Abstract
The gold standard for breast cancer treatment is surgery, but many women may desire to avoid surgery if possible. The purpose of this study was to evaluate whether breast cancer could be cured with modern sophisticated radiation techniques with good cosmetic outcome. We have treated 18 patients with operable breast cancer by conventional whole-breast irradiation followed by stereotactic body radiotherapy (primary tumor only) or intensity-modulated radiotherapy (tumor plus axillary nodes) boost. The planned doses were 50 Gy in 25 fractions, 18 to 25.5 Gy in 3 fractions, and 20 Gy in 8 fractions, respectively, for the 3 modalities. Stereotactic body radiotherapy was delivered with 7 to 9 coplanar and noncoplanar fixed beams, and intensity-modulated radiotherapy was given by tomotherapy. Chemotherapy and/or hormone therapy was used depending on the stage and receptor status. In 9 recent patients, hydrogen peroxide was intratumorally injected twice a week before whole-breast irradiation. All treatments were well tolerable and there were no grade ≥3 toxicities. With a median follow-up period of 35 months (range, 8-120 months), only 1 patient developed local recurrence and 2 patients developed distant metastasis. Overall survival, progression-free survival, and local control rates were 93%, 85%, and 92%, respectively, at 3 years. In 50% of the patients, the irradiated breast became better rounded, and the position of the nipple of the irradiated breast became ≥1 cm higher compared to that of the unirradiated breast. Thus, the treated breasts may be more aesthetically favorable than before irradiation in these patients. This may become a treatment option for patients with operable breast cancer.
Keywords: whole-breast irradiation, stereotactic radiotherapy, intensity-modulated radiotherapy, tomotherapy, hydrogen peroxide, KORTUC
Introduction
Surgery is the gold standard of treatment for breast cancer. For early-stage breast cancer, breast-conserving surgery followed by postoperative radiotherapy has been commonly performed. Recently, the application of total mastectomy followed by prosthetic breast reconstruction has also been spreading for both early and advanced breast cancer. Even after breast-conserving surgery, however, the shape of the breast is never the same as before surgery and there remain permanent scars, which sometimes cause pain. Furthermore, even with the development of mammary prostheses, the reconstructed breast is an artificial one. Therefore, many women may wish to conserve their breasts by avoiding surgery if possible. Among the nonsurgical methods to treat breast cancer, radiofrequency ablation and focused ultrasound have been investigated,1,2 but they are not widely available at present.
Radiation therapy has been used to treat patients with inoperable breast cancer, but most of these treatments were palliative ones.3 Concurrent chemoradiotherapy yielded complete responses in a large proportion of patients, but the results were considered inferior to those obtained by surgery.4,5 Also, conventional radiation therapy was primarily employed for early operable breast cancer, but the outcome was not satisfactory compared to surgery.6 However, with the development of modern radiation therapy techniques, curing breast cancer with a high probability might have become possible. With conventional radiotherapy alone, delivering sufficient doses to control breast cancer may often be difficult because of the occurrence of severe radiation dermatitis. However, using the sophisticated technique of stereotactic irradiation or intensity-modulated radiotherapy (IMRT), tumoricidal doses might be deliverable without severe complications.
We started such treatment in 2007 according to the strong wishes of patients. We elaborated our treatment protocol as follows: whole-breast irradiation with a conventional dose of 50 Gy in 25 fractions is given first and then a stereotactic body radiotherapy (SBRT) boost is given in 3 fractions (tested doses: 18-25.5 Gy) when pretreatment examination reveals no lymph node (LN) metastases. When axillary LN metastasis is present, boost irradiation is given by tomotherapy with doses of 20 Gy in 8 fractions. So far, we have treated 18 patients, and this article reports the excellent cosmetic as well as clinical outcomes using this treatment.
Materials and Methods
Study Design
This study started as a dose-seeking pilot study for the SBRT boost. Our consistent policy during the study period was to deliver 50 Gy in 25 fractions to the whole breast first, since this has been the standard treatment at our institution for patients undergoing breast-conserving surgery. Furthermore, it was decided to deliver booster doses by SBRT when no LN metastases were present; 18 to 25.5 Gy in 3 fractions was investigated. Since only 1 or 2 patients per year visited us for this treatment before 2015, only 1 or 2 patients were tested for the low SBRT doses of 6 and 6.5 Gy per fraction. After starting the study, patients with axillary LN metastases also wished to receive this treatment; in these cases, the booster dose was given by IMRT with tomotherapy and the dose was fixed at 20 Gy in 8 fractions. One patient with no axillary LN metastasis was treated with the IMRT boost, since the tumor location (just beneath the nipple) was considered to be more suitable to tomotherapy. The biologically effective dose-10 Gy (BED10) of our treatment was 89 to 107 Gy for the whole-breast irradiation (50 Gy) plus SBRT boost (6-8.5 Gy × 3 fractions) and 85 Gy for the whole-breast irradiation followed by IMRT boost (2.5 Gy × 8 fractions).
After establishing the standard SBRT boost dose as 21 Gy in 3 fractions and the IMRT booster dose as 20 Gy in 8 fractions, the study was continued as a pilot study of the intratumoral injection of hydrogen peroxide (Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas [KORTUC]) during whole-breast irradiation.7,8 The studies were approved by the institutional review board (approval numbers: NCU-0701, 42-14-0010) and written informed consent was obtained from all patients.
Eligibility and Patients
To be eligible, patients had to have (1) histological proof of breast cancer, (2) a concrete wish to avoid any type of surgery, (3) no distant metastases on F-18 fluoro-deoxyglucose positron emission tomography, and (4) no other active malignancy. Patients were considered ineligible if they were pregnant or had a history of any surgery or radiation therapy to the breast. Between October 2007 and January 2015, 9 patients entered the first part of the study, and between July 2015 and June 2017, 9 patients entered the pilot study of KORTUC treatment. Thus, outcomes of the 18 patients are herein reported. All were female and their ages ranged from 32 to 80 years (median, 48 years). Their characteristics are shown in Table 1. Since the aim of this treatment was to conserve the breast as it was, neoadjuvant chemotherapy was performed whenever necessary according to the wishes of the patients, and adjuvant hormonal therapy was given to hormone-receptor-positive patients unless they refused it.
Table 1.
Patient and Tumor Characteristics.
| Age (years) | Median (range) | 48 (32-80) |
| Laterality | Right/left | 8/10 |
| Stage | IA/IIA/IIB/IIIA/IIIC | 6/7/2/2/1 |
| TNM classificationa | T1bN0M0/T1cN0M0/T2N0M0/T2N1M0/T3N1M0/T3N3M0 | 2/4/7/2/2/1 |
| Histology | IDC/DCIS/scirrhous/unclassified | 12/3/1/2 |
| Maximum tumor diameter (mm), median (range) | 22 (9-57) | |
| Hormone receptor | ER & PR (+)/both (-)/unknown | 11/5/2 |
| HER2 receptor | + / - /unknown | 3/12/3 |
| Triple negative BCA | Yes/no/unknown | 2/14/2 |
Abbreviations: BCA, breast cancer; DCIS, ductal carcinoma in situ; ER, estrogen receptor; IDC, invasive ductal carcinoma; PR, progesterone receptor.
a TNM classification according to the American Joint Committee on Cancer, 8th edition (2018).
Treatment
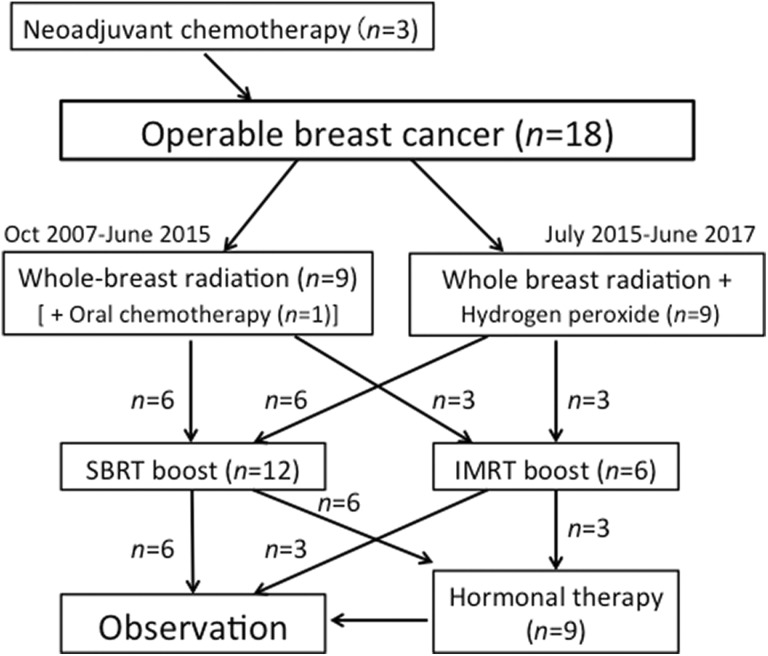
The study flowchart and treatment details are summarized in Figure 1 and Table 2. The patients received all treatments in the supine position. Whole-breast irradiation was delivered first with tangential opposed fields using 2-Gy daily fractions up to 50 Gy with 4- or 6-MV X rays from a linear accelerator. Planning for whole-breast radiation and SBRT was performed on Eclipse Version 7.5 (Varian Medical Systems, Palo Alto, California).9 For whole-breast irradiation, the planning target volume (PTV) covered the superior border at the manubriosternal joint and the inferior border at 1 cm below the inframammary line; the medial border was usually the midline of the sternum, and the lateral border was the midaxillary line, excluding the outermost 2 mm from the superficial skin surface.10 Regarding regional nodal irradiation, the lower part of the ipsilateral axillary LN area was included in all cases. The upper part of the ipsilateral axillary LN area was also included when metastases to the LNs were present. Two beam angles for tangential irradiation were determined by an attending radiation oncologist. When skin reactions were severe, dose reduction of up to 6 Gy was permitted.
Figure 1.
Study flowchart and treatment details.
Table 2.
Treatment Details.
| Whole-breast dose (Gy) | Median (range) | 50 (44-50) |
|---|---|---|
| Hydrogen peroxide injection | Yes:no | 9:9 |
| Boost method | SBRT:IMRT | 12:6 |
| SBRT dose (Gy/fr) | 18/3:19.5/3:21/3:17/2:25.5/3 | 2:1:5:2:2 |
| IMRT dose (Gy/fr) | 17.5/7:20/8 | 1:5 |
| Hormone therapy | Yes:no | 9:9 |
| Chemotherapy | Yes:no | 4:14 |
Abbreviations: fr, fractions; IMRT, intensity-modulated radiotherapy; SBRT, stereotactic body radiotherapy.
Our SBRT method for breast cancer followed the method for lung cancers, and it was described in detail previously.11 Patients were fixed in the supine position with an immobilization device, which was the same as that used for lung SBRT. The PTV was the gross tumor volume plus 5- to 7-mm margins. Registration of the breast contour was performed with computed tomography (CT) in the first and third SBRT sessions, and in addition, it was also performed by megavoltage portal imaging before every SBRT treatment. Seven to 9 coplanar and noncoplanar beams were used. The SBRT dose was prescribed to the isocenter. No special fixation method nor fiducial markers were used for the breast; it was confirmed that respiratory movement of the breast was 5 mm or less in all directions. Our tomotherapy IMRT method was described in detail previously.12,13 The same fixation method as in the SBRT boost was used. The PTV covered the primary tumor and metastatic axillary nodes with 5- to 7-mm margins. The TomoHelical and TomoDirect modes were used in 3 patients each. The field width was 2.5 cm in 5 cases and 5 cm in 1, all with the dynamic jaw mode.13 The pitch was 0.25 to 0.5 (median, 0.43) and the modulation factor was 1.5 to 3 (median, 2). Registration with megavoltage CT was performed at every fraction. When skin reactions were severe, omission of one fraction of SBRT or IMRT was allowed.
Kochi Oxydol-Radiation Therapy For Unresectable Carcinomas treatment was performed for all 9 patients encountered after July 2015. Beginning at the sixth to eighth fraction of whole-breast irradiation, 6 mL of 0.5% hydrogen peroxide (Oxydol, Ken-ei Pharmaceutical Co Ltd, Osaka, Japan) dissolved in sodium hyaluronate was injected into the breast tumor under CT guidance twice a week (Monday and Wednesday or Thursday) up to 7 or 8 times, according to the previous investigators.14,15 Hydrogen peroxide was not administered during SBRT or IMRT boost. This was part of a phase I toxicity evaluation study. Systemic chemotherapy was used in 4 patients; used agents were 5-fluorouracil, epirubicin, cyclophosphamide, paclitaxel, doxorubicin, docetaxel, S-1, and trastuzumab.
Evaluation
Follow-up examinations were performed at intervals of 2 months until 1 year after treatment, 3 or 4 months until 3 years, 4 or 6 months until 5 years, and 6 to 12 months thereafter. At every visit, CT or magnetic resonance imaging was performed in addition to physical examination and a blood test. F-18 Fluoro-deoxyglucose positron emission tomography was performed whenever necessary. Overall survival, progression-free survival, and local control rates were calculated by the Kaplan-Meier method from the start of radiation therapy. Common Terminology Criteria for Adverse Events version 4.0 was used to score toxicity. During treatment and data analyses, the investigators were not blinded and no randomization was performed.
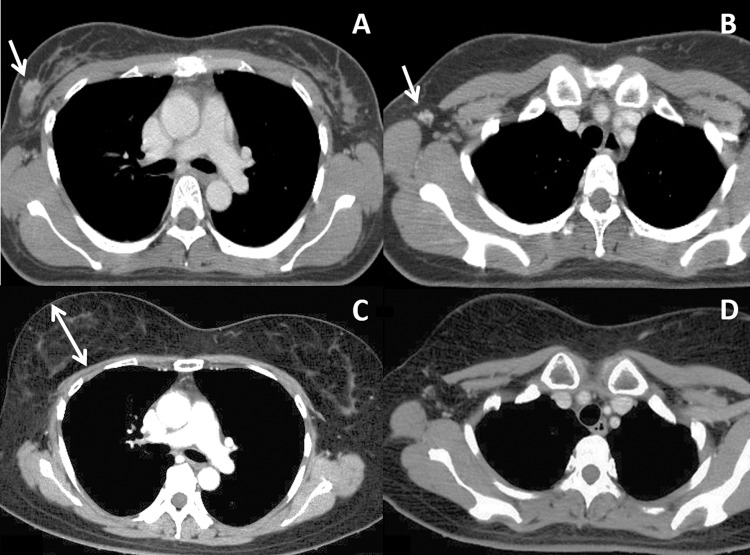
To evaluate the configuration of the irradiated breast, we devised a method using inspection and CT. At inspection, the positions of the bilateral nipples were compared. On CT, the breast height was measured on the slices of the maximal breast size as the distance from the rib cage to the most anterior part of the breast (excluding the nipple) on the axis perpendicular to the rib cage, as shown later in Figure 2. This measurement was conducted using CT images taken between 12 and 18 months after radiation therapy. The difference in the breast height between the irradiated and unirradiated sides was examined using the software StatView version 5 (SAS Institute Inc, Cary, North Carolina).
Figure 2.
Computed tomography (CT) images of a patient before and 5 years after treatment. A and B, Before treatment. Arrows indicate a mammary tumor (A) and an axillary lymph node (B). C and D, Five years after treatment, both the breast tumor and axillary lymph node are controlled. Note the marked enlargement of the right irradiated breast. The double-headed arrow in Figure 2C indicates the breast height on CT defined in this study.
Results
Patients and Treatment
Between October 2007 and June 2017, a total of 18 Japanese patients were treated (Table 1); 9 were treated in the boost dose-seeking study and the other 9 were treated in the phase I study of KORTUC. No grade ≥3 toxicities were observed, and after the dose-seeking study, the standard dose was determined to be 50 Gy in 25 fractions over 5 weeks for whole-breast irradiation followed by SBRT boost with 21 Gy in 3 fractions twice a week or IMRT boost with 20 Gy in 8 fractions 5 times a week in the KORTUC study. Regarding additional radiation doses to organs at risk delivered by the boost treatment, the mean lung dose was 0.1 to 1.6 Gy (median, 0.7 Gy) by the SBRT boost and 0.4 to 1.9 Gy (median, 1.3 Gy) by the IMRT boost. In the patients with left breast cancer, the maximum dose to the heart was 2.1 to 13.7 Gy (median, 3.9 Gy) by SBRT and 2.9 to 8.6 Gy (median, 5.4 Gy) by IMRT, and the mean heart dose was 0.1 to 1.8 Gy (median, 0.6 Gy) by SBRT and 0.4 to 1.8 Gy (median, 0.8 Gy) by IMRT.
Outcome and Toxicity
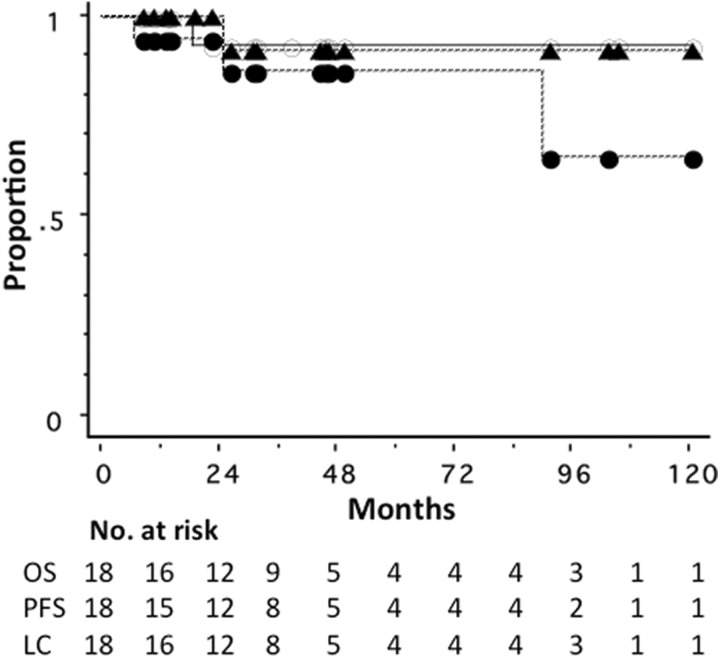
As of February 2018, the median follow-up period for all 18 patients was 35 months (range, 8-120). Of the 18 patients, only 1 patient died due to lung metastases. Another patient developed liver metastasis at 90 months, which was treated by SBRT, and she had no evidence of disease at 6 months after liver SBRT. Only 1 patient developed local recurrence; this patient had a large tumor with the longest diameter of 51 mm at diagnosis, and she was treated with radiation therapy after systemic chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide. She underwent salvage partial resection of the breast followed by chemotherapy and was alive with no evidence of disease at 13 months after local recurrence. All these patients who developed local recurrence or distant metastasis were in the dose-seeking study group. Figure 3 shows overall survival, progression-free survival, and local control curves for all 18 patients. The overall survival, progression-free survival, and local control rates were 93%, 85%, and 92%, respectively, at 3 years (Figure 3).
Figure 3.
Kaplan-Meier overall survival (OS, ^), progression-free survival (PFS, •), and local control curves (LC, ▴) for 18 patients with operable breast cancer.
Grade 2 acute skin toxicity was observed in 11 patients and grade 1 in 7 patients. All recovered and no late skin toxicity was observed. Grade 1 radiation pneumonitis was observed in 7 patients. No other toxicities have been observed to date. Oxydol injection exhibited no toxicity, and KORTUC was safe.
Cosmetic Outcome
Figure 2 shows CT images of a patient before and after treatment. At 5 years, the breast tumor and axillary LN were controlled. The irradiated right breast was markedly enlarged compared to the left unirradiated breast (Figure 2C). This enlargement of the breast became apparent at 10 months after treatment and continued until the last follow-up at 105 months. Figure 4 shows bilateral breasts of another patient at 1 year after treatment. The right nipple (irradiated side) was at a higher position than the left nipple, and the right breast was better rounded than the left breast. These changes in the breast shape became complete within 1 year after treatment and remained stable thereafter.
Figure 4.
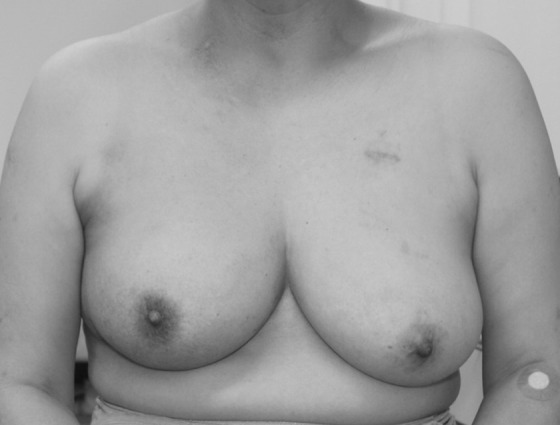
Photograph of a patient with right breast cancer at 1 year after treatment. Note that the nipple of the irradiated right breast is at a higher position than the left nipple. The right breast is better rounded than the left breast.
The positions of the bilateral nipples did not differ significantly before treatment in any patient. After treatment, the position of the nipple on the irradiated side was ≥1 cm higher compared to that of the contralateral nipple in 8 of 16 patients followed for more than 1 year. The mean difference (standard deviation [SD]) between the bilateral nipples was 0.9 (1.0) cm. The mean (SD) height of the breast on CT was 3.9 (1.4) cm for the irradiated breasts and 3.3 (1.2) cm for the unirradiated breasts (n = 16, excluding 2 patients whose follow-up period was less than 1 year; data were normally distributed, P = .0005 by paired t test).
Discussion
Numerous clinical data are now available regarding the correlation between local tumor control and SBRT dose in patients with non-small cell lung cancer (NSCLC). The data show about 90% and 75% to 85% local control rates for T1 and T2 tumors, respectively, when the employed dose corresponds to a BED10 of 100 Gy or higher.16–18 Although the concept of BED10 must be employed very cautiously for hypofractionated SBRT,19 the BED10 of our treatment was 85 to 107 Gy. Breast cancer is a relatively radiosensitive tumor and, in general, may be more radiosensitive than NSCLC.20,21 Therefore, it is not surprising that breast cancers of a few centimeters were controlled by our high-dose irradiation method. Even when the tumor develops local recurrence, salvage surgery may be feasible; indeed, one of our patients underwent salvage breast-conserving surgery successfully. A concern about this treatment is that, if uncontrolled, residual tumor cells may become a cause of distant metastasis during follow-up periods after radiation therapy. This possibility should be evaluated in future studies with more patients and longer follow-up periods.
This study demonstrated an excellent cosmetic outcome for breasts treated with whole-breast irradiation with SBRT or IMRT boost. In addition to the advantages that there were no surgical wounds nor loss of the normal shape due to surgical resection, the position of the nipple on the irradiated side became ≥1 cm higher compared to that of the contralateral nipple in 50% of the patients. However, asymmetry of the bilateral breasts was a problem in these patients. We speculate that this swelling of the irradiated breast is due to lymph edema induced primarily by whole-breast irradiation. The findings of lymph edema were not evident on CT images in every patient, but mild changes were observed. Acute skin reactions were slightly stronger than in patients undergoing whole-breast irradiation alone, but all of them recovered to satisfactory levels. Therefore, this treatment may be an effective option for patients who desire to avoid surgery.
Recently, several studies reported that focal radiotherapy increases the immunogenicity of tumor cells.22,23 If a tumor is resected, antigens disappear from the body, but in radiation therapy, tumors gradually diminish and apoptotic tumor cells may become more immunogenic.24 In addition, only a part of the trunk lies within the irradiation field, so breast irradiation may not compromise host immunity. As a result, a small amount of residual tumor cells in the body may be killed by the host’s immune system. Therefore, the combination of this treatment with immunotherapy warrants future investigation.
The KORTUC treatment has been safely delivered to more than 300 patients in Japan and the United Kingdom, and there was also no problem with safety in our study. The 9 patients treated with KORTUC have developed no local recurrence or distant metastasis to date. Even though the hypoxic fraction may increase when the tumor exceeds 1 cm in diameter,25 it may be argued that small breast cancers less than 3 cm may be controlled without hydrogen peroxide injection if SBRT or IMRT boost is given after 50 Gy whole-breast irradiation and thus oxydol injection is an overtreatment. However, hydrogen peroxide also has an effect of inactivating antioxidative enzymes such as peroxidases and catalases that scavenge radicals produced by irradiation and reduce the therapeutic effect of irradiation.26 We, alongside our patients, wish to control the tumor with a probability of nearly 100%. Therefore, we will continue to use KORTUC for tumors of 2 cm or larger in diameter.
There are several limitations in this study. The patient number is small and the patient population is heterogenous. The study period is long, and miscellaneous treatments are included. All these factors may influence the progression-free and overall survival as well as local control. So, we are now making a new protocol to treat patients with stage I/II breast cancer who refuse surgery with whole-breast irradiation combined with KORTUC and SBRT or IMRT boost.
In conclusion, definitive radiotherapy with SBRT or IMRT boost yielded an excellent cosmetic outcome as well as good local control. In the future, this treatment may replace surgery in patients with early- and middle-stage breast cancer who wish to avoid surgery. Further clinical studies are warranted.
Acknowledgments
The authors are grateful to Drs Satoshi Ishikura, Chikao Sugie, Akifumi Miyakawa, Yusuke Sawada, Taeko Goto, Kento Nomura, Hiroshi Fukuma, Takahiro Tsuchiya, Makoto Higuchi, Yasujiro Hirose, and all other doctors and radiotherapy technologists for their valuable help with this research.
Abbreviations
- BED
biologically effective dose
- CT
computed tomography
- IMRT
intensity-modulated radiotherapy
- KORTUC
Kochi Oxydol-Radiation Therapy For Unresectable Carcinomas
- LN
lymph node
- NSCLC
non-small cell lung cancer
- PTV
planning target volume
- SD
standard deviation
- SBRT
stereotactic body radiotherapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by Grants-in-Aids for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
ORCID iD: Yuta Shibamoto, MD  http://orcid.org/0000-0003-4255-8611
http://orcid.org/0000-0003-4255-8611
References
- 1. Kinoshita T, Iwamoto E, Tsuda H, Seki K. Radiofrequency ablation as local therapy for early breast carcinomas. Breast Cancer. 2011;18(1):10–17. [DOI] [PubMed] [Google Scholar]
- 2. Schmitz AC, Gianfelice D, Daniel BL, Mali WP, van den Bosch MA. Image-guided focused ultrasound ablation of breast cancer: current status, challenges, and future directions. Eur Radiol. 2008;18(7):1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Courdi A, Ortholan C, Hannoun-Levi JM, et al. Long-term results of hypofractionated radiotherapy and hormonal therapy without surgery for breast cancer in elderly patients. Radiother Oncol. 2006;79(2):156–161. [DOI] [PubMed] [Google Scholar]
- 4. Mandilaras V, Bouganim N, Spayne J, et al. Concurrent chemoradiotherapy for locally advanced breast cancer—time for a new paradigm? Curr Oncol. 2015;22(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukai H, Watanabe T, Mitsumori M, et al. Final results of a safety and efficacy trial of preoperative sequential chemoradiation therapy for the nonsurgical treatment of early breast cancer: Japan Clinical Oncology Group Study JCOG0306. Oncology. 2013;85(6):336–341. [DOI] [PubMed] [Google Scholar]
- 6. Harris JR, Connolly JL, Schnitt SJ, Cohen RB, Hellman S. Clinical-pathologic study of early breast cancer treated by primary radiation therapy. J Clin Oncol. 1983;1(3):184–189. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa Y, Kubota K, Ue H, et al. Phase I study of a new radiosensitizer containing hydrogen peroxide and sodium hyaluronate for topical tumor injection: a new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II). Int J Oncol. 2009;34(3):609–618. [DOI] [PubMed] [Google Scholar]
- 8. Takaoka T, Shibamoto Y, Matsuo M, et al. Biological effects of hydrogen peroxide administered intratumorally with or without irradiation in murine tumors. Cancer Sci. 2017;108(9):1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagai A, Shibamoto Y, Yoshida M, Inoda K, Kikuchi Y. Intensity-modulated radiotherapy using two static ports of tomotherapy for breast cancer after conservative surgery: dosimetric comparison with other treatment methods and 3-year clinical results. J Radiat Res. 2017;58(4):529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shibamoto Y, Naruse A, Fukuma H, Ayakawa S, Sugie C, Tomita N. Influence of contrast materials on dose calculation in radiotherapy planning using computed tomography for tumors at various anatomical regions: a prospective study. Radiother Oncol. 2007;84(1):52–55. [DOI] [PubMed] [Google Scholar]
- 11. Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer. 2012;118(8):2078–2084. [DOI] [PubMed] [Google Scholar]
- 12. Sugie C, Shibamoto Y, Ayakawa S, et al. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat. 2011;10(2):187–195. [DOI] [PubMed] [Google Scholar]
- 13. Manabe Y, Shibamoto Y, Sugie C, Hayashi A, Murai T, Yanagi T. Helical and static-port tomotherapy using the newly-developed dynamic jaws technology for lung cancer. Technol Cancer Res Treat. 2015;14(5):583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyatake K, Kubota K, Ogawa Y, Hamada N, Murata Y, Nishioka A. Non-surgical care for locally advanced breast cancer: radiologically assessed therapeutic outcome of a new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II) with systemic chemotherapy. Oncol Rep. 2010;24(5):1161–1168. [DOI] [PubMed] [Google Scholar]
- 15. Ogawa Y, Kubota K, Aoyama N, et al. Non-surgical breast-conserving treatment (KORTUC-BCT) using a new radiosensitization method (KORTUC II) for patients with stage I or II breast cancer. Cancers (Basel). 2015;7(4):2277–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol. 2015;10(6):960–964. [DOI] [PubMed] [Google Scholar]
- 17. Kestin L, Grills I, Guckenberger M, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol. 2014;110(3):499–504. [DOI] [PubMed] [Google Scholar]
- 18. Bi N, Shedden K, Zheng X, Kong FS. Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I non-small cell lung cancer: a systemic review and pooled analysis. Int J Radiat Oncol Biol Phys. 2016;95(5):1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shibamoto Y, Otsuka S, Iwata H, Sugie C, Ogino H, Tomita N. Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res. 2012;53(1):1–9. [DOI] [PubMed] [Google Scholar]
- 20. Rofstad EK, Wahl A, Brustad T. Radiation sensitivity in vitro of cells isolated from human tumor surgical specimens. Cancer Res. 1987;47(1):106–110. [PubMed] [Google Scholar]
- 21. Shibamoto Y, Shibata T, Miyatake S, et al. Assessment of the proliferative activity and radiosensitivity of human tumours using the cytokinesis-block micronucleus assay. Br J Cancer 1994;70(1):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ye JC, Formenti SC. Integration of radiation and immunotherapy in breast cancer—treatment implications. Breast. 2017;38:66–74. [DOI] [PubMed] [Google Scholar]
- 23. Iyer SP, Hunt CR, Pandita TK. Cross talk between radiation and immunotherapy: the Twain shall meet. Radiat Res. 2018;189(3):219–224. doi: 10.1667/RR14941.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandenabeele P, Vandecasteele K, Bachert C, Krysko O, Krysko DV. Immunogenic apoptotic cell death and anticancer immunity. Adv Exp Med Biol. 2016;930:133–149. [DOI] [PubMed] [Google Scholar]
- 25. Shibamoto Y, Yukawa Y, Tsutsui K, Takahashi M, Abe M. Variation in the hypoxic fraction among mouse tumors of different types, sizes, and sites. Jpn J Cancer Res. 1986;77(9):908–915. [PubMed] [Google Scholar]
- 26. Ogawa Y. Paradigm shift in radiation biology/radiation oncology—exploitation of the “H2O2 effect” for radiotherapy using low-LET (linear energy transfer) radiation such as X-rays and high-energy electrons. Cancers (Basel). 2016;8(3):E28 doi:10.3390/cancers8030028. [DOI] [PMC free article] [PubMed] [Google Scholar]