Abstract
Long QT syndrome (LQTS) is an inherited channelopathy which exposes athletes to a risk of sudden cardiac death. Diagnosis is more difficult in this population because: the QT interval is prolonged by training; and the extreme bradycardia frequently observed in athletes makes the QT correction formula less accurate. Based on limited clinical data which tend to demonstrate that exercise, especially swimming, is a trigger for cardiac events, participation in any competitive sports practice is not supported by 2005 European guidelines. However, based on recent retrospective studies and adopting a different medical approach, involving the patient-athlete in shared decision making, the 2015 US guidelines are less restrictive, especially in asymptomatic genotype-positive/phenotype-negative athletes. These guidelines also consider giving medical clearance to competitive sport participation in asymptomatic athletes with appropriate medical therapy.
Keywords: Long QT syndrome, arrhythmia, sudden death, sport, athlete
Congenital long QT syndrome (LQTS) is an inherited cardiac ion channelopathy characterised by a variable degree of QT interval prolongation on ECG and an increased susceptibility to life-threatening ventricular arrhythmias (torsades de pointes and ventricular fibrillation) in the absence of morphological cardiac disease.
LQTS is estimated to affect one in 2,000 individuals.[1] It is usually diagnosed in children and young adults, with a mean age at presentation of 14 years[2]; the annual rate of sudden cardiac death (SCD) in untreated patients is estimated to be between 0.33 %[3] and 0.9 %,[4] and the rate of syncope is 5 %.[4] Mutations in 17 genes have been associated with LQTS[5]. The subtypes of LQTS can be grouped into three categories:
Thirteen genes have been reported in autosomal dominant forms of Romano-Ward syndrome (LQT1–6 and 9–15), which are characterised by an isolated prolongation of the QT interval. Among them, LQT1 (KCNQ1 gene), LQT2 (KCNH2 gene) and LQT3 (SCN5A gene) account for the majority (75 %) of genetically identifiable cases.[6]
Two autosomal dominant forms of LQTS are associated with a phenotype extending beyond cardiac arrhythmia. In addition to the prolonged QT interval, associations include muscle weakness as well as facial dysmorphism in Andersen-Tawil syndrome (LQTS7) and hand/foot, facial and neurodevelopmental features in Timothy syndrome (LQTS8).
There are also two autosomal-recessive forms of LQTS (Jervell and Lange–Nielsen syndrome: JLN 1–2), which are associated with profound sensorineural hearing loss.[6]
There is a variable penetrance in patients with genotype-positive LQTS, resulting in variation in both clinical and ECG manifestations, as well as between family members with the same genotype.[7]
In general, the QT interval is longer in athletes than in non-athletic controls because of the lower resting heart rates associated with athletic training, while the corrected QT (QTc) of the athletic group is within normal limits, although toward the upper limit.[8–10]
Intense sports participation is considered to be a potential risk-taking behaviour for patients with LQTS in general and those with LQT1 in particular. Therefore, correct diagnosis and risk stratification is fundamental to advising patients appropriately about sports practice.
This review will discuss the effect of sport on QT prolongation, the best way to diagnose patients and to attempt accurate risk stratification in athletes. Finally, this study will discuss current evidence on competitive sports participation in athletes with LQTS.
Challenges of QT Measurement in Athletes
Studies have suggested that the ability of cardiologists and even heart rhythm specialists to accurately measure the QTc is suboptimal.[11] The accuracy of computer-generated QTc values is approximately 90 –95 %; the duration of the QT interval therefore should be measured manually from the beginning of the QRS complex to the end of the T wave.
The first difficulty is to define the end of the T wave. This is usually done by drawing the tangent line to the steepest part of the descending portion of the T wave, chosen in a lead where the T wave has the greatest amplitude, taking its intercept with the isoelectric line as the end of the T wave (Figure 1).[12,13] This ‘teach the tangent, avoid the tail’ method will help to exclude low amplitude U waves, which are common in athletes. QT interval should be preferably measured using lead II or V5; the longest value should be considered.
Figure 1: Measure of QT Using The ‘Teach the Tangent, Avoid-the-Tail’ Method.
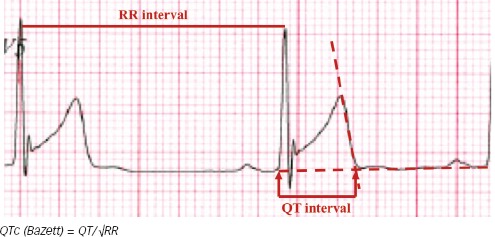
As the QT changes with the heart rate, there are several formulas to correct QT interval with the heart rate, the most used is Bazett’s formula – QTc = QT/√RR – using the RR interval preceding the QT interval measured.[14] As the majority of the available data for congenital LQTS are derived from studies using Bazett’s formula, this is still the recommended way to adjust QT in athletes.[15] Using Bazett’s formula, the QTc interval represents the value of the QT interval normalised for a heart rate of 60 BPM, i.e. a RR interval of 1000 ms. In case of a significant fluctuation in heart rate, as seen in respiratory arrhythmia, it is important to calculate the average QT interval and average RR interval to improve accuracy[15]. The response of the QT interval to a change in heart rate is not instantaneous, with full adaptation taking 1–3 minutes.[16]
Bazett’s formula has been criticised as inaccurate, especially at extreme heart rates of ≤40 BPM and >120 BPM[15]. At slow heart rates, which frequently occur in athletes (due to change in the automatic balance with a lower sympathetic activity and a higher vagal tone at rest), the QTc interval may be underestimated if Bazett’s formula is used (Figure 2).[2,15] If the heart rate is too slow, the ECG should therefore be repeated after a mild aerobic activity to achieve a heart rate closer to 60 BPM where the formula is most accurate; conversely, if the heart rate is too fast, repeating the ECG after a longer resting period should be considered. Holter ECG monitoring is also useful to measure QT interval at a stable heart rate of 60 BPM, where no adjustment for heart rate is needed.
Figure 2: QT Corrected Interval with Bazett’s Formula According to Heart Rate.
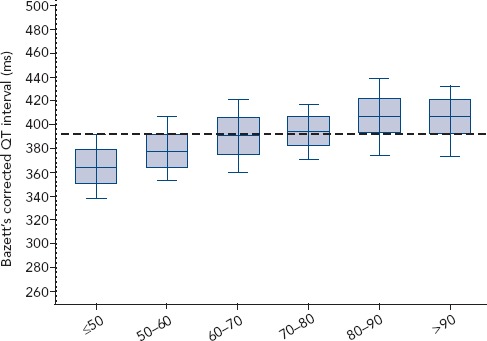
Source: personal data from a cohort of 5,092 French elite athletes
An alternative solution, to avoid using correction formulas, is to use QT/RR scatter diagrams obtained from individual athletes.[17] Using such a diagram might make it easier to measure the QT interval and corresponding RR value of an individual athlete to ascertain if they are in the normal range (Figure 3).
Figure 3: Scatter Diagram of QT/RR.
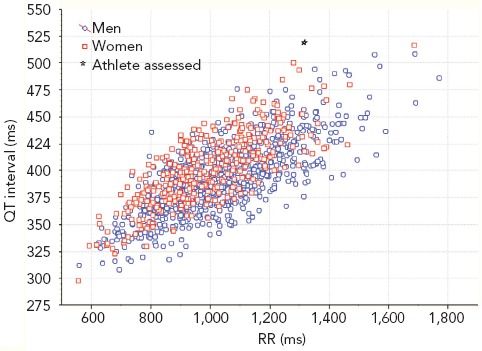
Source: personal data obtained from individual athletes
Which Cut-off Should be Used in Athletes?
Clinical observations have shown that training can increase and detraining decrease the QT interval duration in athletes.[8] Vagal stimulation is increased in athletes, which prolongs the QT interval, independently of the induced bradycardia.[12] An isolated long QT interval in an athlete may result from the effect of delayed repolarisation as a result of increased left ventricular mass.[10]
The cut-off values used to identify whether a QTC interval is prolonged vary in the literature. Incomplete penetration (i.e. where people who carry a genetic mutation do not show the pathological phenotype) has been clearly demonstrated in LQTS. Consequently, there is a remarkable overlap of QT values between normal subjects and those carrying an LQTS mutation at the upper values of QT distribution. Therefore, no screening programme will identify all persons with LQTS.[18,19] The QTc cut-off value used to decide whether further evaluation is needed must be chosen carefully to balance the frequency of abnormal results with the positive predictive value when LQTS is detected incidentally.
QT interval is modulated by sex so different cut-off values are used after puberty.[20] The latest ECG recommendations in athletes consider that QTc values of >470 ms in men and >480 ms in women are the thresholds of QT prolongation that warrant further assessment in asymptomatic athletes.[15] These cut-off values are around the 99th percentile and are consistent with thresholds defined by the 36th Bethesda Conference.[21]
However, not only the duration of QT interval should be considered. Recent European Society of Cardiology (ESC) guidelines[2] emphasised that a Schwartz score of >3 might also be used to diagnose LQTS.[22] This score includes QT interval duration on a resting ECG, occurrence of torsades de pointes, T wave alternans, morphology of the QT, low heart rate for age (which might be difficult to identify in an athlete); clinical history of symptoms such as syncope (especially if occurring during stress), congenital deafness; family history (definite LQTS or unexplained SCD below the age of 30 years among immediate family members) (Table 1).
Table 1: Schwartz score LQTS diagnostic criteria and their values in athletes.
| Schwartz Score | Points | Questionable in Athletes | |
|---|---|---|---|
| Electrocardiographic findings | |||
| A | QTc duration (ms) (Bazett formula) | ||
| ≥480 | 3 | ||
| 460–470 | 2 | x | |
| 450 (in males) | 1 | x | |
| B | Torsades de pointes* | 2 | |
| C | T-wave alternans | 1 | |
| D | Notched T wave in three leads | 1 | |
| E | Low heart rate for age | 0.5 | x |
| Clinical history | |||
| A | Syncope* | ||
| With stress | 2 | ||
| Without stress | 1 | ||
| B | Congenital deafness | 0.5 | |
| Family history | |||
| A | Family members with definite LQTS | 1 | |
| B | Unexplained sudden cardiac death below the age of 30 among immediate family members | 0.5 | |
* Mutually exclusive. Source: Schwartz PJ, et al.[22] With permission from Wolters Kluver.
LQTS is also diagnosed, irrespective of the QT duration, in the presence of a confirmed pathogenic LQTS mutation.
Complementary Exploration for LQTS Diagnosis
Before considering congenital LQTS as a diagnosis, acquired causes of prolongation of QT interval should be excluded. The most frequent causes are the use of QT-prolonging medication, metabolic changes and electrolyte disorders (such as hypokalemia).[23] This abnormality might also be encountered in athletes.
It might be valuable to repeat resting ECG measurements for several days, especially if the QT value is borderline. In endurance athletes, because of the frequent occurrence of bizarre T-wave shapes, it could be useful to repeat resting ECG after a period (2–4 weeks) of complete detraining.
Evaluating the QT dynamic can improve diagnostic accuracy in some patients. An exercise test is useful to identify ventricular arrhythmias related to LQTS and to assess the evolution of QT during recovery.[15] Indeed, a QT duration at 4 minutes of recovery from exercise stress above 480 ms is included in the 2011 update of the Schwartz score.[24–26] Holter ECG monitoring, which is useful to measure QT at a HR of 60 BPM to avoid having to use QT correction formulas, is also used to depict ventricular arrhythmias and to assess the dynamic evolution of QT.[27] Pharmacological tests might also be performed; a paradoxical increase of an uncorrected QT interval during infusion of low-dose epinephrine has been demonstrated in patients with LQT1.[28,29]
The response of the QT interval to the brief tachycardia provoked by standing suddenly is another way to assess QT dynamics.[30] Viskin et al. demonstrated that, in response to brisk standing, patients with LQTS and untrained control subjects responded with similar heart rates, but the response of the QT interval to this tachycardia differed. On average, the QT interval of controls shortened by 21±19 ms whereas the QT interval of LQTS patients increased by 4±34 ms (p<0.001). Since the RR interval shortened more than the QT interval, during maximal tachycardia the corrected QT interval increased by 50±30 ms in the control group and by 89±47 ms in the LQTS group (p<0.001). A similar experiment was performed by Pressler et al., which found that in healthy elite athletes, the QT interval shortened in all athletes by 40±17 ms, so QTc increased by 12±22 ms.[31] Unfortunately, the authors are not aware of a study that assessed athletes with LQTS.
As LQTS is hereditary, clinical familial screening should be considered if the condition is strongly suspected. Because penetrance varies,[7] it might be helpful to assess first degree relatives as well in case of borderline phenotype (with history, clinical examination and a resting ECG) to increase the diagnostic accuracy in the index athlete.
The Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) have issued a statement on genetic testing in LQTS.[6] They recommend genetic testing in any patient where there is a strong clinical suspicion for LQTS based on clinical history, family history and expressed electrocardiographic phenotype (resting 12–lead ECGs and/or provocative stress testing with exercise or catecholamine infusion). In a patient with a QTc>480 ms (prepuberty) or >500 ms (adults) on serial ECG analysis, genetic testing is recommended. In case of QTc values >460 ms (prepuberty) or >480 ms (adults), this might also be considered. As the HRS/EHRA guidelines are not specific to athletes, ECGs should be interpreted with common athlete-specific findings in mind.[15]
Nevertheless, LQTS genetic testing should not be performed systematically without any evidence of LQTS and should be interpreted with caution if diagnosis is borderline. Indeed, the significant rate of rare variants of uncertain significance in the LQT 1–3 genes complicates correct mutation identification and shows that LQTS genetic testing should be carried out based upon clinical suspicion rather than being ordered indiscriminately.[32] A cascade familial genetic screening should be performed if the index athlete has a positive genotype.
Risk Stratification in Athletes
Risk stratification is difficult, especially in the setting of competitive sport. In the general adult population, the most important predictors of outcome are QT interval duration (≥500 ms), male sex in childhood but female sex in adulthood, and a history of cardiac events including syncope.
Genotype is also associated with outcome and with different arrhythmia triggers. LQT1 genotype seems to be associated with a more positive prognosis, especially with a better response to beta-blocker therapy.[3,33,34] Exercise is the most important trigger of arrhythmia in this form of LQTS. Schwartz et al. demonstrated that exercise, especially swimming, was the trigger in 62 % of cardiac events in LQT1, 13 % of LQT2 and 13 % of LQT3. Furthermore, exercise was found to be the trigger of 68 % of lethal cardiac events in LQT1. Emotional stress and auditory stimuli were specific triggers of cardiac events in LQT2, and events were most likely to occur at sleep or at rest in LQT3.[35] These differences in triggers of cardiac events make competitive sport disqualification more questionable in LQT2–3.
Patients with LQT1 with malfunctioning IKs channels are expected to shorten their QT intervals during tachycardia less effectively than normal individuals. A major catecholamine release, as happens during intense exercise, without a proper QT adaptation sets the stage for early afterdepolarisations, which may then lead to torsades de pointes via re-entry.[35] This concept is supported by an experimental model for LQT1 in which IKs blockade greatly increases the probability of torsades de pointes in the presence of catecholamines. This study also demonstrated the protective effect of beta-blockers, as they prevent the actions of isoproterenol to increase transmural dispersion of repolarization and to induce torsades de pointes.[36]
The exact mechanism of the specific arrhythmogenic effect of swimming in people with LQT1 is unclear. The hypothesis is that autonomic conflict plays a role. The sympathetic nervous system is activated because of physical effort and the cold shock response, while the parasympathetic nervous system is activated by the diving response induced by face immersion and voluntary apnoea. This concomitant activation of both sympathetic and parasympathetic autonomic systems may explain why swimming seems to precipitate premature ventricular contractions.[37] Epinephrine QT stress testing[29] and cold-water face immersion[38] demonstrate a paradoxical prolongation in the QT interval in LQT1. Furthermore, the consequences of a syncope during swimming are more severe that one during dry land activities because of the risk of drowning.[39]
Because people with LQT1 are more susceptible to cardiac events during exercise than those with LQT2–3, genotype identification might be relevant to assess risks related to sports. The correlation between genotype and ST–T morphologies on the ECG is not always clear. Zhang et al. demonstrated that typical ST–T patterns were present in 88 % of LQT1 and LQT2 gene carriers but in only in 65 % of those carrying LQT3; with ECG analysis, the mean sensitivity/specificity for LQT1, LQT2 and LQT3 was 61 %/71 %, 62 %/87 % and 33 %/98 % respectively[40]. Therefore, genotyping might be used to improve risk stratification, keeping in mind that in about one-third of cases there is a failure to identify mutations.[40] Complicating our interpretation of genotype is the complexity of variable penetrance and modifier genes that are relatively poorly understood, which may account for the pleiotropy between different families with the same mutation.
Nevertheless, recent retrospective studies temper previous conclusions. Johnson and Ackerman demonstrated the absence of any lethal sport-related event in a cohort of 353 athletes whose LQTS syndrome was well managed. However, the majority of patients did not participate in competitive sports or chose to discontinue sport (63 %). The remaining 130 patients (37 %) chose to continue competitive sports; most of them (87 %) were treated with beta-blockers and 20 (15 %) had an ICD implanted. Just one 9-year-old child experienced two sport-related events and an appropriate ICD shock was delivered; there was non-adherence to beta-blocker medication.[41]
Another retrospective study was conducted by Chambers et al.,[42] in 172 children with LQTS, of whom 66 (38 %) exercised on a recreational basis and 106 (62 %) competitively. No syncopal events were reported during competitive exercise, and no cardiac arrests or deaths were reported during recreational or competitive exercise, but four patients experienced exertional syncope during recreational sport. The same results were demonstrated by Aziz et al. on a retrospective cohort of 103 children with LQTS involved in recreational (75 %) or competitive sport practice (25 %). No patients experienced LQTS symptoms during sports participation.[43]
These studies had limitations. They were retrospective, involved young subjects, and sports with the highest cardiovascular demand were poorly represented (only a few class IIIB and IIIC sports), with few subjects practising at a national/professional level. Assessing the suitability of competitive/professional sport to young adults with LQTS might be a different task from advising children regarding participation in normal sport activities.
As these studies state, optimal treatment is warranted. As the ESC guidelines recommend, beta-blockers are the cornerstone of treatment and are recommended in patients with a diagnosis of LQTS; they should also be considered in carriers of a causative LQTS mutation who have a normal QT interval. ICD implantation with the use of beta-blockers is recommended in those with LQTS with previous cardiac arrest, and should be considered in patients who have experienced syncope and/or ventricular tachycardia while receiving an adequate dose of beta-blockers.[2]
Eligibility for Competitive Sport Participation According to European and US Guidelines
Several guidelines on the eligibility of athletes with LQTS for competitive sport exist, and conclusions by different experts are not the same on both sides of the Atlantic.
The ESC recommendations for competitive sport participation, published in 2005, are the most restrictive[44]. These state that congenital LQTS is a contraindication for any type of sports, even without documented major arrhythmic events. In 2015, ESC guidelines for the management of ventricular arrhythmia and prevention of SCD recommended the avoidance of strenuous swimming, especially in LQT1, but no other kinds of sports were mentioned.[2]
The more recent US guidelines on suitability and disqualification recommendations for competitive athletes in cardiac channelopathies, proposed in 2015, are less restrictive.[45] Of course, experts recommend symptomatic athletes should not compete and that a comprehensive evaluation should be performed by a heart rhythm specialist or genetic cardiologist with sufficient experience and expertise with LQTS. For an athlete with symptomatic LQTS or an ECG with manifest LQTS, competitive sports participation (except competitive swimming in a previously symptomatic person with LQT1) may be considered after treatment has been implemented and appropriate precautionary measures taken, assuming the athlete has been asymptomatic on treatment for at least 3 months. Athletes and their families should be given information on the potential risks of competitive sports participation.
In asymptomatic genotype-positive/phenotype-negative athletes, the experts concluded that it was reasonable for them to participate in all competitive sports as long as they took precautionary measures. These include the avoidance of QT-prolonging drugs, electrolyte/hydration replenishment and avoidance of dehydration, avoidance or treatment of training-related heat exhaustion or heat stroke, as well as acquiring a personal automatic external defibrillator (AED) and establishing an emergency action plan with school or team officials. Pundi et al. evaluated retrospectively the efficacy of AEDs for prevention of sudden cardiac arrest in children with LQTS. The rate of needing an external defibrillator rescue was relatively low (three AED rescues in 1,700 patient-years). Irrespective of the external defibrillator used (personal, community or hospital AED) or the person delivering the shock (medical provider or parent/school personnel), the AED was successful in recognising and treating the LQTS-triggered ventricular arrhythmia appropriately.[46] Furthermore, Drezner et al. demonstrated that survival rates were higher in schools with an established emergency action plan for sudden cardiac arrest than in those without (79 % against 44 %; odds ratio 4.6) and if an onsite AED was used compared to an offsite AED provided by emergency medical services (80 % versus 50 %; OR 4.0).[47]
Why are the guidelines so different? It may be argued that the European guidelines were written before the most recent studies on LQTS and sport.[41,42] The variation might also reflect a cultural contrast between the US and Europe regarding personal freedom to pursue life’s goals versus the role of the state to ensure the safety of its population.[48] What is acceptably safe is not solely a medical decision – it is also a social and ethical question.[49] In some European countries, for example France or Italy, physicians are asked to certify that an athlete is fit to compete without any restriction. This legal position will lead to a paternalistic model, where the athlete’s autonomy is limited to what the physician will consider for the good of the patient, regardless of the will of the athlete. In the US, the physician is asked for medical advice on risk stratification, and the athlete is responsible for the final decision after having received thorough information, which lead to a more modern shared decision-making or informed decision model.[50] In the US, under the latest US guidelines, the return to play of an athlete with LQTS will have to involve the school and or team official, who can refuse to accept a medical decision.[51]
Conclusion
There is still only a paucity of firm prospective data that can guide practitioners in making decisions about sports participation in athletes with LQTS. Nevertheless, current ESC guidelines are probably too restrictive. In the era of precision medicine, the recommendation of disqualifying every single athlete with LQTS athlete, regardless of any risk stratification or even genotype-positive/phenotype-negative athletes, is probably too restrictive.
Clinical Perspective
Appropriate LQTS cut-off values in athletes are 470 ms in men and 480 ms in women
Beta-blockers are the cornerstone therapy for patients with a clinical diagnosis of LQTS and should also be considered in genotype-positive/phenotype-negative patients.
A comprehensive evaluation, with risk stratification according to age, sex, genotype and symptoms should be performed, with referral to a cardiologist with specialist skills in LQTS and/or sports cardiology.
Current European guidelines (2005) recommend that patients with LQTS should be excluded from any competitive sports. The more recent US guidelines (2015) are less restrictive, especially in athletes who are genotype-positive/phenotype-negative and asymptomatic.
References
- 1.Schwartz PJ, Stramba-Badiale M, Crotti L et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–7. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;2015;36:2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Schwartz PJ, Napolitano C et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Schwartz PJ, Crampton RS et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–44. doi: 10.1161/01.CIR.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 5.Nakano Y, Shimizu W et al. Genetics of long-QT syndrome. J Hum Genet. 2016;61:51–5. doi: 10.1038/jhg.2015.74. [DOI] [PubMed] [Google Scholar]
- 6.Ackerman MJ, Priori SG, Willems S et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. Heart Rhythm. 2011;8:1308–39. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Priori SG, Napolitano C, Schwartz PJ et al. Low penetrance in the Long-QT syndrome: clinical impact. Circulation. 1999;99:529–33. doi: 10.1161/01.CIR.99.4.529. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano C, Bloise R, Priori SG et al. Long QT syndrome and short QT syndrome: how to make correct diagnosis and what about eligibility for sports activity. J Cardiovasc Med. 2006;7:250–6. doi: 10.2459/01.JCM.0000219317.12504.5f. [DOI] [PubMed] [Google Scholar]
- 9.Kapetanopoulos A, Kluger J, Maron BJ et al. The congenital long QT syndrome and implications for young athletes. Med Sci Sports Exerc. 2006;38:816–25. doi: 10.1249/01.mss.0000218130.41133.cc. [DOI] [PubMed] [Google Scholar]
- 10.Basavarajaiah S, Wilson M, Whyte G et al. Prevalence and significance of an isolated long QT interval in elite athletes. Eur Heart J. 2007;28:2944–9. doi: 10.1093/eurheartj/ehm404. [DOI] [PubMed] [Google Scholar]
- 11.Viskin S, Rosovski U, Sands AJ et al. Inaccurate electrocardiographic interpretation of long QT: The majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569–74. doi: 10.1016/j.hrthm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Funck-Brentano C, Jaillon P et al. Rate-corrected QT interval: techniques and limitations. Am J Cardiol. 1993;72:17B–22B. doi: 10.1016/0002-9149(93)90035-B. [DOI] [PubMed] [Google Scholar]
- 13.Lepeschkin E, Surawicz B et al. The measurement of the Q-T interval of the electrocardiogram. Circulation. 1952;6:378–88. doi: 10.1161/01.CIR.6.3.378. [DOI] [PubMed] [Google Scholar]
- 14.Bazett HC et al. An analysis of the time relations of electrocardiograms. Heart. 1920;7:355–70. [Google Scholar]
- 15.Sharma S, Drezner JA, Baggish A et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2017;39:1466–80. doi: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 16.Toivonen L et al. More light on QT interval measurement. Heart. 2002;87:193–4. doi: 10.1136/heart.87.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik M, Färbom P, Batchvarov V et al. Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart. 2002;87:220–8. doi: 10.1136/heart.87.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43:657–62. doi: 10.1136/bjsm.2008.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taggart NW, Haglund CM, Tester DJ et al. Diagnostic miscues in congenital long-QT syndrome. Circulation. 2007;115:2613–20. doi: 10.1161/CIRCULATIONAHA.106.661082. [DOI] [PubMed] [Google Scholar]
- 20.Rautaharju PM, Zhou SH, Wong S et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiology. 1992;8:690–5. [PubMed] [Google Scholar]
- 21.Maron BJ, Zipes DP et al. Eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005;45:1318–1375. doi: 10.1016/j.jacc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz PJ, Moss AJ, Vincent GM et al. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88:782–4. doi: 10.1161/01.CIR.88.2.782. [DOI] [PubMed] [Google Scholar]
- 23.Ernstene AC, Proudfit WL et al. Differentiation of the changes in the Q-T interval in hypocalcemia and hypopotassemia. Am Heart J. 1949;38:260–72. doi: 10.1016/0002-8703(49)91334-4. [DOI] [PubMed] [Google Scholar]
- 24.Chattha IS, Sy RW, Yee R et al. Utility of the recovery electrocardiogram after exercise: a novel indicator for the diagnosis and genotyping of long QT syndrome? Heart Rhythm. 2010;7:906–11. doi: 10.1016/j.hrthm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Sy RW, van der Werf C, Chattha IS et al. Derivation and validation of a simple exercise-based algorithm for prediction of genetic testing in relatives of LQTS probands. Circulation. 2011;124:2187–94. doi: 10.1161/CIRCULATIONAHA.111.028258. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Crotti L et al. QTc Behavior during exercise and genetic testing for the Long-QT Syndrome. Circulation. 2011;124:2181–4. doi: 10.1161/CIRCULATIONAHA.111.062182. [DOI] [PubMed] [Google Scholar]
- 27.Mauriello DA, Johnson JN, Ackerman MJ et al. Holter Monitoring in the evaluation of congenital long QT syndrome. Pacing Clin Electrophysiol. 2011;34:1100–4. doi: 10.1111/j.1540-8159.2011.03102.x. [DOI] [PubMed] [Google Scholar]
- 28.Vyas H, Hejlik J, Ackerman MJ et al. Epinephrine QT stress testing in the evaluation of congenital Long-QT syndrome: diagnostic accuracy of the paradoxical QT response. Circulation. 2006;113:1385–92. doi: 10.1161/CIRCULATIONAHA.105.600445. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu W, Noda T, Takaki H et al. Diagnostic value of epinephrine test for genotyping LQT1, LQT2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm. 2004;1:276–83. doi: 10.1016/j.hrthm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Viskin S, Postema PG, Bhuiyan ZA et al. 1 The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55:1955–61. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pressler A, Vogel A, Scherr J et al. Applying the ‘Viskin test’: QT interval in response to standing in elite athletes. Int J Cardiol. 2012;154:93–4. doi: 10.1016/j.ijcard.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 32.Kapa S, Tester DJ, Salisbury BA et al. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation. 2009;120:1752–60. doi: 10.1161/CIRCULATIONAHA.109.863076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer AJ, Moss AJ, McNitt S et al. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329–37. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 34.Goldenberg I, Moss AJ et al. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz PJ, Priori SG, Spazzolini C et al. genotype-phenotype correlation in the long-qt syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.CIR.103.1.89. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu W, Antzelevitch C et al. Cellular basis for the ECG features of the LQT1 form of the Long-QT syndrome: effects of β-Adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–22. doi: 10.1161/01.CIR.98.21.2314. [DOI] [PubMed] [Google Scholar]
- 37.Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol. 2012;590:3219–30. doi: 10.1113/jphysiol.2012.229864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshinaga M, Kamimura J, Fukushige T et al. Face immersion in cold water induces prolongation of the QT interval and T-wave changes in children with nonfamilial long QT syndrome. Am J Cardiol. 1999;83:1494–7. doi: 10.1016/S0002-9149(99)00131-9. [DOI] [PubMed] [Google Scholar]
- 39.Choi G, Kopplin LJ, Tester DJ et al. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation. 2004;110:2119–24. doi: 10.1161/01.CIR.0000144471.98080.CA. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Timothy KW, Vincent GM et al. Spectrum of ST-T–Wave patterns and repolarization parameters in congenital Long-QT syndrome: ECG findings identify genotypes. Circulation. 2000;102:2849–55. doi: 10.1161/01.CIR.102.23.2849. [DOI] [PubMed] [Google Scholar]
- 41.Johnson JN, Ackerman MJ et al. Return to play? Athletes with congenital long QT syndrome. Br J Sports Med. 2012;47:28–33. doi: 10.1136/bjsports-2012-091751. [DOI] [PubMed] [Google Scholar]
- 42.Chambers KD, Beausejour Ladouceur V, Alexander ME et al. Cardiac events during competitive, recreational, and daily activities in children and adolescents with long QT syndrome. J Am Heart Assoc. 2017;6((9)):e005445. doi: 10.1161/JAHA.116.005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aziz PF, Sweeten T, Vogel RL et al. Sports participation in genotype positive children with long QT syndrome. JACC Clin Electrophysiol. 2015;1:62–70. doi: 10.1016/j.jacep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelliccia A, Fagard R, Bjørnstad HH et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–45. doi: 10.1093/eurheartj/ehi325. [DOI] [PubMed] [Google Scholar]
- 45.Ackerman MJ, Zipes DP, Kovacs RJ et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 10: the cardiac channelopathies. J Am Coll Cardiol. 2015;66:2434–28. doi: 10.1016/j.jacc.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 46.Pundi KN, Bos JM, Cannon BC et al. Automated external defibrillator rescues among children with diagnosed and treated long QT syndrome. Heart Rhythm. 2015;12:776–81. doi: 10.1016/j.hrthm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Drezner JA, Toresdahl BG, Rao AL et al. Outcomes from sudden cardiac arrest in US high schools: a 2-year prospective study from the national registry for AED use in sports. Br J Sports Med. 2013;47:1179–83. doi: 10.1136/bjsports-2013-092786. [DOI] [PubMed] [Google Scholar]
- 48.Pew Research Center. The American-western European values gap. Pew Research Centers Global Attitudes Project. 2011. pp. 1–25.
- 49.Pelliccia A et al. Long QT syndrome, implantable cardioverter defibrillator (ICD) and competitive sport participation: when science overcomes ethics. Br J Sports Med. 2014;48:1135–6. doi: 10.1136/bjsports-2013-092441. [DOI] [PubMed] [Google Scholar]
- 50.Providencia R, Teixeira C, Segal OR et al. Empowerment of athletes with cardiac disorders: a new paradigm. Europace. 2017;339:1623–9. doi: 10.1093/europace/eux268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turkowski KL, Bos JM, Ackerman NC et al. Return-to-play for athletes with genetic heart diseases. Circulation. 2018;137:1086–8. doi: 10.1161/CIRCULATIONAHA.117.031306. [DOI] [PubMed] [Google Scholar]


