Abstract
This data article contains the truncated view of the transition states for methyl radical attacking camptothecin at the site of 9, 10, 11, 12 and 14 in acidic conditions obtained from quantum computation of Gaussian 09 with B3LYP/6–31+G(d,p) level, also the truncated view of transition states for H abstraction by singlet O2 from sites of 9, 10, 11 and 12 of the intermediates of methyl combination with camptothecin and that by triplet O2 from site 9 of the intermediate of methyl combination with camptothecin in acidic condition are included. The corresponding parameters of reaction rate constant calculation for the formation of methyl radical from acetaldehyde, the first and second step of radical substitution of camptothecin under acidic conditions are listed. The data of the basic parameters for the computation of the total energy of the spin-projection of singlet oxygen, and the S2 values for the reactants, transition states and intermediates in the free radical substitution reaction of camptothecin are also included.
Specifications Table
| Subject area | Chemistry |
| More specific subject area | Molecular graphics and modeling |
| Type of data | graph, figure, table |
| How data was acquired | By ChemBio3D Ultra 12.0 |
| Data format | Raw |
| Experimental factors | Some transition state structures come from computation of Gaussian 09 |
| Data source location | Tianjin, China. |
| Data accessibility | No |
Value of the data
-
•
To facilitate the reader׳s understanding of this study.
-
•
Extend readers׳ knowledge about the free radical reaction of camptothecin.
-
•
To lay a foundation for further study on the mechanism of free radical substitution of natural medicines.
1. Data
Data provided in this article are based on computation performed applying Gaussian 09 [1] at B3LYP/6–31+G(d, p) level [2], [3] and the figures are treated using ChemBio3D Ultra 12.0 [4]. The corresponding parameters for the calculation of reaction rate constant, for the computation of the total energy of the spin-projection of singlet oxygen, and the S2 values for the reactants, transition states and intermediates in the free radical substitution reaction of camptothecin are included.
2. Experimental design, materials and methods
The truncated 3D structures of the reactants, transition states (TSs) and intermediates in the reaction in Fig. 1, Fig. 2, Fig. 3 are all sketched using ChemBio3D Ultra 12.0 based on the TS optimization of the corresponding transition states with Gaussian 09 at B3LYP/6–31+G(d,p) level.
Fig. 1.
The truncated view of TSac (9), TSac (10), TSac (11), TSac (12), and TSac (14) corresponding for the transition states of methyl radical attacking camptothecin at the site of 9, 10, 11and 12 in acidic conditions respectively.
Fig. 2.
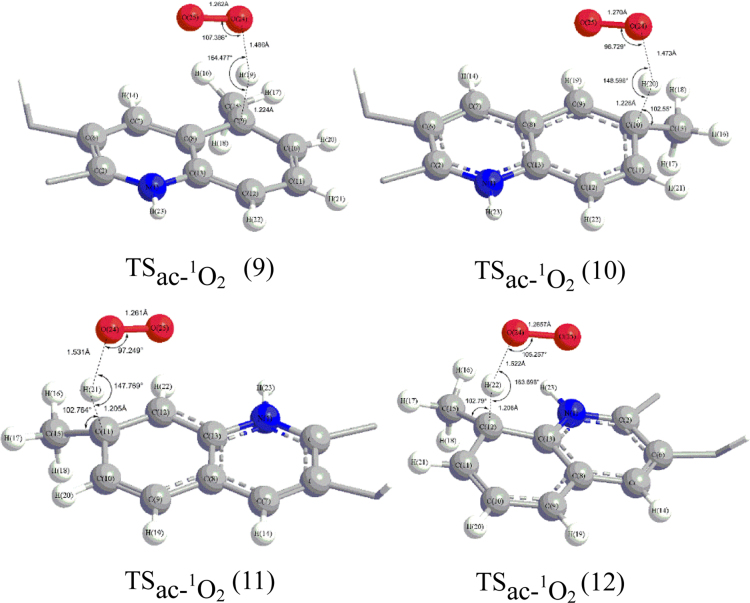
The truncated view of TSac-1O2 (9), TSac-1O2 (10), TSac-1O2 (11), and TSac-1O2 (12) corresponding for transition states of H abstraction by singlet 1O2 from sites of 9, 10, 11 and 12 of the intermediate of methyl combination with camptothecin in acidic condition respectively.
Fig. 3.
The truncated view of TSac-3O2 (9) corresponding for the transition state of H abstraction by triplet O2 from site 9 of the intermediate of methyl combination with camptothecin in acidic condition.
In this paper, some abbreviations were used for the convenience of description, the reactants, transition states, intermediates and products are expressed by R, TS, M and P respectively, and the Arabic numerals indicate the order. The neutral reaction conditions are denoted by n, and the acidic reaction conditions are represented by ac. The singlet O2 and the triplet O2 are denoted by 1O2 and 3O2, respectively.
FTS stands for the absolute value of the frequency of the transition state. κ is the tunnel effect correction factor. It can be calculated by Wigner method based on the virtual frequency of the transition state. ΔE is the static potential threshold on the minimum energy response path (MEEP). QA and QB are the partition functions of the reactants A and B. And QTS is the partition function for the transition state.
The molecular structures and the tables are shown below:
Fig. 1 shows the truncated view of the transition states for methyl radical attacking camptothecin at the site of 9, 10, 11, 12 and 14 in acidic conditions. The truncated view of transition states for H abstraction by singlet O2 from sites of 9, 10, 11 and 12 of the intermediates of methyl combination with camptothecin in acidic condition is shown in Fig. 2. The structure of transition states for H abstraction by triplet O2 from site 9 in acidic condition is illustrated in Fig. 3.
The corresponding parameters for the calculation of reaction rate constant of the formation of methyl radical from acetaldehyde, the first and second step of radical substitution of camptothecin under acidic conditions are listed in Table 1, Table 2, Table 5 respectively. The data of the basic parameters for the computation of the total energy of the spin-projection of singlet oxygen, and the S2 values for the reactants, transition states and intermediates in the free radical substitution reaction of camptothecin are also included in Table 3, Table 4 respectively.
Table 1.
The corresponding parameters of reaction rate constant calculation for the formation of methyl radical from acetaldehyde.
| Step | FTS (cm−1) | κ | QAa | QTS | ΔE (kcal/mol) |
|---|---|---|---|---|---|
| 1 | −148.84 | 1.022 | 1.24 × 1014 | 4.52 × 1013 | 3.215 |
| 2 | −247.14 | 1.059 | 2.51 × 1015 | 5.47 × 1012 | 19.70 |
Represents that the reactant of acetaldehyde molecule.
Table 2.
The corresponding parameters of reaction rate constant calculation for the first step of radical substitution of camptothecin under acidic conditions.c (298.15 K).
| Site | FTS1 (cm−1) | κ | QA1a | QB1b | QTS1 | ΔE1 (kcal/mol) |
|---|---|---|---|---|---|---|
| 7 | −280.81 | 1.027 | 1.27 × 1010 | 4.47 × 1022 | 2.50 × 1024 | 5.42 |
| 9 | −396.00 | 1.054 | – | – | 2.68 × 1024 | 8.28 |
| 10 | −412.99 | 1.059 | – | – | 2.25 × 1024 | 9.16 |
| 11 | −368.16 | 1.047 | – | – | 2.19 × 1024 | 8.16 |
| 12 | −444.43 | 1.068 | – | – | 2.46 × 1024 | 6.88 |
| 14 | −362.43 | 1.045 | – | – | 2.43 × 1024 | 7.35 |
Represents that the protonated camptothecin molecule.
Represents that the methyl radical.
“-” represents that the value is same as above.
Table 5.
The corresponding parameters of reaction rate constant calculation for the second step of radical substitution of camptothecin under acidic conditions.* (298.15 K).
| Site | FTS2 (cm−1) | κ | QA2 | QB2 | QTS2 | ΔE2 (kcal/mol) |
|---|---|---|---|---|---|---|
| 7 | −1396.24 | 1.054 | 1.02 × 1029 | 8.75 × 108 | 1.63 × 1025 | 8.35 |
| 9 | −1114.48 | 1.429 | 1.93 × 1029 | – | 1.22 × 106 | 5.76 |
| 10 | −399.56 | 1.055 | 1.65 × 1027 | – | 1.94 × 1025 | 0.98 |
| 11 | −300.07 | 1.031 | 2.64 × 1030 | – | 2.15 × 1025 | 3.26 |
| 12 | −922.35 | 1.294 | 3.19 × 1028 | – | 9.57 × 1025 | 6.04 |
a represents that the intermediate produced by the first step reaction.
b represents that the singlet oxygen.
* “-” represents that the value is same as above.
Table 3.
The basic parameters for the computation of the total energy of the spin-projection of singlet oxygen.
| α | ||||||
|---|---|---|---|---|---|---|
| −150.324 | −150.311 | −150.317 | 2.009 | 1.004 | 2 | 1 |
Table 4.
S2 values for the reactants, transitions and intermediates in the reaction.
| Site |
Step 1 |
Step 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
Neutral conditions |
Acidic condition |
Acidic condition |
|||||||
| Rna | TSnb | Mnc | Raca | TSacb | Macc | Rac-1O2a | TSac-1O2b | Pac-1O2c | |
| 7 | – | 0.7828 | 0.7843 | – | 0.7673 | 0.7678 | 0.76784 | 0.7662 | 0.7539 |
| 9 | – | 0.7883 | 0.7797 | – | 0.7813 | 0.7715 | 0.7715 | 0.7666 | 0.7539 |
| 10 | – | 0.7862 | 0.7867 | – | 0.7850 | 0.7844 | 0.7844 | 0.7775 | 0.7539 |
| 11 | – | 0.7835 | 0.7803 | – | 0.7738 | 0.7663 | 0.7663 | 0.8108 | 0.7539 |
| 12 | – | 0.7533 | 0.783 | – | 0.7883 | 0.7824 | 0.7824 | 0.7690 | 0.7539 |
| 14 | – | 0.7533 | 0.7667 | – | 0.7853 | 0.7561 | – | – | – |
| CH3 | 0.7533 | – | – | 0.7533 | – | – | – | – | – |
| 1O2 | – | – | – | – | – | – | 1.004448 | – | – |
* “-” represents that the value does not exist.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21272171).
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.07.004.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.07.004.
Transparency document. Supplementary material
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H. Gaussian Inc.; Wallingford, CT: 2013. Gaussian 09, Revision D. 01. [Google Scholar]
- 2.Becke A.D. Density‐functional thermochemistry. III. The role of exact exchange. J Chem. Phys. 1993;98:5648–5653. [Google Scholar]
- 3.Stephens P.J., Devlin F.J., Chabalowski C.F., Frisch M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. Chem. Phys. Lett. 1994;98:247–257. [Google Scholar]
- 4.Pro C.D. CambridgeSoft; Cambridge, MA: 2009. Version 12.0. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material