Abstract
Banana peel (BP) is a major waste produced by fruit processing industries. Pre-treatment of BP at different temperatures led to 40% reduction in saponin at 100 °C (from 9.5 to 5.7 mg/g). Sequential mixed culture of Phanerochaete chrysosporium (P. chrysosporium) and Candida utilis (C. utilis) gave highest protein enrichment (88.93 mg/g). There is 26% increase in protein synthesis (from 88.93 to 111.78 mg/g) after media screening. Inclusion of KH2PO4, FeSO4·7H2O, wheat flour and sucrose in the media contributed positively to protein synthesis, while elevated concentration of urea, peptone, K2HPO4, KCl, NH4H2PO4, and MgSO4.7H2O are required to reach optimum protein synthesis. Total soluble sugar (TSS), total reducing sugar (TRS) and total carbohydrate (CHO) consumption varied with respect to protein synthesis in all experimental runs. Optimum protein synthesis required 6 days and inclusion of 5% sucrose, 0.6% NH4H2PO4, 0.4% KCl, and 0.5% MgSO4·7H2O as concentration media constituents to reach 140.95 mg/g protein synthesis equivalent to 300% increase over the raw banana peel protein content (35.0 mg/g).
Keywords: Banana peel, Candida utilis, Phanerochaete chrysosporium, Solid state fermentation, Protein
Introduction
Fruits wastes generation is increasing in developing nations due to agricultural subsidies and supermarkets quality specifications. In fact, the Food and Agricultural Organization (FAO) reported over 6.5 million tons of banana wastes in India alone (Arumugam and Manikandan 2011). Similar trend is obvious in other developing economies of sub-Saharan Africa and Southeast Asia. The phenomenal increase in banana peels and other fruits wastes by hospitality industry alone is a concern to governments (Jayabalan et al. 2010; Saheed et al. 2016).
Fruit wastes from food processing industries could be abundant but contain some nitrogen, vitamins and minerals. This poor nutritional composition restricted the wastes as basal feed ingredient (Kasapidou et al. 2015). Banana peel (BP)—a common solid waste contains sugars, minerals (potassium, sodium, calcium and iron), lignin, cellulose and hemicellulose. The nutrient composition suggests that BP could support microbial growth if inherent saponin that inhibits fungus growth in fermentation processes is reduced. Therefore, pretreatment step is a necessary prior to protein enrichment via solid-state fermentation (SSF).
In SSF of agro-residues, the most commonly used method involves monoculture cultivation of edible fungi. A novel approach to ensure robust consumption of nutrients, improved microbial growth and product synthesis is sequential fungal-yeast co-culture with assumption of synergistic actions under SSF. Sequential SSF co-culture involves cultivation of two microbes such that the second microbe is inoculated days after the first. Interestingly, sequential cultivation of physiologically different fungi or bacteria cultures abound in literature but information concerning performance of fungal-yeast sequential culture requires further study especially for protein enrichment of BP under SSF (Ahmed et al. 2010; Rajoka et al. 2011).
Edible bacidiomycete fungi and yeast cells could function wholly as animal feed supplement due to their high content of vitamin B-complex and low nucleic acid (Zayed 2018). Lignin degrading microorganisms have attracted interests due to their profound lignin degradation under SSF. Phanerochaete chrysosporium (P. chrysosporium), is capable of producing biomass rich in most essential amino acids at acceptable standards (Nitayavardhana et al. 2013). The lignolytic fungi could synthesize lignin degrading extracellular enzymes such as lignin peroxidase, manganese peroxidase and polyoxal oxidase (Castoldi et al. 2014). These three industrially important lignolytic enzymes are effective in degrading cellulose, xylan and plant polymer lignin (Elisashvili et al. 2008). The mechanism of fungal lignin degradation entails the release of carbon dioxide (CO2) and water (H2O) after depolymerization in the presence of free oxygen molecules (Cameron et al. 2000).
Yeasts are widely consumed in fermented foods such as Tofu (the cheese of Asia from soy) and Tempe (soy product) (Bakar et al. 2011). Moreover, Candida utilis (C. utilis), a hydrolytic enzyme producing yeast, is extensively cultivated on agro-residues for its aromatic flavor, higher nutritive value and non-toxigenic properties. Its extracellular enzymes are capable of degrading mono-saccharides, disaccharides and lignolytic agro wastes materials (Ke et al. 2011; Ionuț et al. 2017).
This investigation focused on the conversion of BP to protein rich biomass by edible bacidiomycete fungi and yeast under sequential SSF after environmental friendly substrate pretreatment. Next step involves design and optimization of the nutritional need of the cultivated microorganisms for improved protein synthesis.
Materials and methods
Substrate collection, pre-treatment and preparation for solid state fermentation
Fresh banana peels were collected from local banana juice processors in Kuala Lumpur, Malaysia and was oven dried immediately at 60 °C for 48 h to arrest any form of decay by microbial enzymes or adverse chemical reactions. After drying, raw BP was placed in 500 ml beaker up to 70% v/v, filled with tap water before subjecting independent triplicate samples to temperature treatment from 20 to 100 °C at 20 °C interval for 60 min in temperature controlled water bath. The saponin content released was then determined according to method described (Hudson and Ei -Difrawi 1979). Dried (10–12% moisture content) pre-treated peels were grinded into pre-optimized 2 mm pore size with a grinding machine (Model D-79219 Staufen, IKA-WERKE GMBH & Co KG Germany) and stored in airtight container for 60 days at most.
Inoculum preparation
The fungi culture (Phanerochaete chrysosporium ATCC 20696 and Panus tigrinus M609RQY) was grown on potato dextrose agar (PDA, Merck, Germany) plates at 32 °C for 7 days, washed with 25 ml sterilized distilled water, then filtered with Whatman No.1 filter paper and spore suspension was collected in 100 ml Erlenmeyer flask. A haemocytometer was used to maintain the spore density at 1.0 × 108 spores/ml. Excess inoculum was stored at 4 °C for further use. Yeast culture (Candida utilis) maintained on PDA plates for 4–5 days and was washed with 25 ml of pre-sterilized distilled water into 250 ml Erlenmeyer flask without prior filtration. One loop full of cells was grown for 24 h on yeast extract 1%, peptone 0.5% and glucose 1% (YEPG) solution and cell density corrected to 1.0 × 107 and stored at 4 °C in a chiller.
Solid state fermentation and media development
Solid state fermentation (SSF) process comprise 6.0 g substrate (30%) and 14.0 g total liquid (70%) containing inoculum (added after sterilization), mineral supplements (0.1% KH2PO4 and 0.25% NH4H2PO4) to maintain 70% moisture content in 250 ml Erlenmeyer flasks of 20 g working volume. Sequential SSF of P. chrysosporium and each of the three yeast cells involves cultivation of P. chrysosporium for 3 days after which yeast cells (C. cylindracea, S. cerevisae and C. utilis) are inoculated and both allowed grow until the end of fermentation period. Media constituent was screened using Plackett Burman design to determine high and low values of each constituent (Plackett and Burman 1946). Eleven factors comprising nitrogen source (organic and inorganic), carbon source and mineral source were selected and the experiment carried out in triplicate was designed accordingly (Table 1).
Table 1.
Plackett Burman design for selected media factors for improved protein production
| Run | Solid content (%) | Urea (%) | Peptone (%) | K2HPO4 (%) | KH2PO4 (%) | KCl (%) | NH4H2PO4 (%) | FeSO4·7H2O (%) | MgSO4·7H2O (%) | WF (%) | SC (%) | Response (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 (72.5) | 1 (0.6) | − 1 (0.3) | 1 (0.15) | 1 (0.9) | − 1 (0) | 1 (0.5) | − 1 (0.3) | − 1 (0.3) | − 1 (0) | 1 (6) | 98.41 |
| 2 | − 1 (62.5) | − 1 (0) | − 1 (0.3) | 1 (0.15) | 1 (0.9) | 1 (0.6) | − 1 (0) | 1 (0.7) | 1 (0.9) | − 1 (0) | 1 (6) | 106.88 |
| 3 | 1 (72.5) | 1 (0.6) | 1 (0.7) | − 1 (0.05) | 1 (0.9) | 1 (0.6) | − 1 (0) | 1 (0.7) | − 1 (0.3) | − 1 (0) | − 1 (0) | 87.49 |
| 4 | − 1 (62.5) | 1 (0.6) | 1 (0.7) | 1 (0.15) | 1 (0.9) | − 1 (0) | − 1 (0) | − 1 (0.3) | 1 (0.9) | 1 (4) | 1 (6) | 98.81 |
| 5 | 1 (72.5) | 1 (0.6) | − 1 (0.3) | 1 (0.15) | − 1 (0.5) | − 1 (0) | − 1 (0) | 1 (0.7) | 1 (0.9) | 1 (4) | − 1 (0) | 83.81 |
| 6 | − 1 (62.5) | − 1 (0) | 1 (0.7) | − 1 (0.05) | 1 (0.9) | − 1 (0) | 1 (0.5) | 1 (0.7) | − 1 (0.3) | 1 (4) | − 1 (0) | 93.26 |
| 7 | 1 (72.5) | − 1 (0) | − 1 (0.3) | 1 (0.15) | 1 (0.9) | 1 (0.6) | 1 (0.5) | − 1 (0.3) | 1 (0.9) | 1 (4) | − 1 (0) | 111.78 |
| 8 | − 1 (62.5) | 1 (0.6) | 1 (0.7) | − 1 (0.05) | − 1 (0.5) | 1 (0.6) | 1 (0.5) | − 1 (0.3) | 1 (0.9) | − 1 (0) | − 1 (0) | 106.10 |
| 9 | − 1 (62.5) | 1 (0.6) | − 1 (0.3) | 1 (0.15) | − 1 (0.5) | 1 (0.6) | 1 (0.5) | 1 (0.7) | − 1 (0.3) | 1 (4) | 1 (6) | 88.46 |
| 10 | 1 (72.5) | − 1 (0) | 1 (0.7) | − 1 (0.05) | − 1 (0.5) | 1 (0.6) | − 1 (0) | − 1 (0.3) | − 1 (0.3) | 1 (4) | 1 (6) | 101.75 |
| 11 | 1 (72.5) | − 1 (0) | 1 (0.7) | − 1 (0.05) | − 1 (0.5) | − 1 (0) | 1 (0.5) | 1 (0.7) | 1 (0.9) | − 1 (0) | 1 (6) | 103.62 |
| 12 | − 1 (62.5) | − 1 (0) | − 1 (0.3) | − 1 (0.05) | − 1 (0.5) | − 1 (0) | − 1 (0) | − 1 (0.3) | − 1 (0.3) | − 1 (0) | − 1 (0) | 106.63 |
WF wheat flour, SC sucrose
Analytical methods
Protein content and total soluble sugar (TSS) content of the product were determined according to described methods (Lowry et al. 1951; Dubois et al. 1956). Total carbohydrate (CHO) content of the product was determined using phenol–sulphuric acid method. 100 mg of the sample was hydrolysed with 2.5 N HCl for 3 h the solution was neutralized with sodium carbonate. Phenol–sulphuric acid reagent were added and absorbance read at 490 nm (Dubois et al. 1956). Total reducing sugar (TRS) was determined as described (Miller 1959). 100 mg of sample was extracted with 80% hot ethanol. DNS reagent was added and the mixture was boiled (~ 100 °C) for 5 min on a temperature regulated water heater, samples were cooled before absorbance was measured at 510 nm. Ash content of BP and product was determined according to the suggested method (Poorter and Bergotte 1992) while moisture content (MC) was measured according to AOAC Official Method 950.46.
Results and discussion
Substrate pre-treatment
Pretreatment process is an important unit operation when converting lignocellulosic agro-residues to new products. Microbial growth inhibiting agent—saponin had profound removal as result showed increased saponin removal by aqueous pretreatment at elevated temperature (Table 2). Mere washing removed 5.3% saponin from BP while 20% reduction happened when soaked in tap water at room temperature for 60 min. Gradual but increased reduction was recorded in the plant glycoside as pretreatment temperature reached 100 °C at 20 °C interval. Saponin is temperature, salt concentration, and pH sensitive in aqueous phase (Güçlü-Üstündağ and Mazza 2007). The saponin reduction in BP could be caused by high glycoside solubility in water, concentration gradient and possible lignocellulosic fibers modification (Oberoi et al. 2011; Salihu et al. 2015).
Table 2.
Pre-treatment of banana peels at different treatment and physical conditions
| Treatment | Saponin concentration (mg/g) |
|---|---|
| Raw | 9.50 ± 1.41 |
| Normal washing | 9.00 ± 0.21 |
| Soaking | 7.50 ± 0.07 |
| After fermentation | 1.65 ± 0.58 |
| Treatment at 20 °C | 7.90 ± 1.06 |
| Treatment at 40 °C | 6.50 ± 0.71 |
| Treatment at 60 °C | 6.50 ± 0.71 |
| Treatment at 80 °C | 6.17 ± 0.35 |
| Treatment at 100 °C | 5.70 ± 1.77 |
Effect of saponin concentration on microbial growth
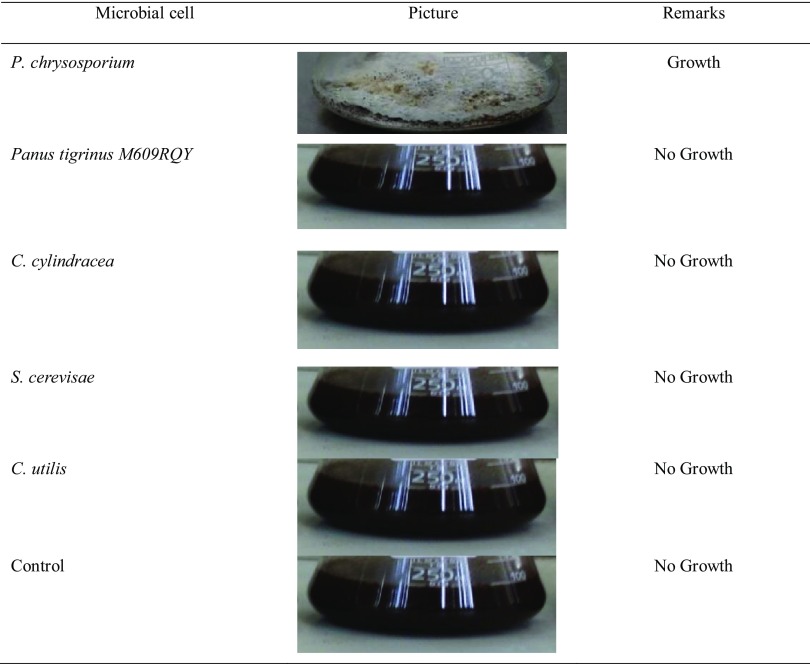
To ascertain the effect of saponin on the growth of selected microorganisms, the strains were inoculated on raw dried BP. Result showed that only P. chrysosporium successfully grow on the un-treated substrate (Table 3). The inability of other microbes to grow on raw BP could be attributed to the presence of branched-chain tri-saccharide moiety without oxygen-containing groups at C2 and C12 of the saponin molecules (Miyakoshi et al. 2000; Francis et al. 2002). However, P. chrysosporium demonstrated ability to metabolize recalcitrant chemicals via extracellular secretion of enzymes that can breakdown complex organics but not plant sterols (Zacchi et al. 2000; Shi et al. 2014). However, all selected strains grew well at 40% saponin reduction (from 9.5 to 5.70 mg/g) after boiling BP at 100 °C for 60 min.
Table 3.
growth characteristics of selected microbial cells on the pretreated substrate
Effects of microbial grouping on protein synthesis
Protein synthesis increased in all treatments as the fermentation period increased but sequential SSF of P. chrysosporium and C. utilis gave the highest (88.93 mg/g) protein synthesis. Synthesis by P. chrysosporium and Saccharomyces cerevisiae (S. cerevisiae) followed, while combination of P. chrysosporium and Candida cylindrisae (C. cylindrisae) produced least protein (Table 4). P. chrysosporium and C. utilis protein enrichment reached 150% increase compared with control. This performance suggested synergistic metabolism of sugars present in BP. Similar trend was reported when Archnioutus sp. and Candida utilis were grown by sequential SSF on corn stover (Ahmed et al. 2010). The high protein synthesis could be influenced by extracellular production of lignocellulosic enzymes (lignin peroxidase and manganese peroxidase) by P. chrysosporium leading to the conversion of complex sugar to forms that are easily consumed by yeast cells (Villas-Bôas et al. 2003; Ke et al. 2011). The was no measurement of protein synthesis profile of C. utili alone, S. cerevisiae alone and C. cylindricea alone since no growth was recorded for them even after pretreatment to remove saponin.
Table 4.
Screening of Potential Microbes for improved protein synthesis
| Organisms | Protein content (mg/g) dry weight | |||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 10 | |
| P. chrysosporium | 19.68 ± 3.58 | 40.17 ± 5.59 | 40.17 ± 0.14 | 78.27 ± 0.44 |
| P. chrysosporium /C. utilis | 8.23 ± 0.32 | 18.82 ± 0.15 | 18.82 ± 0.6 | 88.93 ± 3.38 |
| P. chrysosporium /S. cerevisiae | 7.24 ± 1.27 | 25.29 ± 0.67 | 25.29 ± 4.52 | 85.43 ± 4.53 |
| P. chrysosporium/C. cylindricea | 13.58 ± 1.60 | 27.70 ± 0.77 | 68.41 ± 0.22 | 84.16 ± 1.35 |
| Control | 2.35 ± 0.08 | 5,4 ± 0.03 | 8.3 ± 0.08 | 35.0 ± 1.27 |
Selection of media parameters
To identify required media constituents, eleven media parameters that cover carbon source, nitrogen and mineral salt undergo twelve experimental runs according to Plackett Burman design to determine individual contribution to protein synthesis. Highest protein synthesis (111.78 mg/g) corresponded to low concentration of five (KH2PO4, solid content, FeSO4·7H2O, WF and sucrose) media parameters and high concentration of another five (peptone, K2HPO4, KCl, NH4H2PO4 and MgSO4·7H2O) in run 7 (Table 1). Microbial protein synthesis could depend on the concentration and elemental nature of media components of the growing media (Ruqayyah et al. 2011).
Effects and significance of media concentrations on protein synthesis
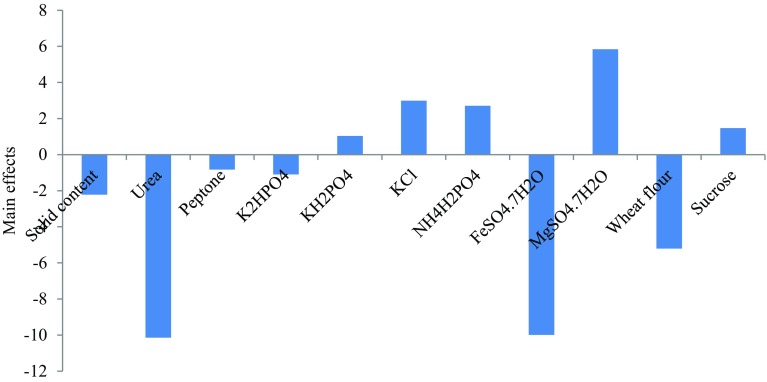
High concentrations of KH2PO4, KCl, NH4H2PO4, MgSO4·7H2O and sucrose favoured protein synthesis while low levels of FeSO4·7H2O, wheat flour (WF), solid content, urea, peptone and K2HPO4 are required (Fig. 1). Easy metabolism of potassium ion (K+) ammonium ion (NH4+) and phosphate ion (PO4+) in NH4H2PO4 and KH2PO4 could trigger growth dependent product synthesis via profound hyphae growth, spore formation and hydrolytic yeast cells processes (Rajendran et al. 2007; Jamal et al. 2008; Ruqayyah et al. 2011). Positive influence of MgSO4·7H2O and FeSO4·7H2O could be caused by increased mycelia branching of P. chrysosporium resulting from actions of Mg+, Fe3+ and SO42− that fovoured secretion of enzymes and co-factors (Ikram-ul et al. 2004). Similarly, the positive contribution of sucrose to protein synthesis by sequential culture of P. chrysosporium and Candida utilis on BP was expected since the microbes required the sugar to trigger growth and product formation. Similar observation has been reported by other researchers (Molla et al. 2004).
Fig. 1.
Main effects of media parameters for optimal protein production by Placket-Burmann experimental design
Urea, peptone, wheat flour, FeSO4·7H2O and solid content contributed negatively to protein synthesis. This observation suggested that P. chrysosporium and Candida utilis preferred inorganic nitrogen source (NH4H2PO4) to organic sources though other white rot fungus could respond differently (Ruqayyah et al. 2011). The microbes performance was independent of FeSO4·7H2O, K2HPO4 and solid content. This response of microbes to certain micronutrients and substrate moisture could be case dependent (Rosma and Cheong 2007; Gad et al. 2010; Hu et al. 2012).
Analysis of variance (ANOVA) showed significant contribution of FeSO4·7H2O, MgSO4·7H2O and wheat flour to protein synthesis (Table 4). Profound influence contribution of FeSO4·7H2O and MgSO4·7H2O to protein synthesis suggested the importance of micronutrients to growth and performance of P. chrysosporium and C. utilis (Jamal et al. 2008). NH4H2PO4 though contributed to protein synthesis, the effect was not significant.
Impact of media screening on chemical analysis of fermentation product
Total carbohydrate (CHO), total reducing sugar (TRS), total soluble sugar (TSS) and ash content of the product varied across experimental runs (Table 5). Sugar concentrations varied greatly across experimental runs suggesting a direct relationship between the microbes and the substrate sugar under sequential SSF (Saheed et al. 2013, 2016). CHO and other sugars generally reduced with corresponding protein synthesis. However, the reduction pattern differ and this suggested that the fungal-yeast system utilization is media design dependent (Yin et al. 2012). The ash content varied between 8.17 and 10.57% in the product; thus it falls within the acceptable level (AboSiada et al. 2017).
Table 5.
Result of nutritional parameters based on experimental design
| Run | Protein content (mg/g) DW | Ash content (%) | TSS (mg/g) | TRS (mg/g) | CHO (mg/g) |
|---|---|---|---|---|---|
| 1 | 98.41 ± 2.47 | 9.51 ± 0.16 | 41.44 ± 10.61 | 161.47 ± 0.97 | 249.84 ± 10.83 |
| 2 | 106.89 ± 18.00 | 8.83 ± 0.27 | 29.56 ± 0.00 | 168.00 ± 1.88 | 238.44 ± 2.21 |
| 3 | 87.49 ± 9.45 | 9.43 ± 0.25 | 22.34 ± 1.24 | 167.13 ± 2.92 | 235.31 ± 0.00 |
| 4 | 98.81 ± 14.40 | 9.37 ± 0.52 | 19.28 ± 0.22 | 167.25 ± 2.74 | 250.00 ± 12.37 |
| 5 | 83.41 ± 8.32 | 8.80 ± 0.30 | 12.53 ± 1.72 | 163.04 ± 0.09 | 251.09 ± 3.31 |
| 6 | 93.26 ± 2.70 | 8.17 ± 0.60 | 33.53 ± 1.90 | 194.90 ± 5.97 | 242.50 ± 7.95 |
| 7 | 111.78 ± 9.90 | 10.01 ± 0.76 | 33.53 ± 3.49 | 186.92 ± 0.84 | 258.44 ± 5.30 |
| 8 | 106.10 ± 9.22 | 9.77 ± 0.17 | 38.88 ± 4.60 | 185.41 ± 1.06 | 234.84 ± 13.04 |
| 9 | 88.46 ± 2.47 | 10.57 ± 0.07 | 32.63 ± 0.88 | 174.86 ± 0.63 | 272.03 ± 13.04 |
| 10 | 101.75 ± 14.85 | 9.00 ± 0.10 | 39.31 ± 0.53 | 181.81 ± 3.49 | 204.06 ± 16.79 |
| 11 | 103.62 ± 0.22 | 9.93 ± 0.57 | 35.13 ± 0.35 | 179.01 ± 0.09 | 235.78 ± 0.66 |
| 12 | 106.63 ± 5.62 | 9.86 ± 0.34 | 50.38 ± 3.80 | 171.16 ± 0.27 | 235.00 ± 0.00 |
TSS total soluble sugar, TRS total reducing sugar, CHO total carbohydrate, DW dry weight
Effect of fermentation period and solid content on protein synthesis
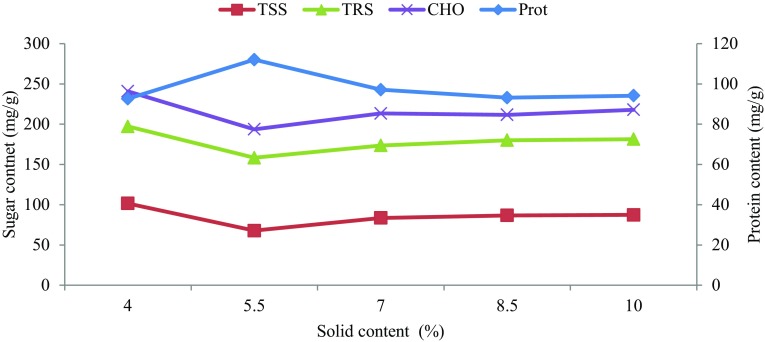
There was gradual increase in protein synthesis from day 2 and climaxed at day 6. This trend is typical of a batch process and has been reported during sequential SSF of Arachniotus sp. and Candida utilis on corn stover (Ahmed et al. 2010). The reduced protein synthesis between day 8 and 10 was consistent with other investigations and could be caused by substrate depletion, exhaustion of limiting nutrients and cell death (Jamal et al. 2012; Salihu et al. 2015). Protein enrichment increased sharply between 20 and 27.5% solid content; then declined (Fig. 2). Similar pattern was reported for white rot fungi possibly due to the ligninolytic activities of P. chrysosporium and Schzophyllum commune grown on fruit wastes (Saheed et al. 2013; Jamal et al. 2014).
Fig. 2.
Optimization of solid content for protein production (diamond) and relationship with total soluble sugar (square), total reducing sugar (triangle) and total carbohydrate (×)
Effects of micronutrients and sucrose on protein synthesis
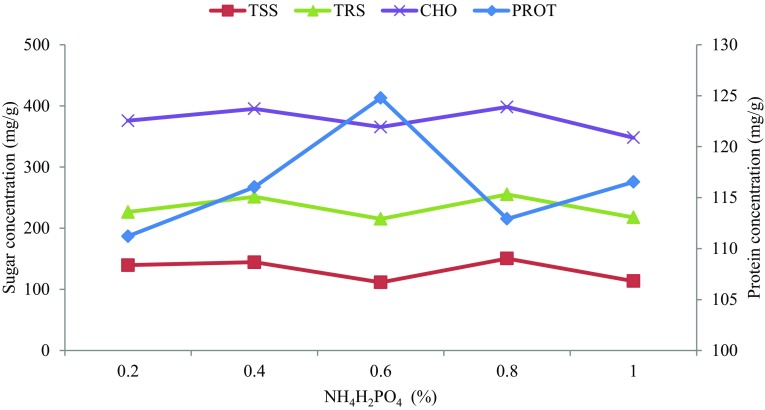
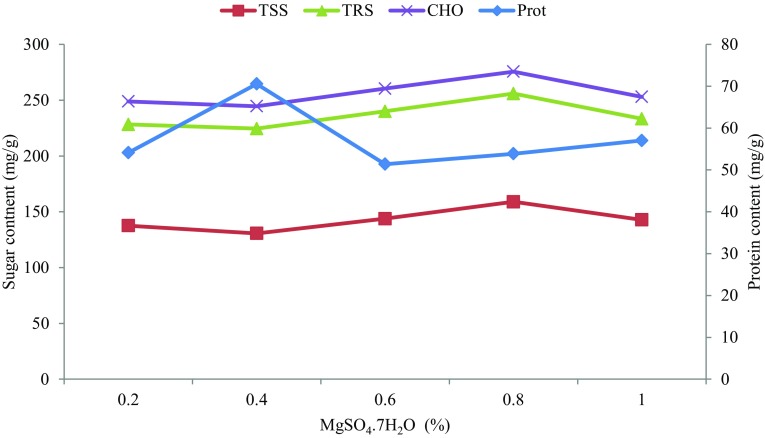
KCl, NH4H2PO4 and MgSO4·7H2O were selected to determine individual optimum concentration for improved protein synthesis using one-factor-at-a-time (OFAT) method. Gradual increase in KCl concentration resulted in elevated protein synthesis until 0.5%; albeit, synthesis dropped sharply with further increase in KCl concentration (Fig. 3). NH4H2PO4 salt concentration of 0.6% supported increased protein synthesis to 112.92 mg/g—the highest value recorded among the optimized factors (Fig. 4). The presence of ammonium and phosphate (NH4+, PO42−) ions in the salt could trigger the high synthesis. NH4+ and PO42− have been reported to enhance microbial growth and product synthesis (Rosma and Cheong 2007). Optimum protein synthesis due to MgSO4·7H2O was recorded at 0.4% inclusion but declined at higher concentration (Fig. 5). Increased concentration of MgSO4·7H2O under solid state fermentation has been reported to hamper protein synthesis of microorganisms (Ruqayyah et al. 2011).
Fig. 3.
Optimization of potassium chloride concentration (KCl) (diamond) and relationship with total soluble sugar (square), Total reducing sugar (triangle) and total carbohydrate (×)
Fig. 4.
Optimization of ammonium Di-hydrogen phosphate (NH4H2PO4) (diamond) and relationship with total soluble sugar (square), Total reducing sugar (triangle) and total carbohydrate (×)
Fig. 5.
Optimization of magnesium sulphate concentration (MgSO4·7H2O) (diamond) and relationship with total soluble sugar (square), Total reducing sugar (triangle) and total carbohydrate (×)
Protein synthesis increase slowly as the concentration of sucrose increased between 1% and 3% but climaxed at 5% before decline with further increase (Fig. 6). Overall, the amount of protein synthesized as effect of sucrose was the highest among all the media factors investigated. This profound effect could be due to ease of sucrose metabolism by microbes (especially yeast and fungi imperfecti) leading to growth and product synthesis (Castillo et al. 1994; Dhanasekaran et al. 2011).
Fig. 6.
Optimization of sucrose (diamond) and relationship with total soluble sugar (square), Total reducing sugar (triangle) and total carbohydrate (×)
There is a direct relationship between microbial growth and sugar consumptions since in all cases, the sugar content of the SSF product decreased either greatly or marginally throughout the fermentation period (Figs. 2, 3, 4, 5, 6). Corresponding dip in sugar at optimum protein synthesis signaled peak of microbial activity and product synthesis. Interestingly, cultivated microbes generally consumed maximum sugar at optimum sucrose content followed by KCl and solid content while least sugar was consumed at optimum MgSO4·7H2O. TSS and CHO are the most susceptible to microbial attack and could be responsible for the corresponding increased protein synthesis. TRS metabolism was highest at optimum NH4H2PO4 (Table 6). High consumption of CHO and TSS at optimal sucrose content suggested ease of attack that could trigger cellular growth with corresponding product synthesis (Okamoto et al. 2011). Action of the white rot fungi—P. chrysosporium and simple sugar consuming yeast (C. utilis) could trigger elevated CHO and TSS consumption due to the secretion of lignocellulosic and hydrolytic enzymes needed to convert lignin and cellulose to simple sugars for microbial growth and product synthesis (Ahmed et al. 2010; Shi et al. 2014). Bioethanol and protein enriched cassava waste production in a batch SSF process involving fungi and yeast cells showed increased sugar consumption as a function of product synthesis (Dhillon 2011; Rajoka et al. 2011; Ruqayyah et al. 2013).
Table 6.
Effect of microbial protein production on as function of sugar consumption
| Media constituent | |||||
|---|---|---|---|---|---|
| Solid content | KCl | NH4H2PO4 | MgSO4·7H2O | Sucrose | |
| Protein difference | 16.48 | 32.13 | 39.03 | 16.43 | 33.11 |
| Sugars consumed | |||||
| CHO (mg/g) | 8.25 | 37.52 | 24.52 | 0.52 | 39.44 |
| TRS (mg/g) | 5.10 | 2.85 | 14.87 | 3.12 | 1.52 |
| TSS (mg/g) | 33.75 | 5.62 | 28.15 | 6.87 | 13.20 |
TSS total soluble sugar, CHO carbohydrate, TSS total soluble sugar
Conclusion
The results of this research indicated that bioling of dried banana peel under aqueous condition removed sufficient amount of saponin that previously hinder fungal-yeast growth. Cultivated microbes required micronutrients and sucrose to further increase protein enrichment of the substrate in many folds; simple and complex sugars in the substrate serve as carbon source consumed for enhanced product formation at optimum media constituent concentrations. Sequential SSF proved efficacious in improving convertion of banana peel to high proetein product capabe of use as animal feed protein supplement.
Acknowledgements
The investigators thanked Research Management Center of International Islamic University Malaysia for their financial support in making this work a reality.
Compliance with ethical standards
Conflict of interest
There are no financial/commercial or any other conflicts of interest among authors.
References
- AboSiada OA, Negm MS, Basiouny ME, Fouad MA, Elagroudy S. Nutrient enrichment of agro-industrial waste using solid state fermentation. Microbiol Res J Int Br Microbiol Res J Ghana. 2017;22:1–11. [Google Scholar]
- Ahmed S, Ahmad F, Hashmi AS. Production of microbial biomass protein by sequential culture fermentation of Arachniotus sp., and Candida utilis. Pakistan J Biotechnol. 2010;42:1225–1234. [Google Scholar]
- Arumugam R, Manikandan M. Fermentation of pretreatedd hydrolyzatess of banana and mango fruit wastes for ethanol production. Asian J Exp Biol Sci. 2011;2:246–256. [Google Scholar]
- Bakar A, Hashim M, Hamid A. MiniReview Popular fermented foods and beverages in Southeast Asia. Int Food Res J. 2011;18:475–484. [Google Scholar]
- Cameron M, Timofeevski S, Aust S. Enzymology of Phanerochaete chrysosporium with respect to the degradation of recalcitrant compounds and xenobiotics. Appl Microbiol. 2000;54:751–758. doi: 10.1007/s002530000459. [DOI] [PubMed] [Google Scholar]
- Castillo MR, Gutierrez-Correa M, Linden JC, Tengerdy RP. Mixed culture solid substrate fermentation for cellulolytic enzyme production. Biotechnol Lett. 1994;16:967–972. doi: 10.1007/BF00128635. [DOI] [Google Scholar]
- Castoldi R, Bracht A, de Morais G, Baesso M. Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: study of degradation patterns and saccharification kinetics. Chem Eng. 2014;258:240–246. doi: 10.1016/j.cej.2014.07.090. [DOI] [Google Scholar]
- Dhanasekaran D, Lawanya S, Saha S. Production of single cell protein from pineapple waste. Innov Rom Food Biotechnol. 2011;8:26–32. [Google Scholar]
- Dhillon GS, Oberoi HS, Kaur S, Sunil Brar SK. Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind Crops Prod. 2011;34:1160–1167. doi: 10.1016/j.indcrop.2011.04.001. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Elisashvili V, Kachlishvili E, Penninckx M. Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J Ind. 2008;38:1531–1538. doi: 10.1007/s10295-008-0454-2. [DOI] [PubMed] [Google Scholar]
- Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Gad AS, Hasan EA, Aziz AA. Utilization of Opuntia ficus indica waste for production of Phanerochaete chrysosporium bioprotein. J Am Sci. 2010;6:208–216. [Google Scholar]
- Güçlü-Üstündağ Ö, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 2007;47:231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- Hu C-C, Liu L-Y, Yang S-S. Protein enrichment, cellulase production and in vitro digestion improvement of pangolagrass with solid state fermentation. J Microbiol Immunol Infect. 2012;45:7–14. doi: 10.1016/J.JMII.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Hudson BJF, Ei-Difrawi EA. The sapogenins of the seeds of four lupin species. J Plant Foods. 1979;3:181–186. doi: 10.1080/0142968X.1979.11904227. [DOI] [Google Scholar]
- Ikram-ul H, Ali S, Qadeer MA, Iqbal J. Citric acid production by selected mutants of Aspergillus niger from cane molasses. Bioresour Technol. 2004;93:125–130. doi: 10.1016/j.biortech.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Ionuț SA, Cristina-Gabriela G, Lidia F, Moroi AM, Franck K, Yassine Elena G. Successful fodder yeast production from agro-industrial by products through a statistical optimization approach. Rom Biotechnol Lett. 2017;22:12671–12679. [Google Scholar]
- Jamal P, Alam MZ, Salleh NU. Media optimization for bioproteins production from cheaper carbon source. J Eng Sci Technol. 2008;3:124–130. [Google Scholar]
- Jamal P, Saheed O, Alam Z. Bio-valorization potential of banana peels (Musa sapientum): an overview. Asian J Biotechnol. 2012;4:1–14. doi: 10.3923/ajbkr.2012.1.14. [DOI] [Google Scholar]
- Jamal P, Saheed OK, Alam MdZ, Muyibi SA, Karim MIA. Protein enrichment through synergistic activities of fruit wastes using white rot fungi under submerged state bioconversion. J Pure Appl Microbiol. 2014;8:839–844. [Google Scholar]
- Jayabalan R, Malini K, Sathishkumar M, Swaminathan K, Yun S-E. Biochemical characteristics of tea fungus produced during kombucha fermentation. Food Sci Biotechnol. 2010;19:843–847. doi: 10.1007/s10068-010-0119-6. [DOI] [Google Scholar]
- Kasapidou E, Sossidou E, Mitlianga P. Fruit and vegetable co-products as functional feed ingredients in farm animal nutrition for improved product quality. Agriculture. 2015;5:1020–1034. doi: 10.3390/agriculture5041020. [DOI] [Google Scholar]
- Ke L, Wu Q, Zhang D. Bioconversion of rape straw into a nutritionally enriched substrate by Ganoderma lucidum and yeast. African J Biotechnol. 2011;10:5648–5653. doi: 10.5897/AJB10.2353. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Miller G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Miyakoshi M, Tamura Y, Masuda H, Mizutani K, Tanaka O, Ikeda T, Ohtani K, Kasai R, Yamasaki K. Antiyeast steroidal saponins from Yucca schidigera (Mohave yucca), a new anti-food-deteriorating agent. J Nat Prod. 2000;63:332–338. doi: 10.1021/np9904354. [DOI] [PubMed] [Google Scholar]
- Molla A, Fakhru’l-Razi A, Hanafi M, Zahangir Alam M. Optimization of process factors for solid-state bioconversion of domestic wastewater sludge. Int Biodeterior Biodegradation. 2004;53:49–55. doi: 10.1016/J.IBIOD.2003.09.003. [DOI] [Google Scholar]
- Nitayavardhana S, Issarapayup K, Pavasant P, Kumar S. Bioresource Technology Production of protein-rich fungal biomass in an airlift bioreactor using vinasse as substrate. Bioresour Technol. 2013;133:301–306. doi: 10.1016/j.biortech.2013.01.073. [DOI] [PubMed] [Google Scholar]
- Oberoi HS, Vadlani PV, Saida L, Sunil Hughes JD. Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Manag. 2011;31:1576–1584. doi: 10.1016/j.wasman.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nitta Y, Maekawa N, Yanase H. Direct ethanol production from starch, wheat bran and rice straw by the white rot fungus Trametes hirsuta. Enzyme Microb Technol. 2011;48:273–277. doi: 10.1016/j.enzmictec.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrika. 1946;33:305–325. doi: 10.1093/biomet/33.4.305. [DOI] [Google Scholar]
- Poorter H, Bergotte M. Chemical composition of 24 wild species differing in relative growth rate. Plant Cell Environ. 1992;15:221–229. doi: 10.1111/j.1365-3040.1992.tb01476.x. [DOI] [Google Scholar]
- Rajendran A, Thirugnanam M, Thangavelu V. Statistical evaluation of medium components by Plackett–Burman experimental design and kinetic modeling of lipase production by Pseudomonas fluorescens. Indian J Biotechnol. 2007;6:469–478. [Google Scholar]
- Rajoka MI, Ahmed S, Hashmi AS, Athar M. Production of microbial biomass protein from mixed substrates by sequential culture fermentation of Candida utilis and Brevibacterium lactofermentum. Ann Microbiol. 2011;62:1173–1179. doi: 10.1007/s13213-011-0357-8. [DOI] [Google Scholar]
- Rosma A, Cheong M. Effects of nitrogen supplementation on yeast (Candida utilis) biomass production by using pineapple (Ananas comosus) waste extracted medium. Malaysian J Microbiol. 2007;3:19–26. [Google Scholar]
- Ruqayyah TID, Jamal P, Alam Z, Mirghani ES. Valorization of Cassava Peels by the White Rot Fungus Panus tigrinus M609RQY. Aust J Basic Appl Sci. 2011;5:808–816. [Google Scholar]
- Ruqayyah TID, Jamal P, Alam MZ, Mirghani MES. Biodegradation potential and ligninolytic enzyme activity of two locally isolated Panus tigrinus strains on selected agro-industrial wastes. J Environ Manage. 2013;118:115–121. doi: 10.1016/j.jenvman.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Saheed O, Jamal P, Karim M. Cellulolytic fruits wastes: a potential support for enzyme assisted protein production. J Biol Sci. 2013;13:379–385. doi: 10.3923/jbs.2013.379.385. [DOI] [Google Scholar]
- Saheed OK, Jamal P, Karim MIsmailA, Alam Md, Zahangir Muyibi SA. Utilization of fruit peels as carbon source for white rot fungi biomass production under submerged state bioconversion. J King Saud Univ Sci. 2016;28:143–151. doi: 10.1016/j.jksus.2015.08.002. [DOI] [Google Scholar]
- Salihu A, Abbas O, Sallau AB, Alam MZ. Agricultural residues for cellulolytic enzyme production by Aspergillus niger: effects of pretreatment. 3 Biotech. 2015;5:1101–1106. doi: 10.1007/s13205-015-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Chinn MS, Sharma-Shivappa RR. Interactions between fungal growth, substrate utilization, and enzyme production during solid substrate cultivation of Phanerochaete chrysosporium on cotton stalks. Bioprocess Biosyst Eng. 2014;37:2463–2473. doi: 10.1007/s00449-014-1224-3. [DOI] [PubMed] [Google Scholar]
- Villas-Bôas SG, Esposito E, de Mendonça MM. Bioconversion of apple pomace into a nutritionally enriched substrate by Candida utilis and Pleurotus ostreatus. World J Microbiol Biotechnol. 2003;19:461–467. doi: 10.1023/A:1025105506004. [DOI] [Google Scholar]
- Yin Y, Gao Q, Zhang F, Li Z. Medium optimization for the high yield production of single (+)-terrein by Aspergillus terreus strain PF26 derived from marine sponge Phakellia fusca. Process Biochem. 2012;47:887–891. doi: 10.1016/J.PROCBIO.2012.02.005. [DOI] [Google Scholar]
- Zacchi L, Burla G, Zuolong D, Harvey PJ. Metabolism of cellulose by Phanerochaete chrysosporium in continuously agitated culture is associated with enhanced production of lignin peroxidase. J Biotechnol. 2000;78:185–192. doi: 10.1016/S0168-1656(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Zayed Mona S. Enhancement the feeding value of rice straw as animal fodder through microbial inoculants and physical treatments. International Journal of Recycling of Organic Waste in Agriculture. 2018;7(2):117–124. doi: 10.1007/s40093-018-0197-7. [DOI] [Google Scholar]