Abstract
Thyroid cancer, with the exception of anaplastic thyroid cancer, typically has very favorable outcomes with the standard therapy. However, those that persist, recur, or metastasize are associated with a worse prognosis. Targeted therapy with kinase inhibitors has shown promise in advanced cases of thyroid cancer, and currently five drug regimens are approved for use in clinical practice in the treatment of differentiated, medullary, and anaplastic thyroid cancer, with more options in the pipeline. However, one of the greatest dilemmas is when and how to initiate one of these drugs, and this is discussed herein.
Keywords: anaplastic thyroid cancer, BRAF inhibitors, differentiated thyroid cancer, immunotherapy, kinase inhibitors, medullary thyroid cancer
Thyroid cancer is the most common endocrine malignancy, and its incidence continues to rise in the United States [1]. All thyroid cancers, except for medullary thyroid cancer (MTC), are derived from the follicular cells. Differentiated thyroid cancer (DTC) has three basic subtypes, papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and Hurthle cell thyroid cancer (HCTC), and account for 95% of all thyroid cancers [2]. Poorly differentiated thyroid cancers (PDTCs) also fall into the category of DTC. Well-differentiated thyroid cancers generally have an indolent course, as these cells are structurally more similar to benign thyroid cells [3]. DTC is usually curable with surgery, selective use of radioactive iodine (131I) (RAI) ablation, and thyroid hormone suppressive therapy [4].
Despite the rising incidence of DTC, <10% of cases will develop locally advanced or metastatic disease; ~67% of these advanced cases are radioiodine refractory (RAIR) [5]. When metastases occur in DTC, further surgery or RAI ablation may be curative in a few patients, and a higher dose of thyroid hormone replacement slows the progression of disease.
MTC derives from malignant transformation of C cells (the parafollicular neuroendocrine cells adjacent to the follicular cells within the thyroid) and accounts for ~4% of all thyroid cancers [2]. It is primarily treated with surgery. In metastatic or recurrent MTC, surgery and/or other localized therapies, such as liver ablation/embolization or intensity-modulated radiation therapy may be beneficial.
Anaplastic thyroid cancer (ATC) is rare (encompassing <2% of all thyroid cancers) and has the highest mortality rate of all the thyroid cancers. Until recently, this disease was thought of as untreatable unless caught in a very early stage. However, recent advances, discussed herein, have started to change this perception [6].
This review summarizes the rationale for using systemic therapy in advanced thyroid carcinoma, listing the approved drugs and their clinical indication. Relevant clinical trials and their preliminary data are also presented.
1. Targetable Genetic Alterations and Signaling Pathways in Thyroid Cancer
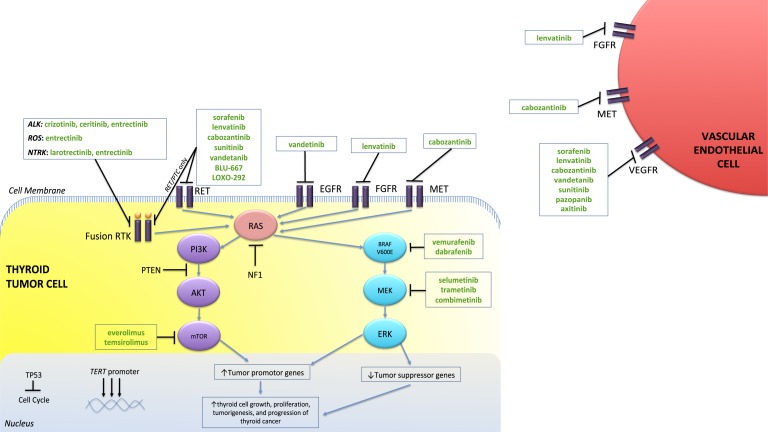
Aberrant signaling pathways have been implicated in the onset, progression, and invasiveness of thyroid cancer. Studies have reported a high frequency of activating somatic alterations of genes encoding components of the MAPK and phosphatidylinositol 3-kinase (PI3K) signaling pathways (Fig. 1). The most common of genetic changes in PTC are point mutations in BRAF (40%) and RAS (38%) genes [7–11]; rearrangement of the RET/PTC proto-oncogene occurs in ~10% to 20% [12]. Genetic rearrangements and mutations in anaplastic lymphoma kinase (ALK) and neurotrophic tropomyosin receptor kinase (NTRK)1-3 are also present, although rare, only ~1% to 2% [13, 14]. The incidence of NTRK or ALK in PTC increases postradiation exposure, especially in those affected by Chernobyl or the atomic bombs in Japan, respectively [15, 16]. These mutations are almost always mutually exclusive [17], suggesting similar or redundant downstream effects.
Figure 1.
Schematic of the two commonly activated pathways and genetic aberrations involved in thyroid cancer, as well as the targeted therapies studied in thyroid cancer. The MAPK and the PI3K pathways are activated by the receptor tyrosine kinase (RTK). Increased activity of the receptor tyrosine kinases (purple bars) by growth factors, as well as mutations along the pathway, results in oncogenic activation of the MAPK pathway, leading to activation of MEK and ERK and, subsequently, tumor progression. The PI3K pathway is negatively regulated by PTEN and is activated by mutations in PIK3CA, PTEN, AKT, and mTOR. The PI3K pathway can also be activated by RAS and NF1 mutations, as there is a cross-talk mechanism with the MAPK pathway. TP53 is a tumor suppressor gene and plays a role in apoptosis. TERT activation via TERT promotor mutations promotes cell immortality. Patients with TERT promotor mutations that coexist with BRAF or RAS mutations have a worse prognosis. In green are drugs that can potentially target along these signaling pathways to shrink tumors or halt their growth. AKT, protein kinase B.
Papillary tumors with the BRAF mutations are associated with reduced gene expression involved with iodine metabolism, including genes for the sodium/iodide symporter (NIS), apical iodide transporter, thyroperoxidase, and thyroglobulin (Tg); instead, there is increased GLUT-1 expression [18]. This may explain why certain tumors are less radioiodine avid (due to inability to metabolize iodine) and more visible on 18-fluorodeoxyglucose positron emission tomography (18FDG-PET) imaging, via BRAF-mediated GLUT-1 expression [19]. BRAF mutations were thought to be associated with more aggressive disease, demonstrating extrathyroidal extension, thyroid capsular invasion, lymph node metastases, and increased disease persistence and recurrence [20–22]. However, follow-up studies have shown that this increased risk is associated with BRAF and telomerase reverse transcription (TERT) promoter coexisting mutations vs BRAF alone [23].
In FTC, point mutations of RAS and rearrangements of PPARγ/PAX8 genes are the most common oncogenic alteration; also, mutations in members of the PI3K pathway, such as PTEN deletion/mutation and PIK3CA, have also been reported at low frequencies [24].
PDTC and ATC are characterized by a higher number of mutations where overrepresentation of these is thought to lead to the more aggressive phenotype [25]. Both ATC and PDTC also demonstrate a high prevalence of the TP53 and TERT promoter mutations, which is usually associated with greater aggressiveness [26]. The most common genetic alterations found in MTC cells are the RET-activating point mutations that are present at the germline level in 95% of hereditary forms and 45% of sporadic cases [27]. Other oncogenic alterations found in sporadic MTCs are RAS mutations, mainly the HRAS and KRAS mutations, which have been reported in ~17% [28].
Enhanced activity of the MAPK and PI3K signal transduction cascade due to a mutation can lead to uncontrolled cell growth, and hence this is the molecular rationale to halt this proliferation. Knowing the underlying alterations and their downstream effects, the concept of “targeting” these mutations has become a keen area of interest in the development of new treatments. These kinase inhibitors (KIs) are drugs designed to block either cell-membrane kinase receptors or the intrinsic enzymes responsible for revving the MAPK or PI3K pathways in an effort to stop the tumor’s growth [29–33].
Angiogenesis is also a very important process in tumor development, lending itself as an attractive target for therapy [24]. The vascular endothelial growth factor (VEGF) promotes angiogenesis and is overexpressed in the setting of intratumoral hypoxia via hypoxia-inducible factor-1α (HIF1α). This transcription factor HIF1α is not only upregulated by hypoxia, but also by MAPK and PI3K/AKT pathways. An important target of HIF1α is the MET receptor, which is highly expressed in many thyroid cancers (both in DTC and MTC), promoting angiogenesis, cellular motility, invasion, and metastasis [34, 35].
2. Systemic Therapies
A. Multikinase Inhibitors
Currently, there are four drugs—all oral multitargeted tyrosine KIs [multikinase inhibitors (MKIs)]—approved in the treatment of DTC and MTC. Sorafenib and lenvatinib are Food and Drug Administration (FDA) approved for advanced RAIR DTC and PDTC, and vandetanib and cabozantinib are approved for recurrent or metastatic MTC (Table 1) [36–39].
Table 1.
Four Approved KIs Based on Their Phase III Outcomes
| Drug | Sorafenib (DECSION) [36] | Lenvatinib (SELECT) [37] | Vandetanib (ZETA) [38] | Cabozantinib (EXAM) [39] |
|---|---|---|---|---|
| Tumor | DTC-RAIR | DTC-RAIR | MTC | MTC |
| Targets | VEGFR, c-Kit, RET, PDGFR, RAF | VEGFR, c-Kit, RET, PDGFR, FGFR, RET | VEGFR, c-Kit, RET, EGFR, RET | VEGFR, c-Kit, ERT, MET, RET |
| Patients, N | 417 | 392 | 331 | 330 |
| PR, % | 12.2 | 64.8 | 45 | 28 |
| Median PFS, mo | 10.8 | 18.3 | 30.5 | 11.2 |
| Side effects, % | PPE (76) | Hypertension (67) | Diarrhea (56) | Diarrhea (63) |
| Diarrhea (68) | Diarrhea (59) | Skin rash (45) | PPE (50) | |
| Alopecia (67) | Fatigue (59) | Nausea (33) | Weight loss (47) | |
| Skin rash (50) | Anorexia (50) | Hypertension (32) | Anorexia (45) | |
| Fatigue (49) | Weight loss (46) | Black box warning: | Nausea (43) | |
| Weight loss (46) | Nausea (41) | QT prolongation | Fatigue (40) | |
| Hypertension (40) | Stomatitis (35) | Black box warning: | ||
| Anorexia (31) | GI ulcers and hemorrhage |
Abbreviation: FGFR, fibroblast growth factor receptor; EGFR, endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; PPE, palmer-plantar erythrodysesthesia; PR, partial response; VEGFR, vascular endothelial growth factor receptor.
A-1. Sorafenib
Sorafenib is an MKI targeting the VEGF receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and RAF kinases (although, it is a weak BRAF inhibitor). The medication was approved by the FDA in 2013 on the basis of a phase 3, 1:1 randomized, placebo-controlled trial (DECISION trial) involving 417 patients with locally advanced or metastatic RAIR DTC who were kinase inhibitor (KI) and chemotherapy naive. Patients in the sorafenib arm showed a longer progression-free survival (PFS) than patients in the placebo arm [10.8 months vs 5.8 months, respectively; hazard ratio (HR), 0.59; 95% CI, 0.45 to 0.76; P < 0.0001] with a median duration of response of 10.2 months. A partial response (PR) was seen in 12% of treated patients. At time of progression of disease, patients on the placebo arm had the ability to cross over. Although PFS was longer, sorafenib did not improve overall survival (OS). Observed adverse events (AEs) in sorafenib-treated patients included palmar-plantar erythrodysesthesia (PPE), diarrhea, alopecia, skin rash, fatigue, weight loss, hypertension, and anorexia [36]. The starting dose of sorafenib is 400 mg twice daily without food and should be continued until there is no longer a benefit with the drug or side effects occur. Dose reduction or interruption occurred in 64% and 66%, respectively, in the DECISION trial [36], with a planned median dose exposure of 81% for sorafenib. Dose reductions (by 200-mg decrements) or interruptions were based on the severity of the AEs, usually for recurrent grade 2 events [40]. As this was the first KI approved for thyroid cancer, several other studies combining sorafenib with other medications have been investigated and are discussed later.
A-2. Lenvatinib
Lenvatinib targets VEGFR1–3, fibroblast growth factor receptors (FGFR) 1–4, PDGFRα, RET, and c-Kit signaling pathways [41–43]. Based on the positive outcomes of a phase 2 trial [44], the phase 3, multicenter, double-blinded, 2:1 randomized, placebo-controlled study (SELECT trial) evaluated the efficacy of lenvatinib on RAIR DTC patients with progressive disease. Just as in DECISION, patients in the placebo group with disease progression could cross over to receive open-label lenvatinib.
Outcomes of the study showed that the 261 patients treated with lenvatinib showed a significantly longer PFS than did the 131 patients on placebo (18.3 vs 3.6 months, respectively; HR, 0.21; 99% CI, 0.14 to 0.31; P < 0.001). Even in patients who had been previously treated with a tyrosine KI, the PFS was impressively long at 15.1 months. The response rate (RR) in this trial was 64.8% in the lenvatinib-treated group, with 63% achieving a PR and 1.5% achieving a complete response (CR); RR in the placebo group was 1.5%. The median OS was not reached at the time of the initial publication [37]. However, on further analysis, an improved OS was observed in patients’ age >65 years compared with placebo [45] and in the follicular histotype compared with papillary [46]. Hypertension, diarrhea, fatigue, and decreased appetite were the most common AEs seen in SELECT. Dose reductions from the initial 24 mg were needed in nearly 70% of patients, with discontinuation of drug in ~14% [37].
Given its RET targeting potential, lenvatinib has also been studied in a phase 2 trial for advanced MTC where 36% achieved a PR and PFS was 9 months [47]. In a single-arm, open-label, Japanese phase 2 trial of all thyroid cancers, 17 ATC patients initially placed on maximum 24 mg of lenvatinib saw a median PFS of 7.4 months (95% CI, 1.7 to 12.9) and median OS was 10.6 months (95% CI, 3.8 to 19.8) [48]. This phase 2 trial was expanded to the United States (NCT02657369) and has closed early, although awaiting preliminary results.
A-3. Vandetanib
Vandetanib selectively targets RET, VEGFR, and EGFR signaling [49, 50] and was originally examined in the treatment of locally advanced or metastatic MTC. Based on promising outcomes from a phase 2 trial [51], this led the way to the phase 3 study, ZETA, where patients with advanced MTC were randomly assigned 2:1 to vandetanib or placebo; in cases of disease progression while on study, patients could elect open-label vandetanib. Three hundred thirty-one patients were included, and there was a significant prolongation of PFS in the vandetanib group (30.5 vs 19.3 months; HR, 0.46; 95% CI, 0.31 to 0.69; P < 0.001). Statistically significant differences were also seen in objective RR (P < 0.001), disease control rate (P = 0.001), and biochemical response (P < 0.001) [38]. The results of ZETA led to vandetanib being the first FDA-approved medication for the treatment of locally advanced or distant metastatic and unresectable MTC in the United States (2011) and Europe (2013). One major flaw in the design of the study was that there was no requirement for disease progression prior to enrollment. Thus, patients with indolent disease could have been treated on this clinical trial.
Common observed AEs included diarrhea, rash, nausea, hypertension, and headache [38]. However, vandetanib does have a black box warning that it may prolong the QT interval, induce torsades de pointes, and cause sudden death [52]. Vandetanib can only be prescribed by physicians who have completed the risk evaluation and mitigation strategies program because of this black box warning. Patients with history of QT prolongation, bradyarryhthmias, uncompensated heart failure, or difficult to control electrolyte abnormalities should choose an alternative option. The recommended starting dose is 300 mg daily with or without food, and can be adjusted by 100-mg reductions based on the severity or frequency of AEs, and held in the setting of QT prolongation >500 ms until <450 ms [52].
Vandetanib is currently under investigation for its utility in advanced, RAIR DTC, motivated by positive outcomes in a double-blinded phase 2 study showing a statistically significant increase in PFS compared with placebo (11.1 vs 5.9 months; HR, 0.63; one-sided P = 0.008) [53]. In the double-blinded, 1:1 placebo-controlled phase 3 trial (VERIFY trial) (NCT01876784), preliminary results of the 238 DTC patients enrolled showed no statistically significant difference in PFS in the vandetanib arm (10.0 vs 5.7 months, P = 0.08), although a trend favoring vandetanib was seen [54].
A-4. Cabozantinib
Cabozantinib is an inhibitor of MET, VEGFR2, and RET [55]. It has been studied in pretreated patients in a phase 1 dose-escalation basket study where 10 of 35 (29%) MTC patients had confirmed PR [56]. The phase 3 double-blinded study (EXAM trial) enrolled patients with unresectable locally advanced or metastatic MTC and demonstrated statistically longer PFS in the cabozantinib arm vs placebo (11.2 vs 4 months, respectively; HR, 0.28; 95% CI, 0.19 to 0.40; P < 0.001) [39]. Following this, cabozantinib was approved for use in advanced MTC in the United States. Although the PFS was longer in the ZETA study, this difference may be explained by the different levels of severity of the patients, as shown by the difference in survival of the placebo groups (19.3 months in ZETA and 4.0 months in the EXAM study). Most common AEs observed in the EXAM study included diarrhea, PPE, weight loss, suppressed appetite, nausea, and fatigue, all of which resulted in dose reductions in ~80% of patients, and discontinuation of drug in 16% of patients [39]. Further analysis is currently underway in progressive, metastatic MTC patients comparing the efficacy of 60 mg vs 140 mg of cabozantinib (NCT01896479). Cabozantinib also carries a black box warning for gastrointestinal (GI) perforations, fistulas, and hemorrhage and should be avoided or used cautiously in patients with prior external beam radiation therapy (EBRT) to the neck or with GI mucosal injury [57].
Although no difference in OS was seen initially in either arm of the EXAM trial, further analysis at a minimum follow-up period of 42 months showed a 5.5-month prolongation in OS compared with placebo, which was not statistically significant [58]. In subgroup analyses, the greatest benefit was seen in those harboring an RET M918T or RAS mutations [58, 59]. In the RET M918T-treated group, median OS (44.3 vs 18.9; HR, 0.60; 95% CI, 0.38 to 0.94; P = 0.03) and PFS (13.9 vs 4 months; HR, 0.15; 95% CI, 0.08 to 0.28; P < 0.0001) were longer compared with placebo. Patients harboring RAS mutations showed a trend toward improved OS (HR, 0.37; 95% CI, 0.10 to 1.42) and PFS (HR, 0.15; 95% CI, 0.02 to 1.10). However, this was a small subgroup (13 patients in the cabozantinib arm and 3 in the placebo arm) and differences between both groups were not statistically significant [58].
Early studies have also shown tremendous promise of cabozantinib in the treatment of advanced DTC, especially given its targetable sites overlapping with receptors expressed in PTC. In a single-arm, open-label phase 1 trial, a PR was observed in 5 of 11 (45%) patients previously treated with a VEGFR inhibitor [60]. This led to a single-arm phase 2 trial of 25 RAIR DTC patients who failed a prior VEGFR agent; PR was noted in 10 of 25 (40%, with median time to PR of 2 months) and median duration of 11.3 months [61]. Although the phase 1 study initiated the dose at 140 mg, >70% of the patients required dose reductions due to intolerance, and hence the phase 2 study starting dose was 60 mg, with upwards titration to 80 mg daily in patients who failed to achieve a response [61].
A-5. Other MKIs
Axitinib, sunitinib, and pazopanib are a similar class of MKIs to those above that have been studied in DTC [62–64] but are not approved for this indication. All of these are antiangiogenic (target VEGFR) but have additional targets that may contribute to their efficacy. Table 2 shows the responses in DTC trials.
Table 2.
Other Tyrosine KIs in DTC
A clinical trial combining intensity-modulated radiation therapy plus paclitaxel with or without pazopanib in patients with ATC was recently completed, but results have not been presented at this time (NCT01236547).
B. Selective Inhibitors
B-1. BRAF and/or MEK inhibitors
BRAF inhibitors, in combination with MEK inhibitors, have been studied in BRAF mutated thyroid cancer. Most recently, based on the phase 2 trial, the combination of the BRAF inhibitor, dabrafenib, plus the MEK inhibitor, trametinib, was FDA approved for BRAF V600E mutated, unresectable/locally advanced ATC. This global open-label trial enrolled 16 locally advanced or metastatic ATC patients, who received the drugs during a median of 47 weeks (range, 4 to 120 weeks). All patients previously received radiation and/or surgery; six previously received systemic therapy. Eleven of 16 (69%) patients achieved an overall response, with 7 ongoing responses. The median PFS and OS were not reached, but at 1 year, 80% of patients were alive [65]. Although these results are certainly very encouraging, note that the clinical trial enrolled patients with excellent performance status, who were able to swallow and who could withstand a wash-out period after radiation. These patients may not necessarily represent most ATC patients. Iyer et al. [66] published “real-world” experience with targeted therapy that included six dabrafenib/trametinib-treated patients. Although PRs were seen in three patients, the median PFS and OS were 5.2 and 9.3 months, respectively, suggesting that better treatments are needed for these patients. The first case report of an ATC patient treated with neoadjuvant dabrafenib/trametinib plus immunotherapy was recently published [67]. This strategy is being studied as an exploratory endpoint in a clinical trial (NCT03181100). We recommend that all ATC patients, regardless of mutation status, continue to be enrolled in clinical trials when feasible.
Combination of dabrafenib and trametinib has also been studied in BRAF V600E mutated PTC patients. Fifty-three BRAF mutated PTC patients were enrolled in a phase 2 study, randomized to two treatment arms: dabrafenib alone (arm A) vs combination dabrafenib and trametinib (arm B). Responses were seen in 11 of 22 (50%) in arm A and 13 of 24 (54%) in arm B, and the median PFS was 11.4 and 15.1 months, respectively. No statistical difference was seen between either treatment modality, and overall the drugs were well tolerated [68].
Vemurafenib was the first BRAF inhibitor to be studied in BRAF V600E mutated, RAIR PTC in an open-label nonrandomized phase 2 trial. Patients were divided into two cohorts: KI naive (cohort 1) and previous history of treatment with VEGFR2 inhibitors (cohort 2). The primary endpoint was response to therapy. This trial enrolled 51 patients. After a median follow-up time of 18.8 months, a PR was seen in 10 of 26 (38.4%) of patients in cohort 1 (95% CI, 20.2 to 59.4) and 6 of 22 (27.3%) patients in cohort 2 (95% CI, 10.7 to 50.2) [69].
Based on preclinical results, it has been proposed that rebound activation of the receptor tyrosine kinase HER2/HER3 may contribute to development of resistance; hence, HER inhibition may sensitize cells to BRAF inhibition [70]. This concept was evaluated in a phase 1 study of 21 BRAF mutated RAIR DTC patients, where lapatinib, an HER inhibitor, was combined with dabrafenib (NCT01947023). A PFS of 18 months was seen as the best outcome, and an RR of 58% (12 of 21 patients) was reported. Overall this combination seemed to be well tolerated, with reported single grade 3 AEs of lymphocytosis and uveitis only [71]. Further investigation with this combination is needed.
The common AEs of BRAF inhibitors are fatigue, fever, arthalgias, anemia/leukopenia, diarrhea, and several dermatologic findings, including PPE [72] and squamous cell carcinoma of the skin [69, 73]. Squamous cell carcinoma related to dabrafenib or vemurafenib is generally well differentiated and less aggressive [74]. BRAF inhibitors lead to cutaneous malignancies by paradoxically activating MAPK signaling, leading to accelerated growth of premalignant skin cancers with wild-type BRAF [75–77]. This can be prevented by the addition of an MEK inhibitor and they are FDA approved in combination for melanoma (trametinib plus dabrafenib and cobimetinib plus vemurafenib). MEK inhibitor AEs are similar and include fatigue, maculopapular rash, edema, and acneiform rash [78]
Redifferentiation therapy.
More than 40% of PTC tumors harbor a BRAF V600E mutation and these tumors tend to be less sensitive to conventional RAI therapy after surgery due to suppressed NIS expression [19]. Preclinical studies have shown that redifferentiation of cells is possible with BRAF and/or MEK inhibition, thereby promoting NIS expression and RAI avidity [79]. Thus, redifferentiation therapy—restoring the ability of thyroid cancer cells to capture RAI—with BRAF or MEK inhibitors has become a hot topic in DTC.
MEK inhibition alone, which is downstream from BRAF on the MAPK pathway, has been shown to treat RAIR disease and reintroduce radioiodine avidity by redifferentiating the metastatic thyroid cancer cells [78]. This study of 20 patients with DTC received a 4-week course of selumetinib (not currently commercially available), after which eight patients subsequently qualified for RAI based on dosimetry. Five patients achieved a PR, whereas the remaining three achieved SD with regression, and all patients showed a reduction in Tg levels. Interestingly, the outcomes were most favorable in RAS-mutated cases [78]. Two trials of selumetinib vs placebo are currently underway, investigating either a 3-week (NCT02393690, phase 2) or 5-week (NCT01843062, phase 3) course of selumetinib or placebo followed by RAI treatment and monitored postablation at set intervals. Unfortunately, in a recent announcement by the sponsor, AstraZeneca, the phase 3 trial, NCT01843062, failed to meet its primary endpoint of improvement in the rate of CR compared with placebo [80].
BRAF inhibition alone has also been prospectively studied specifically in BRAF V600E mutated PTC, where 6 of 10 patients demonstrated new RAI uptake after receiving dabrafenib. They then received 150 mCi of I-131, and after 3 months, two patients attained a PR, and four had SD. Tg levels also decreased in four of six patients 3 months after RAI [81].
A retrospective study evaluated 13 patients with RAIR disease who were treated with an MEK or BRAF inhibitor, based on whether they had a RAS or a BRAF mutation, respectively; this was to determine whether this redifferentiated the cells to respond to RAI ablation. Of the nine (69%) patients who subsequently qualified to receive RAI ablation based on adequate uptake on the diagnostic whole-body scan, three achieved a PR (two BRAF, one NRAS), two with SD and at least 20% regression of tumor (one BRAF, one RAS), and four patients had no change in tumor size. The median follow-up period was 8.3 months after RAI ablation. A reduction in Tg levels was also noted in this group, including one patient whose Tg became undetectable. Long-term follow-up data are lacking for this therapeutic strategy, and therefore patients should continue to enroll in clinical trials to better understand the safety and efficacy of utilizing RAI during KI treatment [82].
B-2. Mammalian target of rapamycin (mTOR) inhibitors
The PI3K/Akt/mTOR pathway is downstream of RAS. Activation of this pathway mostly occurs in more advanced thyroid cancers [26]. Everolimus and temsirolimus are inhibitors of mTOR and have been studied in several phase 2 clinical trials of all thyroid cancer subtypes [29, 30, 32]. These drugs are not KIs. They are derivatives of the drug rapamycin, which is used as an immunosuppressant agent in solid organ transplants. These rapamycin derivatives have improved pharmacokinetics with less immunosuppressive effects compared with their predecessor; however, patients on mTOR inhibitors are still susceptible to infections [83]. Other AEs may include mucositis, anorexia, pancytopenia, hyperglycemia, liver function test abnormalities, and rarely pneumonitis.
In the most recently published everolimus trial by Hanna et al. [29], 50 patients were enrolled: 33 DTC, including 13 HCTC; 10 MTC; and 7 ATC. Progression within the previous 6 months was a requirement for enrollment to the trial. The primary endpoint was PFS. Six percent of patients (one of each histologic type) achieved a PR and 74% experienced stable disease. The median PFS was 12.5 months for the entire cohort. The median PFS in DTC and MTC was 12.9 and 13.1 months, respectively. Whereas RRs were low, PFS in the DTC and MTC cohorts were remarkable given that these were patients with rapidly progressive disease. The median PFS in the ATC cohort was not specified, but four of seven patients progressed rapidly after starting everolimus. It appears that there may be a subset of ATC patients who benefit from everolimus given that one ATC patient achieved a nearly complete PR for 18 months and one had stable disease for 26 months. These patients’ tumors harbored a TSC2 and an NF1 driver mutation, respectively [29]. A second-generation mTOR inhibitor, MLN0128, is currently being studied in thyroid cancer and specifically in ATC (NCT02244463).
Everolimus has also been used in combination with other agents. In a trial combining everolimus and sorafenib, 55% of patients achieved a PR (21 of 38), which is higher than previous trial reports of single-agent sorafenib [33]. HCTC patients had the highest RR and therefore this combination is being studied in that patient population (NCT02143726). Similarly, a combination of sorafenib and temsirolimus was studied in follicular-derived thyroid cancer, where PR was seen in 8 of 36 (22%) patients [26].
B-3. Selective RET inhibitors
In patients with RET-driven cancers, such as RET point mutations in MTC or RET rearrangements in PTC and PTC-derived PDTC and ATCs, therapies have primarily centered on multikinase inhibition, resulting in numerous off-target toxicities. Preclinical studies of selective RET inhibition have successfully shown activity against RET oncogene variants [84]. Recently, two selective RET inhibitor trials have been reported. BLU-667 is a potent and selective RET inhibitor (NCT03037385). The trial has enrolled 31 thyroid cancer patients (29 with MTC and 2 with PTC). The overall RR (ORR) was 40% in thyroid cancer patients. Overall, the drug was well tolerated with constipation, elevated AST and ALT, hypertension, fatigue, and peripheral edema being the most common AEs. Most AEs were grade 1 [85].
LOXO-292 is the other selective RET inhibitor studied in a phase 1 trial (NCT03157128). Twenty-seven thyroid cancer patients (20 MTC and 7 PTC) were included. Preliminary results showed a 45% ORR in RET-mutant MTC patients and 100% ORR in RET fusion PTC patients. Overall, the drug was well tolerated with few AEs reported, with only six treatment-emergent AEs reported in >10% of patients (fatigue, diarrhea, constipation, dry mouth, nausea, and dyspnea) [86]. Planned phase 2 expansion studies are underway for both selective RET inhibitors.
B-4. Other selective inhibitors
The existence of gene fusions in NTRK, ALK, and ROS1 in a subset of PTC, PDTC, and ATC patients has added to the understanding of the genetic basis of thyroid cancer and may be associated with more aggressive disease [87, 88]. Hence, identifying patients who may benefit from targeted therapy to one or more of these sites are of growing interest.
Currently, there are two NTRK inhibitors available. Larotrectinib (LOXO-101) is a selective pan-NTRK (A, B, and C) inhibitor. In a phase 1 basket trial of patients harboring this NTRK fusion, 5 of 55 patients with thyroid cancer were included. There was a 78% RR in the entire population, and all five thyroid cancer patients demonstrated a response: four showed a PR and one achieved CR. Overall, larotrectinib was tolerable, with abnormal liver function tests, fatigue, vomiting, dizziness, and nausea reported as the most common AEs [89]. Note that no patients with NTRK mutation or amplification responded to the NTRK inhibitor [90]. Information on the specific types of thyroid cancer patients included are lacking from this publication. A basket trial with larotrectinib continues to enroll patients with NTRK1–3 fusions. Larotrectinib is currently enrolling a phase 2 expansion study (NCT02576431).
Entrectinib is also a potent inhibitor of NTRK, but it also targets ROS1 and ALK. It has demonstrated early and excellent responses in phase 1 studies of colorectal, non–small cell lung carcinoma, melanoma, and renal cell carcinoma harboring NTRK fusions, including complete resolution of brain metastases in a lung cancer patient [91, 92]; no thyroid cancer patients were included in these trials. An open-label, multicenter, global, phase 2 basket study of entrectinib (NCT02568267) is enrolling patients with tumors (including PTC) harboring gene rearrangements in NTRK, ROS1, or ALK. Early reports of a PTC patient with an ROS1 fusion, who failed standard therapy as well as a TKI, was enrolled in this trial; within 6 months of treatment resolution of her distant metastases was noted, including brain lesions [93].
Currently, there is only one case report of an ATC patient with an ALK rearrangement, who has been successfully treated with crizotinib (an ALK inhibitor) after failing standard therapy. An open-label study of ceritinib, an ALK inhibitor, is currently recruiting ATC patients (NCT02289144), although preliminary results are not available.
B-5. Immunotherapy
Alterations of the immune system can lead to suppression of the immune response [94]. For example, malignant cells that survive the immune system surveillance may evolve to augment their expression of programmed death-ligand 1 and 2 (PD-L1/2), which can bind to its receptor, programmed cell death protein 1 (PD-1), on T-cells, resulting in an exhausted T-cell and inefficient immune response. PD-L1 is expressed in DTC and ATC [95, 96] and may even be a prognostic marker in ATC [97]. With the successful outcomes seen in melanoma and other solid tumors leading to FDA approval of several immunotherapies, many studies are underway to improve durability and expanded response in other cancer types. A phase 1 basket trial (NCT02054806) with pembrolizumab, an anti-PD1 drug, included a small group of DTC patients, where 2 of 22 (9%) patients achieved a PR to pembrolizumab monotherapy with a durable RR [98]. Spartalizumab, an anti-PD1 drug, was studied in an expansion trial in ATC patients. Of 30 evaluable patients, 5 (17%) achieved a PR by RECIST [99] 1.1 [100].
Studies involving combination targeted therapy with immunotherapy are underway. The phase 2 combination therapy involving lenvatinib and pembrolizumab is in collaboration with the International Thyroid Oncology Group (NCT02973997). Combination immunotherapy with nivolumab (anti-PD-1) plus ipilimumab (inhibitor of cytotoxic T lymphocyte A4, CTLA4) for all types of thyroid cancer is currently under investigation (NCT03246958). Another phase 2 study is enrolling ATC and PDTC patients to various targeted therapies in combination with atezolizumab, an anti-PD-L1 drug (NCT03181100). The targeted therapy is selected based on the driver mutation present in the tumor. Additionally, ongoing trials investigating the combination of EBRT given to a metastatic site in combination with immunotherapy in solid organ tumors have expanded to involve thyroid cancer as well (NCT02239900, NCT03122496).
3. Initiating Systemic Therapy
A. Overview
Although the approval and use of KIs filled an unmet need in the treatment of advanced thyroid cancer, they come with many side effects. In light of this, a key challenge is when to initiate systemic therapy and in which patients. KIs are not curative; they are cytostatic. Patients with DTC should have RAIR disease before considering systemic therapy [101, 102]. The rate of progression of their thyroid cancer should also be considered [4]. Rising Tg or calcitonin/CEA levels should not be the sole identifier of progressive disease, but should prompt more frequent imaging, including surveillance for new metastases (e.g., brain, bone, liver) [103, 104].
B. Focal Therapies
Bone lesions, particularly when they are threatening limb function or causing neurologic compromise, should initially be addressed by focal therapy, such as radiation or surgery whenever amenable. Patients with oligometastatic disease to the bone without other sites of disease requiring systemic therapy may also be managed with focal therapy. Antiresorptive therapy, such as zoledronic acid or denosumab, may also address pain related to bony metastasis. Both drugs may induce hypocalcemia in a patient with a baseline low calcium or 25-hydroxy vitamin D level; hence, it is important to replete both calcium and vitamin D prior to initiation of these antiresorptive drugs.
Solitary lung lesions progressing in the setting of other stable lesions may also be considered for focal therapies. For metastatic MTC to the liver or bone, embolization or cryoablation of these sites may help decrease tumor burden, pain, and potentially the neuroendocrine disease–mediated diarrhea [105].
However, when these treatment options are ineffective and/or contraindicated due to anatomic limitations, or there is a large burden of disease in other sites, initiation of a KI should be considered. Opting to treat earlier rather than later (after tumor invasion or engulfing of nearby structures) may limit the AEs associated with KIs, such as bleeding [106].
C. Timing of Systemic Therapy
Rate of progression, size, and site of disease as well as patient symptoms should all be considered prior to starting a KI [102, 107]. Patients with rapidly growing, multiple large tumors (>1 to 2 cm) defined by RECIST [99] in <6 months should be considered for treatment. In patients with smaller tumors that are rapidly progressing (<6 to 12 months) or those with large tumors that are progressing very slowly (>12 months), it is less clear when to initiate a KI and should be reviewed individually. Patients with a larger tumor burden may not respond as well to a KI, as shown in a subanalysis of the SELECT trial, where patients saw less tumor shrinkage when their initial tumor size was greater than the median population tumor size of 59.1 mm (where tumor size was defined as the sum of diameters of the target lesions) [108]. Starting a KI should ideally occur prior to symptomatic development [102, 109], as this may lead to an improved PFS [110].
It has been suggested that identifying the “inflection point,” that is, a point when the disease volume rate of structural progression (tumor diameter doubling time) clinically becomes significant, may help the clinician pinpoint the earliest time to initiate a KI in an otherwise asymptomatic individual [111]. Additionally, the analysis from an observational study evaluating practice patterns of treating physicians in the initiation of KIs in asymptomatic patients is currently underway [112].
D. Identifying the Optimal Systemic Therapy
A review of the patient’s medical history and performance status should be considered before choosing the therapy. Relative contraindication to antiangiogenics include poor cardiac function, recent myocardial infarction, previous bowel surgery or diverticulitis, invasion of tumor into great vessels, esophagus or airway leading to increased risk of fistula [113], and very low body weight [114]. In these scenarios, starting a less potent antiangiogenic drug (sorafenib and vandetanib), choosing a lower starting dose of the more potent antiangiogenic drugs (lenvatinib and cabozantinib), or choosing a nonantiangiogenic alternative may help prevent further complication. Clinical trials studying lower starting doses of lenvatinib and cabozantinib are underway (NCT02702388, NCT01896479). Alternatively, in patients who do not have these relative contraindications but have rapid progression should be considered for the more potent of the antiangiogenic drugs.
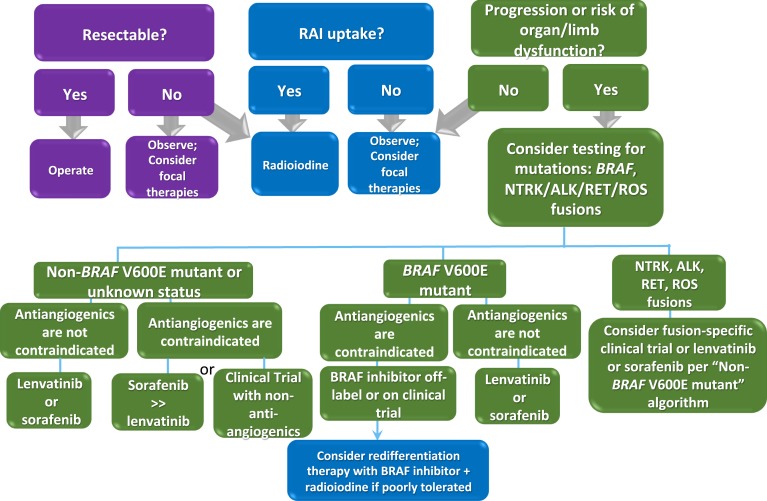
Cabanillas et al. [114] have proposed an algorithm to initiate a KI in selected MTC patients based on their clinical history. Here we propose an algorithm for initiating the optimal KI in DTC patients based on mutation status and clinical history (Fig. 2).
Figure 2.
Algorithm for management of patients with DTC. Patients with active disease should first be considered for surgery and/or RAI. If this is not feasible, or the patient is RAI refractory, observation for progression should be considered, as many patients have indolent disease. Patients who should not be observed are those who in the absence of treatment are at risk for organ (i.e., bronchial obstruction or respiratory failure) or limb dysfunction (i.e., pathologic fracture). These patients should be treated with focal therapies or systemic therapy. In those patients who do require systemic therapy due to clinically significant disease (lesion >1.5 cm) and who have progression within 6 months (12 months, case by case) or who are at risk for organ/limb dysfunction without systemic therapy, molecular testing can help guide treatment. In particular, knowing the BRAF status, as well as the presence of genetic fusions in NTRK, RET, ALK, or ROS, is important, as these are potentially actionable genetic aberrations with selective KIs. In patients who are non–BRAF V600E mutated or where the BRAF status is unknown, a determination regarding whether antiangiogenic drugs will increase the risk of a serious AE, and therefore relatively contraindicated, should be made. Relative contraindications to antiangiogenic drugs include poor cardiac function, recent myocardial infarction, uncontrollable hypertension, large and unhealed wounds, history of colitis, diverticulitis, intestinal perforation, or recent bowel surgery, tumor invading trachea/esophagus/great vessels, hemoptysis or use of anticoagulants, and very low body weight (may be exacerbated with antiangiogenic TKIs). If no contraindication to antiangiogenics exists, regardless of the BRAF status, either one of the FDA-approved drugs for DTC should be initiated. If there is a relative contraindication to antiangiogenics, the less potent of the two drugs, sorafenib, should be favored. Alternatively, a lower dose of lenvatinib can be started. In either scenario, these patients require close follow-up in this setting. If the patient has a relative contraindication to antiangiogenics and is BRAF mutated, a selective BRAF inhibitor should be favored. Particularly in patients with poor tolerance of BRAF inhibitors, radioactive iodine while on the BRAF inhibitor (“redifferentiation therapy”) may be considered. TKIs, tyrosine KI.
E. Management of Side Effects
Monitoring and managing AEs in patients on KIs is critical to be able to maintain patients on the drug. Hypertension, diarrhea, PPE, and weight loss are fairly common AEs associated with antiangiogenics, and their frequencies are listed in Table 3.
Table 3.
Comparison of Common AEs Between Different Antiangiogenic Drugs Studied in Thyroid Cancer
| Antiangiogenic Drug | Hypertension (%) | Diarrhea (%) | Fatigue (%) | PPE (%) | Weight Loss (%) | Other (%) |
|---|---|---|---|---|---|---|
| Axitinib [64] | 28 | 48 | 50 | 15 | 25 | Nausea 33; stomatitis 25; proteinuria 18 |
| Cabozantinib [39] | 32 | 63 | 41 | 50 | 48 | Nausea 43; stomatitis 29 |
| Lenvatinib [37] | 68 | 59 | 59 | 32 | 46 | Nausea 41; stomatitis 36; proteinuria 31 |
| Pazopanib [62] | 51 | 73 | 78 | 5 | 27 | Skin and hair hypopigmentation 76; alopecia 35; elevated ALT 38; elevated bilirubin 24 |
| Sorafenib [36] | 41 | 69 | 50 | 76 | 47 | Rash or desquamation 50; alopecia 67; oral mucositis 23 |
| Sunitinib [63] | 26 | 52 | 52 | 39 | 35 | Mucositis 22; hypopigmentation 13; GI hemorrhage 17; increased ALT 48 |
| Vandetanib [38] | 32 | 56 | 24 | NR | 10 | Nausea 33; QT prolongation 14; acneiform rash 15 |
Data are from the highest phase of trial conducted with the highest number of thyroid cancer patients. All percentages are rounded to the nearest whole number. NR, not reported.
E-1. Cardiovascular AEs
Hypertension is the most common cardiovascular side effect associated with antiangiogenic drugs, and the degree of the hypertension is dose related [115]. Antihypertensives should be initiated or titrated to a blood pressure goal of <140/90 mm Hg [116]. An angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or a β-blocker may be used as first-line therapy for hypertension because these drugs are not metabolized through the cytochrome P450 3A4 (CYP3A4) system [117]. Calcium channel blockers are also effective in these patients. Rapid titration of antihypertensive medications and/or KI dose reduction or interruption may be needed to achieve adequate blood pressure control. Although hypertension is a significant AE, one study showed that lenvatinib-induced hypertension correlated with more favorable outcomes in the SELECT trial, including prolonged PFS, ORR, and tumor reduction size, vs those patients who did not experience hypertension [118].
QT interval prolongation is also a known side effect and KIs should be used with caution, especially in those with a history of QT prolongation or on an antiarrhythmic drug. As mentioned, vandetanib carries a black box warning on QT prolongation (>450 msec), torsades de pointes (all of which could result in sudden death), and is contraindicated in patients with these conditions. Additionally, drugs that may prolong the QT interval, such as amiodarone and erythromycin, should be avoided. As part of the drug safety program, risk evaluation, and mitigation strategies, patients’ ECGs and electrolyte status should be systematically monitored at baseline and 2 to 4 weeks, 8 to 12 weeks, and every 3 months thereafter [119].
The increased risk of bleeding is a direct consequence of VEGFR inhibition. Several risk factors of hemoptysis during antiangiogenic KI treatment may include airway invasion, poorly differentiated pathology, prior EBRT, and thyroidectomy without a neck dissection [120]. Although bleeding is not an absolute contraindication to antiangiogenic treatment, the site and severity of the hemorrhage, as well as therapy benefits, should be taken into account in the evaluation of treatment discontinuation. Conversely, thromboembolic events may also be seen, and although rarely reported, outcomes may be lethal. Management with low molecular mass heparin would be indicated and the decision whether to continue or hold the KI is case-dependent.
E-2. GI AEs
Diarrhea, nausea, mucositis, stomatitis, dysgeusia, and weight loss may all develop with the use of KIs. Supportive therapies such as antidiarrheal and antiemetic medications, or taking a KI with a larger meal and a tall glass of water, may help mitigate some of these symptoms. Mucosal treatment should be driven by prevention (avoiding irritating foods and maintain good oral care) as well as pain control (mouthwashes or analgesics). In more severe situations, dose alterations or discontinuation may be necessary.
GI perforation or fistulas, although rare, are potentially fatal, and cabozantinib carries this black box warning. Risk of developing these conditions after starting a KI may increase with history of EBRT to that site, surgery, or tumor-invading vital structures (such as the trachea, esophagus, and major vasculature) [113].
Hepatotoxicity may also develop, especially seen with pazopanib, and close monitoring of liver function tests throughout therapy is necessary. In grade 3 to 4 cases, discontinue the KI or start at a lower dose.
E-3. Dermatologic AEs
Dermatologic AEs are also common with antiangiogenics, BRAF, and MEK inhibitors. PPE, acneiform rash, alopecia, squamous cell carcinoma, hair color, and texture are often seen. Owing to drug-induced photosensitivity (more common with vemurafenib), minimizing sun exposure through abstinence, wearing appropriate protective clothing, and using broad-spectrum sun-protective factor ≥30 are necessary.
Also known as hand-foot syndrome, PPE is a common side effect of cabozantinib and sorafenib, appearing as desquamating lesions or thick callous formation at areas of pressure points or friction or trauma, and is often considered the most debilitating of the dermatologic AEs. Prevention of PPE is aided by protecting pressure points, keeping skin well moisturized, exfoliating calluses with a keratolytic agent (urea-based creams), and consulting with a podiatrist or dermatologist. Topical creams and oral pain medications, or dose reductions/interruptions, may all help provide symptomatic relief. More details to manage PPE are explained elsewhere [121]. BRAF inhibitors have been known to cause squamous cell carcinoma, and a thorough skin evaluation at baseline and throughout treatment should be performed regularly. The addition of an MEK inhibitor may be protective against some of these cutaneous AEs [122]. MEK inhibitors and vandetanib are known to cause acneiform rash, and may be treated with special soaps or with topical or systemic antibiotics or steroids. Prophylactic antibiotic use may decrease the incidence of acneiform rash [123].
E-4. Renal AEs
Proteinuria is the most frequent renal AE in KI-treated patients, and it has been reported in up to 30% of patients on lenvatinib [37]. This proteinuria is likely due to the KI affecting the glomerular lining. Hypertension and proteinuria are both independent risk factors of adverse cardiovascular outcomes and progression to end-stage renal disease in patients with chronic kidney disease [124]. Patients should be monitored for proteinuria throughout treatment and should the event arise, they could benefit from drugs to reduce proteinuria (i.e., ACE inhibitors) or discontinue the offending agent in severe cases.
E-5. Miscellaneous AEs
All anti-VEGFR drugs impair wound healing, and therefore patients undergoing elective surgeries should be counseled to hold drugs prior to surgery (3 to 5 half-lives). Elevations in TSH are seen with KIs, and therefore thyroid function tests should be monitored carefully in thyroid cancer patients, particularly in DTC patients. Hypothyroidism, from KIs in patients who have had a thyroidectomy and are on stable thyroid hormone doses prior to starting KIs, may be due to impaired absorption of hormonal replacement, increased metabolism of T4, or alterations in negative feedback signaling to the pituitary [125]. Weight loss is common in patients on KIs, particularly with lenvatinib and cabozantinib, and should be monitored throughout treatment.
Drug–drug interactions must also be considered. Many of the KIs are metabolized by CYP3A4. CYP3A4 inducers (i.e., dexamethasone, certain antiepileptics, rifampin, St. John’s wort, and certain HIV medications) can decrease the concentration of KIs, whereas inhibitors (calcium channel blockers, amiodarone, azole antifungals, macrolide antibiotics, HIV antivirals) can increase KI toxicity. A thorough medical reconciliation and assessment of these CYP3A4 inducers and inhibitors should be performed [117].
4. Conclusion
Targeted therapies have offered hope to those with advanced thyroid cancer, with FDA-approved drugs for all three types of thyroid cancers. Although we have come a long way in a relatively short amount of time, several problems remain. First, although these drugs lead to tumor shrinkage in most patients and improved PFS, limited data are available regarding prolonging OS or inducing remission. Second, little is known about quality of life in thyroid cancer patients on systemic therapy. The new, more selective inhibitors of RET and NTRK appear to be less toxic; however, an assessment of quality of life is lacking at this time. Third, resistance to KIs eventually develops and little is known about the mechanisms of resistance or which is the best choice of second-line salvage therapies. It will require serial biopsies (on tissue or blood) and assessment of the evolution of tumor morphology and genetics to answer these questions. Overall, KIs are already providing clinical benefit to patients with advanced thyroid cancer, and through further investigations, the development of better tolerated targeted drugs, combination strategies to minimize resistance to KIs, and possibly even curative strategies are on the horizon.
Acknowledgments
Disclosure Summary: M.E.C. has received research funding from Eisai, Exelixis, Genetech, and Kura and has participated in advisory boards for LOXO, Blueprint, and Ignyta. S.N.R. has nothing to disclose.
Glossary
Abbreviations:
- AE
adverse event
- ALK
anaplastic lymphoma kinase
- ATC
anaplastic thyroid cancer
- CR
complete response
- CYP3A4
cytochrome P450 3A4
- DTC
differentiated thyroid cancer
- EBRT
external beam radiation therapy
- FDA
Food and Drug Administration
- GI
gastrointestinal
- HCTC
Hurthle cell thyroid cancer
- HIF1α
hypoxia-inducible factor-1α
- HR
hazard ratio
- KI
kinase inhibitor
- MKI
multikinase inhibitor
- MTC
medullary thyroid cancer
- mTOR
mammalian target of rapamycin
- NIS
sodium/iodide symporter
- NTRK
neurotrophic tropomyosin receptor kinase
- ORR
overall response rate
- OS
overall survival
- PDGFR
platelet-derived growth factor receptor
- PDTC
poorly differentiated thyroid cancer
- PFS
progression-free survival
- PI3K
phosphatidylinositol 3-kinase
- PPE
palmar-plantar erythrodysesthesia
- PR
partial response
- PTC
papillary thyroid cancer
- RAI
radioactive iodine
- RAIR
radioiodine refractory
- RR
response rate
- Tg
thyroglobulin
- VEGFR
vascular endothelial growth factor receptor
References and Notes
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2. Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, Ehya H, Farrar WB, Haddad RI, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey JC, Olson JA Jr, Parks L, Ridge JA, Shah JP, Sherman SI, Sturgeon C, Waguespack SG, Wang TN, Wirth LJ; National Comprehensive Cancer Network . Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8(11):1228–1274. [DOI] [PubMed] [Google Scholar]
- 3. Elisei R, Molinaro E, Agate L, Bottici V, Masserini L, Ceccarelli C, Lippi F, Grasso L, Basolo F, Bevilacqua G, Miccoli P, Di Coscio G, Vitti P, Pacini F, Pinchera A. Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab. 2010;95(4):1516–1527. [DOI] [PubMed] [Google Scholar]
- 4. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. [DOI] [PubMed] [Google Scholar]
- 6. Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, Gunn GB, Lu C, Ferrarotto R, Lai SY, Cabanillas ME. Patterns of treatment failure in anaplastic thyroid carcinoma. Thyroid. 2017;27(5):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95(8):625–627. [DOI] [PubMed] [Google Scholar]
- 8. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63(7):1454–1457. [PubMed] [Google Scholar]
- 9. Lemoine NR, Mayall ES, Wyllie FS, Farr CJ, Hughes D, Padua RA, Thurston V, Williams ED, Wynford-Thomas D. Activated ras oncogenes in human thyroid cancers. Cancer Res. 1988;48(16):4459–4463. [PubMed] [Google Scholar]
- 10. Suárez HG, Du Villard JA, Caillou B, Schlumberger M, Tubiana M, Parmentier C, Monier R. Detection of activated ras oncogenes in human thyroid carcinomas. Oncogene. 1988;2(4):403–406. [PubMed] [Google Scholar]
- 11. Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98(11):E1852–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grieco M, Santoro M, Berlingieri MT, Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G, Fusco A, Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60(4):557–563. [DOI] [PubMed] [Google Scholar]
- 13. Chou A, Fraser S, Toon CW, Clarkson A, Sioson L, Farzin M, Cussigh C, Aniss A, O’Neill C, Watson N, Clifton-Bligh RJ, Learoyd DL, Robinson BG, Selinger CI, Delbridge LW, Sidhu SB, O’Toole SA, Sywak M, Gill AJ. A detailed clinicopathologic study of ALK-translocated papillary thyroid carcinoma. Am J Surg Pathol. 2015;39(5):652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pierotti MA, Bongarzone I, Borrello MG, Mariani C, Miranda C, Sozzi G, Greco A. Rearrangements of TRK proto-oncogene in papillary thyroid carcinomas. J Endocrinol Invest. 1995;18(2):130–133. [DOI] [PubMed] [Google Scholar]
- 15. Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, Evdokimova VN, Hatch M, Zurnadzy LY, Nikiforova MN, Yue NJ, Zhang M, Mabuchi K, Tronko MD, Nikiforov YE. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120(6):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamatani K, Mukai M, Takahashi K, Hayashi Y, Nakachi K, Kusunoki Y. Rearranged anaplastic lymphoma kinase (ALK) gene in adult-onset papillary thyroid cancer amongst atomic bomb survivors. Thyroid. 2012;22(11):1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, Máximo V, Botelho T, Seruca R, Sobrinho-Simões M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22(29):4578–4580. [DOI] [PubMed] [Google Scholar]
- 18. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, Tosi E, Cavaliere A, Gulino A, Filetti S, Russo D. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92(7):2840–2843. [DOI] [PubMed] [Google Scholar]
- 19. Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAFV600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13(1):257–269. [DOI] [PubMed] [Google Scholar]
- 20. Xing M, Clark D, Guan H, Ji M, Dackiw A, Carson KA, Kim M, Tufaro A, Ladenson P, Zeiger M, Tufano R. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27(18):2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110(1):38–46. [DOI] [PubMed] [Google Scholar]
- 22. Guerra A, Fugazzola L, Marotta V, Cirillo M, Rossi S, Cirello V, Forno I, Moccia T, Budillon A, Vitale M. A high percentage of BRAFV600E alleles in papillary thyroid carcinoma predicts a poorer outcome. J Clin Endocrinol Metab. 2012;97(7):2333–2340. [DOI] [PubMed] [Google Scholar]
- 23. Rusinek DC, Krajewska J, Oczko-Wojciechowska M, Zebracka-Gala J, Zebala-Nozynska E, Chmielik E, Pawlaczek A, Cyplinska R, Handkiewicz-Junak D, Jarzab B. TERT mutations increase risk of treatment failure in BRAF positive papillary thyroid cancer [abstract]. Thyroid. 2017;27(S1):A1–A2. [Google Scholar]
- 24. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haugen BR, Sherman SI. Evolving approaches to patients with advanced differentiated thyroid cancer. Endocr Rev. 2013;34(3):439–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romei C, Casella F, Tacito A, Bottici V, Valerio L, Viola D, Cappagli V, Matrone A, Ciampi R, Piaggi P, Ugolini C, Torregrossa L, Basolo F, Materazzi G, Vitti P, Elisei R. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J Med Genet. 2016;53(11):729–734. [DOI] [PubMed] [Google Scholar]
- 28. Ciampi R, Mian C, Fugazzola L, Cosci B, Romei C, Barollo S, Cirello V, Bottici V, Marconcini G, Rosa PM, Borrello MG, Basolo F, Ugolini C, Materazzi G, Pinchera A, Elisei R. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid. 2013;23(1):50–57. [DOI] [PubMed] [Google Scholar]
- 29. Hanna GJ, Busaidy NL, Chau NG, Wirth LJ, Barletta JA, Calles A, Haddad RI, Kraft S, Cabanillas ME, Rabinowits G, O’Neill A, Limaye SA, Alexander EK, Moore FD Jr, Misiwkeiwicz K, Thomas T, Nehs M, Marqusee E, Lee SL, Jänne PA, Lorch JH. Genomic correlates of response to everolimus in aggressive radioiodine-refractory thyroid cancer: A phase II study. Clin Cancer Res. 2018;24(7):1546–1553. [DOI] [PubMed] [Google Scholar]
- 30. Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, Park CS, Nam KH, Kang SW, Kim MK, Kim SB, Lee SH, Kim HG, Na II, Kim YS, Choi MY, Kim JG, Park KU, Yun HJ, Kim JH, Cho BC. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol. 2013;24(12):3089–3094. [DOI] [PubMed] [Google Scholar]
- 31. Sherman EJ, Dunn LA, Ho AL, Baxi SS, Ghossein RA, Fury MG, Haque S, Sima CS, Cullen G, Fagin JA, Pfister DG. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer. 2017;123(21):4114–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJ, Gelderblom H, Roozen IC, Bos M, Corver WE, van Wezel T, Smit JW, Morreau H, Guchelaar HJ, Kapiteijn E. Everolimus in patients with advanced follicular-derived thyroid cancer: results of a phase II clinical trial. J Clin Endocrinol Metab. 2017;102(2):698–707. [DOI] [PubMed] [Google Scholar]
- 33. Sherman EJ, Ho AL, Fury MG, Baxi SS, Dunn L, Lee JS, Lipson BL, Pfister DG. Combination of everolimus and sorafenib in the treatment of thyroid cancer: update on phase II study [abstract].J Clin Oncol. 2015;33(15 Suppl). Abstract 6069. [Google Scholar]
- 34. Scarpino S, Cancellario d’Alena F, Di Napoli A, Pasquini A, Marzullo A, Ruco LP. Increased expression of Met protein is associated with up-regulation of hypoxia inducible factor-1 (HIF-1) in tumour cells in papillary carcinoma of the thyroid. J Pathol. 2004;202(3):352–358. [DOI] [PubMed] [Google Scholar]
- 35. Ramirez R, Hsu D, Patel A, Fenton C, Dinauer C, Tuttle RM, Francis GL. Over-expression of hepatocyte growth factor/scatter factor (HGF/SF) and the HGF/SF receptor (cMET) are associated with a high risk of metastasis and recurrence for children and young adults with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2000;53(5):635–644. [DOI] [PubMed] [Google Scholar]
- 36. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators . Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. [DOI] [PubMed] [Google Scholar]
- 38. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bayer HealthCare Pharmaceuticals Inc Nexavar® (sorafenib) package insert.Updated June 2015. http://labeling.bayerhealthcare.com/html/products/pi/Nexavar_PI.pdf. Accessed 8 April 2018.
- 41. Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, Tsuruoka A. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340(1):97–103. [DOI] [PubMed] [Google Scholar]
- 42. Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664–671. [DOI] [PubMed] [Google Scholar]
- 43. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14(17):5459–5465. [DOI] [PubMed] [Google Scholar]
- 44. Sherman SI, Jarzab B, Cabanillas ME, Licitra LF, Pacini FR, Martins RA, Robinson B, Ball D, McCaffrey J, Shah MH, Bodenner DL, Allison R, Newbold KL, Elisei R, O’Brien JP, Schlumberger M. A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC) [abstract]. J Clin Oncol. 2011;29(15 Suppl). Abstract 5503.
- 45. Brose MS, Worden FP, Newbold KL, Guo M, Hurria A. Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J Clin Oncol. 2017;35(23):2692–2699. [DOI] [PubMed] [Google Scholar]
- 46. Elisei R, Schlumberger M, Tahara M, Robinson B, Brose M, Ductus C, Zhu J, Newbold K, Kiyota N, Kim SB, Sherman S, Wirth L. Subgroup analysis according to differentiated thyroid cancer histology in phase 3 (SELECT) trial of lenvatinib [abstract]. Oncol Res Treat. 2015;38(Suppl 5):1–270. Abstract 91.
- 47. Schlumberger M, Jarzab B, Cabanillas ME, Robinson B, Pacini F, Ball DW, McCaffrey J, Newbold K, Allison R, Martins RG, Licitra LF, Shah MH, Bodenner D, Elisei R, Burmeister L, Funahashi Y, Ren M, O’Brien JP, Sherman SI. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res. 2016;22(1):44–53. [DOI] [PubMed] [Google Scholar]
- 48. Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, Sasaki T, Suzuki T, Fujino K, Dutcus CE, Takahashi S. Lenvatinib for anaplastic thyroid cancer. Front Oncol. 2017;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62(24):7284–7290. [PubMed] [Google Scholar]
- 50. Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF, Bigley AL, Hennequin LF. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62(16):4645–4655. [PubMed] [Google Scholar]
- 51. Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28(5):767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanofi Genzyme Caprelsa® (vandetanib) package insert. Updated December 2016. www.caprelsa.com/files/caprelsa-pi.pdf. Accessed 4 April 2018.
- 53. Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, Gómez JM, Bonichon F, Leenhardt L, Soufflet C, Licour M, Schlumberger MJ. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13(9):897–905. [DOI] [PubMed] [Google Scholar]
- 54. Brose MS, Fagin J, Wirth L, Elisei R, Sugitani I, Wu Y, Wang Z, Leboulleaux L, Bastholt L, Fuehrer D, Weiss R, Magner J, Bernard J, Laird G, Rana N, Schlumberger M. Impact of TSH levels on response to vandetanib in patients with locally advanced or metastatic differentiated thyroid cancer (DTC) who are refractory or unsuitable for radioiodine (RAI) therapy: a phase III study (VERIFY) [abstract]. Thyroid. 2016;26(Suppl):A-152. Abstract 42.
- 55. Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, Orf J, You A, Laird AD, Engst S, Lee L, Lesch J, Chou YC, Joly AH. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308. [DOI] [PubMed] [Google Scholar]
- 56. Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Müller T, Ratain MJ, Salgia R. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Exelixis Pharmaceuticals Inc Cometriq® (cabozantinib) package insert. Updated January 2018. www.cometriq.com/downloads/Cometriq_Full_Prescribing_Information.pdf. Accessed 4 April 2018.
- 58. Schlumberger M, Elisei R, Müller S, Schöffski P, Brose M, Shah M, Licitra L, Krajewska J, Kreissl MC, Niederle B, Cohen EEW, Wirth L, Ali H, Clary DO, Yaron Y, Mangeshkar M, Ball D, Nelkin B, Sherman S. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28(11):2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sherman SI, Clary DO, Elisei R, Schlumberger MJ, Cohen EE, Schöffski P, Wirth LJ, Mangeshkar M, Aftab DT, Brose MS. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer. 2016;122(24):3856–3864. [DOI] [PubMed] [Google Scholar]
- 60. Cabanillas ME, Brose MS, Holland J, Ferguson KC, Sherman SI. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid. 2014;24(10):1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cabanillas ME, de Souza JA, Geyer S, Wirth LJ, Menefee ME, Liu SV, Shah K, Wright J, Shah MH. Cabozantinib as salvage therapy for patients with tyrosine kinase inhibitor-refractory differentiated thyroid cancer: results of a multicenter phase II international thyroid oncology group grial. J Clin Oncol. 2017;35(29):3315–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC III, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C; Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium . Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11(10):962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16(21):5260–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAFV600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Iyer PC, Dadu R, Ferrarotto R, Busaidy NL, Habra MA, Zafereo M, Gross N, Hess KR, Gule-Monroe M, Williams MD, Cabanillas ME. Real-world experience with targeted therapy for the treatment of anaplastic thyroid carcinoma. Thyroid. 2018;28(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cabanillas ME, Ferrarotto R, Garden AS, Ahmed S, Busaidy NL, Dadu R, Williams MD, Skinner H, Gunn GB, Grosu H, Iyer P, Hofmann MC, Zafereo M. Neoadjuvant BRAF- and immune-directed therapy for anaplastic thyroid carcinoma. Thyroid. 2018;28(7):945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shah M, Wei L, Wirth L, Daniels G, De Souza J, Timmers C. Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma [abstract]. J Clin Oncol. 2017;35(15 Suppl):6022.
- 69. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, Sherman SI, Sherman EJ. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(9):1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3(5):520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sherman EJ, Ho AL, Fagin JA, Haque S, Robinson C, Ghossein RA, Chen HX, Pfister DG. Combination of dabrafenib (DAB) and lapatinib (LAP) for the treatment of BRAF-mutant thyroid cancer [abstract]. J Clin Oncol. 2018;36(Suppl). Abstract 6087.
- 72. Boyd KP, Vincent B, Andea A, Conry RM, Hughey LC. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67(6):1375–1379. [DOI] [PubMed] [Google Scholar]
- 73. Cabanillas ME, Patel A, Danysh BP, Dadu R, Kopetz S, Falchook G. BRAF inhibitors: experience in thyroid cancer and general review of toxicity. Horm Cancer. 2015;6(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. Survival in BRAF V600–mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, Morales T, Aliagas I, Liu B, Sideris S, Hoeflich KP, Jaiswal BS, Seshagiri S, Koeppen H, Belvin M, Friedman LS, Malek S. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464(7287):431–435. [DOI] [PubMed] [Google Scholar]
- 76. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, Bollag G, Kolesnick R, Thin TH, Rosen N, Zanzonico P, Larson SM, Refetoff S, Ghossein R, Fagin JA. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121(12):4700–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. AstraZeneca. H1 2018 Results. Available at: www.astrazeneca.com/content/dam/az/PDF/2018/h1-2018/H1%202018%20Results%20announcement.pdf. Accessed 26 July 2018.
- 81. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21(5):1028–1035. [DOI] [PubMed] [Google Scholar]
- 82. Jaber T, Waguespack SG, Cabanillas ME, Vu T, Santos EB, Dadu R, Busaidy NL. Efficacy of targeted therapy in resensitization to radioactive iodine (RAI) in advanced thyroid cancer: the MD Anderson experience. In: Proceedings of the Endocrine Society’s 99th Annual Meeting and Expo; 1–4 April 2017; Orlando, FL.Endocrine Reviews. Abstract OR39-6. [Google Scholar]
- 83. Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5(8):671–688. [DOI] [PubMed] [Google Scholar]
- 84. Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, Hu W, Cao Q, Sheets MP, Wilson D, Wilson KJ, DiPietro L, Fleming P, Palmer M, Hu MI, Wirth L, Brose MS, Ou SI, Taylor M, Garralda E, Miller S, Wolf B, Lengauer C, Guzi T, Evans EK. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8(7):836–849. [DOI] [PubMed] [Google Scholar]
- 85. Subbiah V, Taylor M, Lin J, Hu M, Ou SI, Brose MS, Garralda E, Clifford C, Palmer M, Evans E, Shi H, Wolf B, Gainor JF. Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase I study of advanced RET-altered solid tumors. In: Proceedings of the American Association for Cancer Research Annual Meeting; 14-18 April 2018; Chicago, IL. Abstract CT043.
- 86. Drilon AE, Subbiah V, Oxnard GR, Bauer TM, Velcheti V, Lakhani NJ, Besse B, Park K, Patel JD, Cabanillas ME, Johnson ML, Reckamp KL, Boni V, Loong HH, Schlumberger M, Solomon B, Cruickshank S, Rothenberg SM, Shah MH, Wirth LJ. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers [abstract]. J Clin Oncol. 2018;36(Suppl). Abstract 102.
- 87. Ritterhouse LL, Wirth LJ, Randolph GW, Sadow PM, Ross DS, Liddy W, Lennerz JK. ROS1 rearrangement in thyroid cancer. Thyroid. 2016;26(6):794–797. [DOI] [PubMed] [Google Scholar]
- 88. Bastos AU, de Jesus AC, Cerutti JM. ETV6-NTRK3 and STRN-ALK kinase fusions are recurrent events in papillary thyroid cancer of adult population. Eur J Endocrinol. 2018;178(1):85–93. [DOI] [PubMed] [Google Scholar]
- 89. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hong DS, Brose MS, Doebele RC, Shaw AT, Dowlati A, Bauer TM, Farago AF, Estrada-Bernal A, Lee AT, Cox MC, Nanda N, Low JA, Burris III. Clinical safety and activity from a phase 1 study of LOXO-101, a selective TRKA/B/C inhibitor, in solid-tumor patients with NTRK gene fusions [abstract]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 16–20 April 2016; New Orleans, LA. Abstract PR13. [Google Scholar]
- 91. Sartore-Bianchi A, Ardini E, Bosotti R, Amatu A, Valtorta E, Somaschini A, Raddrizzani L, Palmeri L, Banfi P, Bonazzina E, Misale S, Marrapese G, Leone A, Alzani R, Luo D, Hornby Z, Lim J, Veronese S, Vanzulli A, Bardelli A, Martignoni M, Davite C, Galvani A, Isacchi A, Siena S. Sensitivity to entrectinib associated with a novel LMNA-NTRK1 gene fusion in metastatic colorectal cancer. J Natl Cancer Inst. 2015;108(1):djv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Farago AF, Le LP, Zheng Z, Muzikansky A, Drilon A, Patel M, Bauer TM, Liu SV, Ou SH, Jackman D, Costa DB, Multani PS, Li GG, Hornby Z, Chow-Maneval E, Luo D, Lim JE, Iafrate AJ, Shaw AT. Durable clinical response to entrectinib in NTRK1-rearranged non-small cell lung cancer. J Thorac Oncol. 2015;10(12):1670–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu SV, Macke LA, Colton BS, Imran SS, Christiansen J, Chow-Maneval E, Hornby Z, Multani PS. Response to entrectinib in differentiated thyroid cancer with a ROS1 fusion. JCO Precis Oncol. 2017;1:1–5. [DOI] [PMC free article] [PubMed]
- 94. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. French JD, Bible K, Spitzweg C, Haugen BR, Ryder M. Leveraging the immune system to treat advanced thyroid cancers. Lancet Diabetes Endocrinol. 2017;5(6):469–481. [DOI] [PubMed] [Google Scholar]
- 96. Dadu R, Para Cuentas E, Rodriguez Canales J, Wistuba I, Zhou S, Williams M, Cabanillas ME. Anaplastic thyroid cancer is a hot immunogenic environment: immunoprofiling of a large cohort of ATC tumors [abstract]. Thyroid. 2016;26(Suppl 1). Abstract OA12. [Google Scholar]
- 97. Chowdhury S, Veyhl J, Jessa F, Polyakova O, Alenzi A, MacMillan C, Ralhan R, Walfish PG. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget. 2016;7(22):32318–32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mehnert J, Varga A, Brose M, Aggarwal R, Lin C, Prawira A, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Pembrolizumab for advanced papillary or follicular thyroid cancer: preliminary results from the phase 1b KEYNOTE-028 study. J Clin Oncol. 2016;33(15 Suppl). Abstract 6069. [Google Scholar]
- 99. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 100. Wirth LJ, Eigendorff E, Capdevila J, Paz-Ares LG, Lin C, Taylor MH, Ramlau R, Butler M, Delord JP, Horvath Z, Gelderblom H, Ascierto PA, Fasolo A, Führer D, Wu H, Bostel G, Cameron S, Faris JE, Varga AI. Phase I/II study of spartalizumab (PDR001), an anti-PD1 mAb, in patients with anaplastic thyroid cancer [abstract]. J Clin Oncol. 2018;36(Suppl). Abstract 6024.
- 101. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. [DOI] [PubMed] [Google Scholar]
- 102. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, Pacini F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2(5):356–358. [DOI] [PubMed] [Google Scholar]
- 103. Laure Giraudet A, Al Ghulzan A, Aupérin A, Leboulleux S, Chehboun A, Troalen F, Dromain C, Lumbroso J, Baudin E, Schlumberger M. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol. 2008;158(2):239–246. [DOI] [PubMed] [Google Scholar]
- 104. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Inoue H, Tomoda C, Yabuta T, Masuoka H. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011;21(7):707–716. [DOI] [PubMed] [Google Scholar]
- 105. Fromigué J, De Baere T, Baudin E, Dromain C, Leboulleux S, Schlumberger M. Chemoembolization for liver metastases from medullary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(7):2496–2499. [DOI] [PubMed] [Google Scholar]
- 106. Ito Y, Suzuki S, Ito K, Imai T, Okamoto T, Kitano H, Sugitani I, Sugino K, Tsutsui H, Hara H, Yoshida A, Shimizu K. Tyrosine-kinase inhibitors to treat radioiodine-refracted, metastatic, or recurred and progressive differentiated thyroid carcinoma [review]. Endocr J. 2016;63(7):597–602. [DOI] [PubMed] [Google Scholar]
- 107. Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JW; European Thyroid Association Task Force . 2012 European Thyroid Association guidelines for metastatic medullary thyroid cancer. Eur Thyroid J. 2012;1(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Robinson B, Schlumberger M, Wirth LJ, Dutcus CE, Song J, Taylor MH, Kim SB, Krzyzanowska MK, Capdevila J, Sherman SI, Tahara M. Characterization of tumor size changes over time from the phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab. 2016;101(11):4103–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schmidt A, Iglesias L, Klain M, Pitoia F, Schlumberger MJ. Radioactive iodine-refractory differentiated thyroid cancer: an uncommon but challenging situation. Arch Endocrinol Metab. 2017;61(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sugino K, Nagahama M, Kitagawa W, Ohkuwa K, Uruno T, Matsuzu K, Suzuki A, Masaki C, Akaishi J, Hames KY, Tomoda C, Ogimi Y, Ito K. Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr J. 2018;65(3):299–306. [DOI] [PubMed] [Google Scholar]
- 111. Tuttle RM, Brose MS, Grande E, Kim SW, Tahara M, Sabra MM. Novel concepts for initiating multitargeted kinase inhibitors in radioactive iodine refractory differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2017;31(3):295–305. [DOI] [PubMed] [Google Scholar]
- 112. Brose MS, Smit J, Lin CC, Pitoia F, Fellous M, DeSanctis Y, Schlumberger M, Tori M, Sugitani I. Timing of multikinase inhibitor initiation in differentiated thyroid cancer. Endocr Relat Cancer. 2017;24(5):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Blevins DP, Dadu R, Hu M, Baik C, Balachandran D, Ross W, Gunn B, Cabanillas ME. Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 2014;24(5):918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cabanillas ME, Hu MI, Jimenez C. Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat—and with which drug—those are the questions. J Clin Endocrinol Metab. 2014;99(12):4390–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Escalante CP, Zalpour A. Vascular endothelial growth factor inhibitor-induced hypertension: basics for primary care providers. Cardiol Res Pract. 2011;2011:816897. [DOI] [PMC free article] [PubMed]
- 116. Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, Remick SC, Steingart R, Tang WH; Cardiovascular Toxicities Panel, Convened by the Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee . Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102(9):596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cabanillas ME, Hu MI, Durand JB, Busaidy NL. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res. 2011;2011:985780. [DOI] [PMC free article] [PubMed]
- 118. Wirth LJ, Tahara M, Robinson B, Francis S, Brose MS, Habra MA, Newbold K, Kiyota N, Dutcus CE, Mathias E, Guo M, Sherman SI, Schlumberger M. Treatment-emergent hypertension and efficacy in the phase 3 study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer. 2018;124(11):2365–2372. [DOI] [PubMed] [Google Scholar]
- 119. Sanofi Genzyme Caprelsa® (vandetanib) tablets REMS program prescriber training pamphlet. Updated May 2017. www.caprelsarems.com/files/caprelsa-rems-prescriber-training-slide-deck.pdf. Accessed 4 April 2018.
- 120. Lamartina L, Ippolito S, Danis M, Bidault F, Borget I, Berdelou A, Al Ghuzlan A, Hartl D, Blanchard P, Terroir M, Deandreis D, Schlumberger M, Baudin E, Leboulleux S. Antiangiogenic tyrosine kinase inhibitors: occurrence and risk factors of hemoptysis in refractory thyroid cancer. J Clin Endocrinol Metab. 2016;101(7):2733–2741. [DOI] [PubMed] [Google Scholar]
- 121. Brose MS, Bible KC, Chow LQM, Gilbert J, Grande C, Worden F, Haddad R. Management of treatment-related toxicities in advanced medullary thyroid cancer. Cancer Treat Rev. 2018;66:64–73. [DOI] [PubMed] [Google Scholar]
- 122. Mattei PL, Alora-Palli MB, Kraft S, Lawrence DP, Flaherty KT, Kimball AB. Cutaneous effects of BRAF inhibitor therapy: a case series. Ann Oncol. 2013;24(2):530–537. [DOI] [PubMed] [Google Scholar]
- 123. Petrelli F, Borgonovo K, Cabiddu M, Coinu A, Ghilardi M, Lonati V, Barni S. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol. 2016;175(6):1166–1174. [DOI] [PubMed] [Google Scholar]
- 124. Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int. 2011;80(12):1271–1277. [DOI] [PubMed] [Google Scholar]
- 125. Illouz F, Braun D, Briet C, Schweizer U, Rodien P. Endocrine side-effects of anti-cancer drugs: thyroid effects of tyrosine kinase inhibitors. Eur J Endocrinol. 2014;171(3):R91–R99. [DOI] [PubMed] [Google Scholar]