.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Palmby T. R., Gavard J., Amornphimoltham P., Zheng Y. and Gutkind J. S. (2008). An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 22, 1829-1838. 10.1096/fj.07-096438 [DOI] [PubMed] [Google Scholar]
- Thomson B. R., Heinen S., Jeansson M., Ghosh A. K., Fatima A., Sung H.-K., Onay T., Chen H., Yamaguchi S., Economides A. N. et al. (2014). A lymphatic defect causes ocular hypertension and glaucoma in mice. J. Clin. Invest. 124, 4320-4324. 10.1172/JCI77162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Nakayama M., Pitulescu M. E., Schmidt T. S., Bochenek M. L., Sakakibara A., Adams S., Davy A., Deutsch U., Lüthi U. et al. (2010). Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483-486. 10.1038/nature09002 [DOI] [PubMed] [Google Scholar]
- Wigle J. T. and Oliver G. (1999). Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769-778. 10.1016/S0092-8674(00)81511-1 [DOI] [PubMed] [Google Scholar]
- Wilson C. W., Parker L. H., Hall C. J., Smyczek T., Mak J., Crow A., Posthuma G., De Mazière A., Sagolla M., Chalouni C. et al. (2013). Rasip1 regulates vertebrate vascular endothelial junction stability through Epac1-Rap1 signaling. Blood 122, 3678-3690. 10.1182/blood-2013-02-483156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Chong D. C., Rankin S. A., Zorn A. M. and Cleaver O. (2009). Rasip1 is required for endothelial cell motility, angiogenesis and vessel formation. Dev. Biol. 329, 269-279. 10.1016/j.ydbio.2009.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Sacharidou A., Fu S., Chong D. C., Skaug B., Chen Z. J., Davis G. E. and Cleaver O. (2011). Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev. Cell. 20, 526-539. 10.1016/j.devcel.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. and Oliver G. (2014). Development of the mammalian lymphatic vasculature. J. Clin. Invest. 124, 888-897. 10.1172/JCI71609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., García-Verdugo J. M., Soriano-Navarro M., Srinivasan R. S., Scallan J. P., Singh M. K., Epstein J. A. and Oliver G. (2012). Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120, 2340-2348. 10.1182/blood-2012-05-428607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L.-C., Baluk P., Srinivasan R. S., Oliver G. and McDonald D. M. (2012). Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am. J. Pathol. 180, 2561-2575. 10.1016/j.ajpath.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Nurmi H., Appak S., Sabine A., Bovay E., Korhonen E. A., Orsenigo F., Lohela M., D'Amico G., Holopainen T. et al. (2014). Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 28, 1592-1603. 10.1101/gad.237677.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.