Abstract
Parental reflective functioning refers to the capacity for a parent to understand their own and their infant’s mental states, and how these mental states relate to behavior. Higher levels of parental reflective functioning may be associated with greater sensitivity to infant emotional signals in fostering adaptive and responsive caregiving. We investigated this hypothesis by examining associations between parental reflective functioning and neural correlates of infant face and cry perception using event-related potentials (ERPs) in a sample of recent mothers. We found both early and late ERPs were associated with different components of reflective functioning. These findings suggest that parental reflective functioning may be associated with the neural correlates of infant cue perception and further support the value of enhancing reflective functioning as a mechanism in parenting intervention programs.
Keywords: parental reflective functioning, infant faces, infant cries, EEG/ERP
Parental reflective functioning represents the capacity of a parent to recognize and understand their infant’s mental states, and how these mental states may relate to behavior. Further, this reflective capacity encompasses both how parents understand their own mental states in the parenting role, and the interplay between their own and their child’s mental states and behavior (Slade, 2005, 2007). Emerging evidence suggests parental reflective functioning may be associated with tolerance of infant distress (Rutherford, Goldberg, Luyten, Bridgett, & Mayes, 2013), parents’ communicative abilities with their infant (Grienenberger, Kelly, & Slade, 2005), and their infant’s attachment security (Slade, Grienenberger, Bernbach, Levy, & Locker, 2005). Therefore, the quality of parental reflective functioning may have multiple significant consequences for both the parent and developing child. For this reason, intervention programs have been developed to foster reflective functioning in mothers to improve the quality of the dyadic relationship (Sadler et al., 2013; Suchman, Decoste, Castiglioni, Legow, & Mayes, 2008). Importantly, parental reflective functioning may support adaptive responding to infant affective cues (Slade, 2005, 2007), which can be measured using both behavioral and brain indices. In this study, we examined the association between this critical parenting faculty and neural sensitivity to infant affective cues in recent mothers.
Reflective functioning was initially conceptualized as a general capacity that underscored the recognition and interpretation of mental states and their interplay with behavior (Fonagy, 1991). Advances of this theory recognized the uniqueness of reflective functioning within parenting contexts (Slade, 2005, 2007). Specifically during the postpartum period, mother-infant communication is limited to a non-verbal level and requires sensitivity to, and interpretation of, infant emotional signals, mental states, and behaviors in a qualitatively different way to other social interactions (Luyten, Fonagy, Lowyck, & Vermote, 2012). Recognizing the broader conceptualization of reflective functioning, the development of more specific measurement tools became necessary to assess this critical capacity. To date, parental reflective functioning has typically been assessed from attachment (Fonagy, Steele, Steele, & Target, 1998) and parenting (Slade, Bernbach, Grienenberger, Levy, & Locker, 2002) interviews. The advantages of these interview-based approaches are the breadth and richness in the qualitative information they provide on the emerging dyadic relationship; however, these interviews are disadvantaged in their applicability to large samples given their need for administration, transcription, and coding, as well as their potential to overlook the multidimensional nature of reflective functioning (Choi-Kain & Gunderson, 2008; Fonagy & Luyten, 2009; Luyten et al., 2012).
In the current study, we employed the Parental Reflective Functioning Questionnaire (PRFQ; (Luyten, Mayes, Nijssens, & Fonagy, under review) - a recently developed reliable and valid self-report measure of parental reflective functioning. The PRFQ identifies three components of parental reflective functioning that are measured across three subscales, encapsulating the multidimensional nature of this capacity. The first assesses parents’ interest and curiosity in their infant’s mental states, with increasing levels thought to be central to mother’s adopting a reflective stance during interactions with her infant. The second subscale assesses the certainty of parents in their understanding of their infant’s mental states - with increasing levels indicating greater certainty in mother’s interpretation of their infant’s mental states. The third subscale captures pre-mentalizing or non-mentalizing modes, which includes developmentally insensitive or malevolent interpretations of infant’s mental states. The PRFQ has been associated with infant attachment security, parental emotional availability, and parenting stress and distress (Luyten et al., under review; Rutherford et al., 2013), and therefore represents a valuable self-report measure of parental reflective functioning.
It is widely held that sensitive and appropriate maternal responding to their infant’s socio-emotional signals may have lasting consequences for their child’s health and well-being (Ainsworth, 1979). Consequently, parental reflective functioning may support sensitivity in detecting and responding to infant affective cues. Consistent with this notion, in an attachment-based intervention targeting parental reflective functioning in at-risk mothers, higher levels of reflective functioning about the self were associated with increased behaviorally-coded maternal sensitivity toward the child (Suchman, DeCoste, Leigh, & Borelli, 2010). More recently, neuroimaging methods have been employed in the assessment of sensitivity to infant cues to extend the longstanding behavioral observational literature. These neuroimaging studies have typically examined neural sensitivity to familiar and unfamiliar infant facial expressions and vocalizations in parent and non-parent samples (Rutherford & Mayes, 2011; Swain, 2011). In particular, measuring event-related potentials (ERPs) has been particularly useful in delineating the neural processing of infant affective cues. This approach is advantageous in providing temporally sensitive insight into stages of processing infant cues that may vary across maternal samples (Maupin, Hayes, Mayes, & Rutherford, 2015), and is an approach that strongly resonates with the intuitive accounts of parenting where sensitive parental responding is so rapid it may be below conscious awareness (Papousek, 2000).
Interpretation of ERP studies of infant cue processing relate to the relative sizes or amplitudes of neural responses elicited by infant faces or cries, with larger amplitudes typically indicative of greater sensitivity and allocation of attention to these stimuli (Maupin et al., 2015). To date, ERP studies have provided insight into the stage of stimulus processing that may be affected by infant-driven factors (e.g., emotional expressions, familiarity) and parent-driven factors (e.g., parent vs. non-parent) when perceiving infant cues. This has included ERP studies examining very early perceptual processing of infant vocalizations and facial expressions, namely the N100 and N170. With respect to infant cry perception, maternal and non-maternal samples have been exposed to infant cry and control (i.e., a word) stimuli, and the N100 elicited by these stimuli was modulated by both the nature of the stimulus (cry vs. word) and maternal status (mother vs. non-mother; (Purhonen, Kilpeläinen-Lees, et al., 2001; Purhonen, Valkonen-Korhonen, & Lehtonen, 2008)). Further, with respect to early face perception, the N170 elicited by infant faces has also showed differences by maternal status (Proverbio, Brignone, Matarazzo, Del Zotto, & Zani, 2006). However, inconsistent results exist regarding the modulation of the N170 by infant emotional expression (Noll, Mayes, & Rutherford, 2012; Proverbio et al., 2006).
Later ERP components relating to infant cue perception have included the P300 - an ERP component that is associated with attentional engagement and continued processing of salient stimuli (Luck, 2005; Ritter & Ruchkin, 1992). Infant familiarity is associated with larger P300 amplitudes (Grasso, Moser, Dozier, & Simons, 2009), and the amplitude of the P300 seems to increase in mothers with greater time and experience with their child (Bick, Dozier, Bernard, Grasso, & Simons, 2013). ERP research has also been used to examine how variation in maternal psychopathology may modulate neural reactivity to infant cues (Doi & Shinohara, 2012; Malak, Crowley, Mayes, & Rutherford, 2015; Noll et al., 2012), as well as differential sensitivity to infant emotional cues in mothers with a history of substantiated neglect toward their child (Rodrigo et al., 2011). Therefore, ERPs may be particularly valuable in probing sensitivity to infant cues in maternal samples.
In the present study, we sought to investigate whether responding on the PRFQ was associated with ERP responses to infant affective cues. Any relationship would be the first evidence of an association between parental reflective functioning and the neural correlates of infant cue perception. Our sample consisted of 63 mothers assessed at 6 months postpartum. These women were presented with unfamiliar infant faces (happy, distress, and neutral expressions) and infant cries (high-distress, low-distress, and a neutral tone), and we examined both early (N170, N100) and late (P300) ERP components elicited by these stimuli. Our inclusion of multiple ERP components allowed the opportunity to investigate at what stage of infant cue processing associations with parental reflective functioning may emerge. Given the cognitive nature of parental reflective functioning, we hypothesized that higher levels of reflective functioning would be associated with the P300 elicited by infant affective cues. However, PRFQ associations with the N100 and N170 would suggest parental reflective functioning might be more generally associated with increased sensitivity to infant emotional signals and influence more stimulus-driven processes.
Methods
Participants
All procedures were approved by the Yale University School of Medicine Human Investigations Committee. Sixty-three mothers (Mean Age = 29 years, SD = 5 years; 32 primiparous; 29 multiparous; 2 did not report parity) were recruited through flyers posted in the local community as part of a larger study on maternal sensitivity to infant cues that involved study visits at 3 months and 6 months postpartum. The women included in this sample completed the PRFQ and EEG session at approximately 6 months postpartum; however, 9 (14%) mothers in the sample completed the PRFQ at the earlier 3 months postpartum visit. The results were comparable with these women included and excluded; therefore, we kept these mothers in the analyses. We employed education in years (M=15 years; SD=3 years) as our proxy for socioeconomic status (Landi, Crowley, Wu, Bailey, & Mayes, 2012; Mayes & Bornstein, 1995). With respect to marital status, 26 mothers were single, 31 were married, 1 mother was divorced, and 5 mothers did not report. Maternal self-reported race/ethnicity was: Caucasian (n=31), African American (n=14), Hispanic/Latino (n=6), Asian American (n=2), Caucasian/African American (n=1), Caucasian/American Indian (n=1), Caucasian/Native Hawaiian (n=1), multi- racial (n=1), and 6 mothers did not report. Mothers were compensated $50 for their participation and received a toy for their infant.
Apparatus and Stimuli
EEG was recorded by Net Station 4.2.1 and 250 Hz sampling rate with high impedance amplifiers (0.1 Hz high-pass, 100 Hz low-pass). Prior to application, a 128 Ag/AgCl electrode sensor net (Electrical Geodesics, Inc.) was soaked in a warm potassium chloride solution. Net electrodes evenly covered the scalp from nasion to inion and from left to right ear. During recording, electrodes were referenced to Cz and impedances were kept below 40 kΩ. E-Prime 1.2 (Schneider, Eschman, & Zuccolotto, 2002) was used to present stimuli on a Pentium-IV computer controlling a 51-cm color monitor (75Hz, 1024 by 768 resolution). Data were collected in a sound-attenuated room with low ambient illumination. Viewing distance was approximately 70 cm.
Stimuli were infant faces and cries that were provided from a former study (Strathearn & McClure, 2002) and have been validated for their emotional content (Landi et al., 2011). Infant cries were 2-second cry samples from healthy infants aged 27–32 days (Gustafson & Green, 1989). From these samples, two high-distress cries and two low-distress cries (one from each infant) were selected (see Appendix for the acoustic properties of cries). A third neutral (220-hz pure tone) auditory stimulus was presented to contrast with the cries. Infant face stimuli (7.63° by 8.07°) included twenty-one colored photographs of unfamiliar happy, neutral, and distressed (i.e., frustration and sadness) faces taken from six infants aged 5–10 months which were balanced for gender and race (Caucasian and African American - reflecting the predominant community demographic). Infant faces were all presented on a black background.
Design and Procedure
A trial sequence consisted of a central fixation cross, stimulus presentation (500 ms for infant faces, 2000 ms for infant cries and tone), and a blank screen. Fixation and blank screen presentations were jittered between 1400–2000 ms. The task consisted of 42 trials for each infant face condition (happy, distressed, neutral) and 42 trials of high-distress cries, 44 trials of low- distress cries, and 40 trials of the neutral tone. There were 42 additional catch trials that were not included in the ERP analysis. During these catch trials, a “?” appeared instead of a fixation cross. This cue prompted participants to respond via button press as to whether the following stimulus was the same or different from the stimulus that was presented on the previous trial. Accuracy was 95% (SD = 0.06%). Mothers completed 8 practice trials prior to the 294 experimental trials that were presented quasi-randomly (7 blocks of 42 trials) with the order of stimulus presentation kept constant. This face-cry paradigm took 30 minutes to complete. The PRFQ was completed after completion of the EEG session.
Measures
The Parental Reflective Functioning Questionnaire (PRFQ; (Luyten et al., under review)) is an 18-item assessment of reflective functioning that was designed to capture parental interest and awareness of their child’s mental states and the relationship between mental states and behavior. There are three subscales: (1) the “interest and curiosity” subscale captures parental interest in their infant’s mental states (e.g., “I am often curious to find out how my child feels”); (2) the “certainty” subscale assesses whether parents recognize that mental states are not transparent (e.g., “I always know why my child acts the way he or she does”); and (3) the “pre-mentalizing” subscale measures non-mentalizing modes (e.g., “When my child is fussy he or she does that just to annoy me”). Each item is rated on a 7-point likert scale, where “1” represents “strongly disagree” and “7” represents “strongly agree”. The measure has good internal consistency across the pre-mentalizing ( =.70), certainty ( =.82) and interest and curiosity ( =.74) subscales, and this three-subscale structure has been replicated in two parental samples and holds for both mothers and fathers (Luyten et al., under review). With respect to construct validity, these subscales are associated with parental and infant attachment, parental emotional availability, and parenting stress and distress (Luyten et al., under review; Rutherford et al., 2013). In this sample, scores for each subscale were: pre-mentalizing (M=1.65, Range=1–4.83), certainty (M=4.23, Range=1.83–6), and interest and curiosity (M=5.92, Range=3–7). Higher scores are indicative of higher levels of each of these aspects of reflective functioning as captured by the PRFQ. Parity (primparous, multiparous) did not differentiate any of the PRFQ subscale scores, p’s>.58, suggesting levels of reflective functioning as measured by the PRFQ were unaffected by this parenting experience variable.
Data Analysis
Net Station 4.5 was used to pre-process EEG data. All files were digitally filtered with a 30 Hz low-pass filter, and segmented into one-second epochs (100 ms before and 900 ms after stimulus onset). Spline interpolation was used to replace any channels with artifacts in more than 40% of trials. Artifact detection was: 200 μν for bad channels, 150 μν for eye-blinks, and 150 μν eye-movements. EEG data were re-referenced to the average reference of all electrodes and baseline-corrected to the 100 ms interval prior to stimulus onset. EEG data were then averaged across each stimulus condition for each participant. Ocular Artifact Removal (OAR; (Gratton, Coles, & Donchin, 1983)), using a blink slope threshold = 14 μV /ms, was applied where participants had fewer than 21 blink and other artifact free trials per condition (n=15). At completion of preprocessing, there were on average 35 trials per condition (SD=4 trials).
N100 (M=87ms - M=171ms) and N170 (M=129ms - M=199ms) time-windows were derived and customized for each participant. The N100 was examined and averaged across 16 central electrode sites (CZ, 6, 7, 13, 31, 32, 38, 54, 55, 62, 80, 81, 88, 106, 107, 113) following visual inspection and existing N100 and infant cry literature (Purhonen, Kilpeläinen-Lees, et al., 2001; Purhonen, Pääkkönen, Yppärilä, Lehtonen, & Karhu, 2001). The N170 was assessed and averaged at 6 electrodes over the left (58, 59, 64, 65, 69, 70) and right (90, 91, 92, 95, 96, 97) posterior scalp sites. These sites overlap with the 10/20 electrode sites T5 and T6 previously used to assess the N170 (Bentin, Allison, Puce, Perez, & McCarthy, 1996), and sites employed in other high-dense array EEG N170 and parenting studies (Malak et al., 2015; Noll et al., 2012). We examined P300 peak amplitude at six electrode sites clustered around Pz (54, 61, 62, 68, 79, 80) consistent with other parenting research (Grasso et al., 2009; Proverbio et al., 2006) during the time window of 200 – 600 ms post-stimulus presentation (Mean Peak Onset=349ms; SD=84ms).
Our data analysis plan first employed a repeated measures analysis of variance (ANOVA) to assess modulation of the N100, N170, and P300 by our independent variables. For the N100 and P300, the within-subjects factors were either auditory stimulus (high-distress cry, low-distress cry, neutral tone) or emotional expression (happy, distress, neutral). For the N170, the within-subjects factors were emotional expression (happy, distress, neutral) and hemisphere (left, right). We next examined the correlations between the PRFQ subscales and the amplitude of the N100, N170, and P300. We then conducted a repeated measures analysis of covariance (ANCOVA) including the PRFQ subscale as a covariate if it was found to be associated with the ERP measures. Effect size is presented as partial eta-squared ( 2partial), where .01 represents a small, .06 represents a medium, and .14 represents a large, effect size (Cohen, 1988). The alpha level was defined as p<.05.
Results
Infant Cries
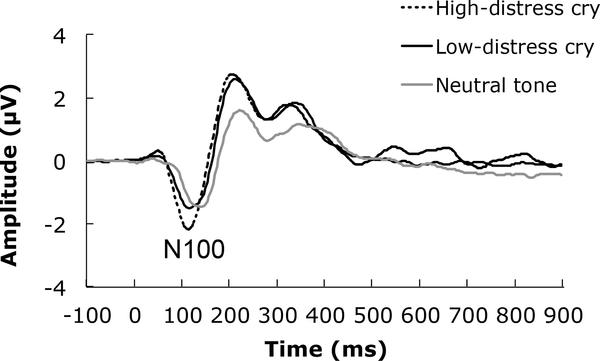
N100. Data from one participant were removed from the N100 analysis given that her mean N100 response was more than 3 SD from the group mean in the neutral tone condition. The grand averaged data for each auditory stimulus are presented in Figure 1. There was a main effect of auditory stimulus, F(2,122) = 12.26, p<.001, 2partial = .17. The N100 was largest in response to the high-distress cry (M=−3.52 μV) relative to the low-distress cry (M=−2.85 μV; t(61)=−4.58,p<.001) and neutral tone (M=−2.84 μV; t(61)=−4.06,p<.001). There was no difference in the N100 elicited by the low-distress cry and neutral tone condition, t<1. We found no correlations between any of the PRFQ subscales and the N100 elicited by each of the auditory stimuli, p’s > .07, r’s < =.24. The N100 does not seem to be reliably associated with the PRFQ subscales.
Figure 1.
Grand averaged ERP waveforms representing the N100 response to infant cries (high-and low-distress) and the neutral tone.
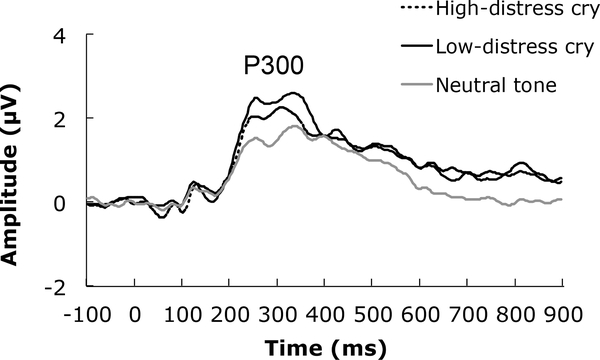
P300. Data from one participant were removed from the auditory P300 analysis given that her mean P300 response was more than 3 SD from the group mean for the high-distress cry condition. The grand averaged P300 data for each auditory stimulus condition are presented in Figure 2. There was a main effect of auditory stimulus, F(2,122) = 10.79, p<.001, 2partial = .15. Relative to the neutral tone (M=3.40 μV), the P300 was larger in response to high-distress (M=4.03 μV; t(61)=3.22, p<.01) and low-distress (M=4.24 μV; t(61)=4.29, p<.001) cries, although the amplitudes of the P300 were comparable between the high- and low-distress cries, t(61)=−1.22, p=.23.
Figure 2.
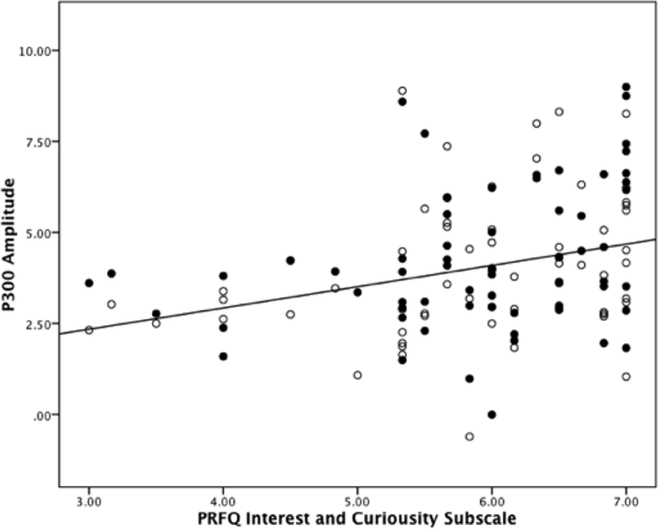
Panel A. Grand averaged ERP waveforms representing the P300 response to infant cries (high- and low-distress) and the neutral tone. Panel B. Scatter plot evidencing the association between the PRFQ interest and curiosity subscale and high-distress (unfilled) and low-distress (filled) cries.
We found no correlations between the pre-mentalizing and certainty subscales and the P300 elicited by infant cries and the neutral tone, p’s > .70, r’s < −.05. No association was observed between the neutral tone and interest and curiosity subscale, r(62)=.03, p=.83. However, there were positive correlations between interest and curiosity and the P300s elicited by high-distress, r(62)=.36, p<.01, and low-distress, r(62)=.32, p=.01, cries (Figure 3). Therefore, we included the interest and curiosity subscale as a covariate in the repeated measures ANCOVA assessing auditory stimuli. The main effect of auditory stimulus remained, F(2,120) = 4.68, p=.01, 2partial = .07, and was qualified by an interaction between auditory stimulus and interest and curiosity, F(2,120) = 7.61,p<.01, 2partial = .11, consistent with the preliminary positive correlations between interest and curiosity and the P300 elicited by infant cries. Interest and curiosity did not reach statistical significance as a covariate, F(1,60) = 3.59,p=.06, 2partial = .06, in this analysis. Taken together, these results suggest the P300 is modulated by the contents of the auditory stimulus and that greater interest and curiosity scores are associated with a larger P300 response to infant cries.
Figure 3.
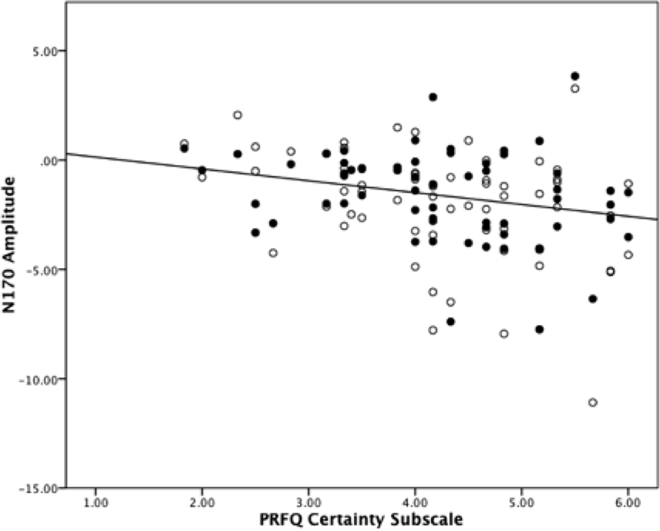
Panel A. Grand averaged ERP waveforms representing the N170 response to infant faces. Panel B. Scatter plot evidencing the association between the PRFQ certainty subscale and happy (unfilled) and neutral (filled) N170 amplitudes in the right and left hemispheres respectively.
Infant Faces
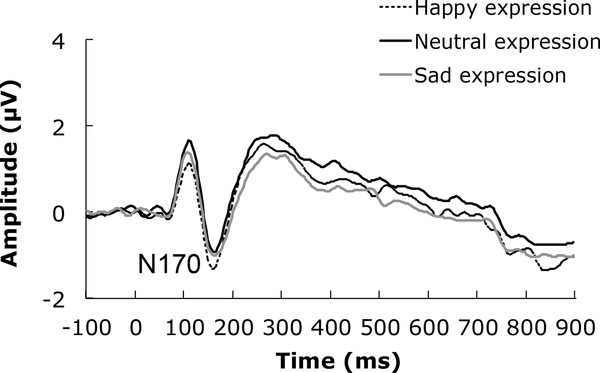
N170. Data from one participant was removed given that her mean N170 response was more than 3 SD from the group mean for the neutral face right hemisphere condition. Grand averaged N170 amplitude data for each infant emotional expression condition are presented in Figure 1. We found no main effect of emotional expression, F(2,122) = 1.51,p=.23, 2partial = .02, hemisphere, F<1, and these variables did not interact, F<1.
We found no correlations between the interest and curiosity and pre-mentalizing subscales and the N170 elicited by emotional faces across each hemisphere, p’s > .15, r’s < .18. However, there were statistically significant correlations between the certainty subscale and the N170 elicited by happy faces in the right hemisphere, r(62)=−.36, p<.01, and neutral faces in the left hemisphere, r(62)=−.32, p=.01 (Figure 4). These negative correlations suggest that as certainty increases, the amplitude of the N170 increases (i.e., it becomes a more negative potential). Therefore, we included certainty as a covariate in a repeated measures ANCOVA assessing emotional expression and hemisphere influences on N170 amplitude. As a consequence, we found a main effect of emotional expression, F(2,120) = 4.94, p<.01, 2partial = .08. The N170 was largest in response to happy (M=−1.95 μV) relative to distress (M=−1.75 μV) and neutral (M=−1.62 μν) faces in this analysis. Only the difference between happy and neutral faces was close to statistical significance, t(61)= −2.00, p=.05, with N170 amplitude between happy and distress faces, and neutral and distress faces being comparable, t’s<1. This main effect was qualified by an interaction with certainty, F(2,120) = 5.23, p<.01, 2partial = .08, where increasing certainty was associated with a greater N170 amplitude for happy, r(62)=−.27, p=.03, and neutral, r(62)=−.32, p=.01, but not distress, r(62)=−.06, p=.65, infant faces. There was a further three-way interaction between emotional expression, certainty and hemisphere, F(2,120) = 3.09,p=.049, 2partial = .05, reflecting the correlation reported in the preliminary analysis between the N170 and happy faces in the right hemisphere and neutral faces in the left hemisphere. There was no main effect of hemisphere, F(1,60) = 1.45, p=.24, 2partial = .02, and the interactions between hemisphere and emotional expression F(2,120) = 2.90, p=.06, 2partial = .05, and hemisphere and certainty, F(2,120) = 1.68, p=.20, 2partial = .03, did not reach statistical significance. Certainty as a covariate also did not reach statistical significance, F(1,60) = 2.91, p=.09, 2partial = .05. Thus, increasing certainty may be associated with greater N170 amplitude to infant emotional faces - particularly when they are happy or neutral in expression.
Figure 4.
Grand averaged ERP waveforms representing the P300 response to infant faces for each emotional expression.
P300. The grand averaged P300 data for each infant emotional expression condition are presented in Figure 4. We found no modulation of the P300 by emotional expression, F(2,124) = 2.09,p=.13, 2partial = .03, and no correlations between any of the PRFQ subscales and the P300 elicited by infant emotional faces,p’s > .10, r’s < .20. These results suggest that infant emotional expression and parental reflective functioning did not modulate the P300 elicited by infant faces.
Discussion
Parental reflective functioning represents a critical faculty that refers to the ability of a parent to recognize and understand their infant’s mental states and how these mental states may be related to behavior (Slade, 2005, 2007). While reflective functioning has been widely implicated in parenting, the extent to which this critical faculty is related to neural processing of infant affective cues is not known. Given the multifaceted nature of parental reflective functioning, and its role in parenting intervention programs, understanding which aspect of this capacity is associated with sensitivity to infant cues is important. Therefore, we investigated whether different components of parental reflective functioning were associated with neural sensitivity to infant faces and cries using event-related potentials (ERPs). We found evidence of relationships between this critical parenting faculty and the amplitude of both early and late ERP components, consistent with the notion that parental reflective functioning may be associated with maternal neural sensitivity to infant cues.
We examined the N100 as an early perceptual marker of cry perception in our maternal sample. Consistent with prior research (Purhonen, Kilpeläinen-Lees, et al., 2001; Purhonen et al., 2008), we found the N100 was modulated by the content of the auditory stimuli. Specifically, the largest N100 was elicited by high-distress infant cries. However, the N100 was not associated with any subscale of the PRFQ, suggesting this very rapid neural response to auditory stimuli may not be associated with parental reflective functioning. Past research suggests the N100 may be an orienting or alerting response to auditory stimuli (Näätänen & Picton, 1987), and therefore the automaticity of N100 generation may not be related to top-down cognitive factors such as reflective functioning. Converging with this N100 amplitude result, we also found the P300 was modulated by auditory stimulus content. The P300 was largest in response to the high- and low- distress cries relative to the neutral tone. Importantly, we also found a positive correlation between the infant cry P300 amplitude and scores on the interest and curiosity subscale of the PRFQ. Notably, this correlation was found for both high- and low-distress cries, but not the neutral tone condition. This specificity is important and suggests that it is the interpersonal nature of the relationship between these variables that may be driving this relationship rather than a more general sensitivity to auditory stimuli. However, it will be necessary to include other non-infant affective stimuli in future studies to ascertain the specificity of this relationship.
A host of studies suggest that the P300 is associated with increased allocation of attention to salient stimuli (Luck, 2005; Ritter & Ruchkin, 1992). Our finding that higher levels of interest and curiosity are associated with larger P300 amplitudes to infant cries suggests that mothers reporting greater levels of reflective functioning in this domain may also allocate more attention toward infant distress. Importantly, the absence of any N100 association with parental reflective functioning suggests that detection or orienting toward cries may not be associated with this cognitive faculty, but it is neural markers of the investment or allocation of attention (i.e., P300) where these relationships begin to emerge - highlighting the value of ERPs in teasing apart the stage of infant cue processing associated with reflective functioning. Notably, this infant cry ERP finding resonates with a previous behavioral study that also employed the PRFQ and examined associations between reflective functioning and tolerance of infant distress (Rutherford et al., 2013). In this latter behavioral study, higher levels of interest and curiosity were associated with greater tolerance of infant distress as measured by persistence times in soothing an inconsolable crying baby simulator.
We found tentative evidence that the N170 was modulated by infant emotional expression, but only when including interest and curiosity in the statistical model. This finding was driven by the N170 being larger in response to happy, relative to neutral, infant faces. Notably, not all studies find N170 modulation by infant emotional expression, with some speculation that the presence or absence of N170 modulation by expression may reflect varying task demands (Malak et al., 2015). Nevertheless, we found that increasing levels of certainty were associated with larger N170 amplitudes elicited by happy and neutral infant faces, primarily in the right and left hemispheres, respectively. The N170 is thought to represent the first recognition of a stimulus as a face in the brain (Rossion & Jacques, 2011) and these results suggest that at this level of structural encoding of facial content, there may be associations with parental reflective functioning. It is interesting to note that this relationship with certainty existed for happy and neutral faces, but was only weak and not statistically significant for distress emotional expressions. Perhaps the saliency of infant distress at this early level of visual processing is greater than happy and neutral infant faces, and the emotional state of the infant is more clearly communicated irrespective of maternal levels of reflective functioning. However, it will be important for future research to replicate and extend this finding to more fully understand this differentiation of distress relative to happy and neutral infant affect and parental reflective functioning.
While these N170 ERP results suggest that mothers’ increasing certainty in their understanding of their infant’s mental states may be associated with increased sensitivity in encoding infant faces, it is also worthwhile noting this may not always be adaptive - especially with respect to higher levels of this capacity. Instead, higher levels of certainty may reflect mothers not being able to recognize the opacity of mental states, the complexity of mental states, and their associations with behavior (Luyten et al., under review). In this sample, the mean certainty score was 4.23, with no participants receiving the maximal score on this measure. Therefore, these findings may reflect a more adaptive sensitivity to infant faces given certainty in mental states, which might explain why parental reflective functioning may be associated with such an early marker of face processing. However, future research that encompasses a broader scope of scores and exploring the potential curvilinear relationship between these variables is warranted - with lower and higher scores on this measure potentially being less adaptive within dyadic relationships and moderate scores being more adaptive (Luyten et al., under review).
Unexpectedly, we did not find a relationship between parental reflective functioning and the P300 elicited by infant emotional faces. This was surprising given the relationship between the P300 elicited by infant cries and levels of interest and curiosity. In the brain, infant faces are rapidly differentiated from adult faces, and the processing of these infant faces may have a modulating effect across neural circuits recruited in face perception (Kringelbach et al., 2008). Given this more rapid encoding of infant cues, it could be that early markers of infant cue processing, including the N170, may be more sensitive to parental reflective functioning than later ERP components. This is an interesting distinction to the stages of infant cry perception, where it is the later P300, and not the early N100, that yields associations with parental reflective functioning. These results further demonstrate the need to examine multiple ERP components as well as sensory modalities in understanding the temporal dynamics of infant cue perception and associations with reflective functioning in parent samples.
Our findings should be considered in light of their limitations and directions for future research. Here we employed a novel self-report measure of parental reflective functioning, the PRFQ. While a reliable and valid measure, it would be valuable to replicate these data with the more widely employed Parent Development Interview (PDI; (Aber, Slade, Berger, Bresgi, & Kaplan, 1985; Slade et al., 2002)) to provide convergent validity to the PRFQ and associations with the ERP data. Recent work has also suggested that parental reflective functioning as measured by the PDI differentiates this capacity as it relates to the self and as it relates to the child (Suchman et al., 2010). Indeed, self-reflective functioning in the latter maternal sample was associated with increased behaviorally coded maternal sensitivity. Presumably, if ERP components provide an indication of maternal sensitivity to infant cues, a similar pattern of responding would be observed between the N170 and P300 with respect to the self-construct, perhaps increasing the validity of employing ERPs as dependent measures in parenting intervention studies. With respect to experimental design, we only employed unfamiliar infant affective cues. Given that the PRFQ requires parents to respond in regard to their own child, the relationship between reflective functioning may be more closely coupled with neural sensitivity to infant cues when those cues are from the mother’s own child. Therefore, the generalizability of the association between parental reflective functioning and neural responses elicited by mothers’ own infant’s affective cues needs to be established. Relatedly, to further validate these data, it would also be important to examine the associations between parental reflective functioning and the neural response generated by other non-infant affective cues, including adult faces and vocalizations (as well as a non-social visual condition that was not included in the present experimental conditions). Finally, given that we recruited a community sample of mothers, it will be important to examine in future research how other variables, including maternal psychopathology, trauma histories, and other demographic factors may play a role in the relationship between parental reflective functioning and the neural correlates of infant cue perception to understand the generalization of these data.
In summary, we report for the first time evidence to suggest that parental reflective functioning may be associated with neural responses to infant affective cues. These findings further validate the importance of parental reflective functioning to the dyadic relationship in suggesting its association with very rapid sensitivity to infant faces and cries. These results also highlight the value of ERPs to understanding mechanisms relevant to parenting and their potential utility as dependent measures in parenting programs designed to change reflective functioning.
Acknowledgments
The authors wish to thank Marion Mayes, Max Greger-Moser, Jasmine Coleman and Amanda Ng for their help with data collection.
Funding Acknowledgment
This work was supported by the NIH (NIDA) grants P01 DA022446 and R01 DA026437, as well as the Anna Freud Centre (UK), the Connecticut Mental Health Center, the National Alliance for Medical Image Computing (NA-MIC) U54 EB005149, and a NIMH T32 postdoctoral fellowship (MH018268). This publication was also made possible by CTSA, Grant Number UL1 RR024139, from the National Center for Research Resources, a component of the NIH, and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of any of the funding agencies.
Appendix:Acoustic properties of cries
The acoustic properties of the cries were as follow, with Praat software http://www.fon.hum.uva.nl/praat/ employed to normalize the cries to the same relative peak intensity:
High-distress cry 1: minimum pitch 129.53 hz; maximum pitch 433.6 hz; mean pitch 351.27 hz; number of bouts 2; mean bout length .97; number of pauses =1, mean pause length 0.09 seconds.
High-distress cry 2: minimum pitch 209.68 hz; maximum pitch 461.32 hz; mean pitch 317.17 hz; number of bouts 1; mean bout length 2.1 seconds; no pauses.
Low-distress cry 1: minimum pitch 297.55 hz; maximum pitch 470.01 hz; mean pitch 348.77 hz; number of bouts 3; mean bout length .47 seconds; number of pauses 3; mean pause length .75 seconds.
Low-distress cry 2: minimum pitch 194.37 hz; maximum pitch 469.82 hz; mean pitch 351.40 hz; number of bouts 9; mean bout length .11 seconds; number of pauses 4; mean pause length .1 seconds.
Footnotes
Declarations of Interests
The authors report that they have no financial conflicts of interest with respect to the content of this manuscript. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Boehringer Ingelheim, Ironwood, Lundbeck, INSYS, Shire and RiverMend Health; has consulted for and has financial interests in Somaxon; has received research support from the NIH, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Ortho-McNeil, Psyadon, Oy-Control/Biotie and Glaxo-SmithKline pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the NIH and other agencies; has edited journals or sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. The other authors reported no biomedical financial interests or other conflicts of interest.
References
- Aber J, Slade A, Berger B, Bresgi I, & Kaplan M (1985). The Parent Development Interview. City Uiniversity of New York. [Google Scholar]
- Ainsworth MS (1979). Infant-mother attachment. American Psychologist, 34(10), 932. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, & McCarthy G (1996). Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience, 8, 551–565. doi: 10.1162/jocn.1996.8.6.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Dozier M, Bernard K, Grasso D, & Simons R (2013). Foster mother-infant bonding: Associations between foster mothers’ oxytocin production, electrophysiological brain activity, feelings of commitment, and caregiving quality. Child Development, 84(3), 826–840. doi: 10.1111/cdev.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Kain LW, & Gunderson JG (2008). Mentalization: ontogeny, assessment, and application in the treatment of borderline personality disorder. Am J Psychiatry, 165(9), 1127–1135. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Doi H, & Shinohara K (2012). Electrophysiological responses in mothers to their own and unfamiliar child’s gaze information. Brain Cogn, 80(2), 266–276. [DOI] [PubMed] [Google Scholar]
- Fonagy P (1991). Thinking about thinking : some clinical and theoretical considerations in the treatment of a borderline patient. International Journal of Psychoanalysis, 72(4), 639–656. [PubMed] [Google Scholar]
- Fonagy P, & Luyten P (2009). A developmental, mentalization-based approach to the understanding and treatment of borderline personality disorder. Development and Psychopathology, 21(Special Issue 04), 1355–1381. doi: doi:10.1017/S0954579409990198 [DOI] [PubMed] [Google Scholar]
- Fonagy P, Steele M, Steele H, & Target M (1998). Reflexive-function manual: Version 50 for application to the adult attachment interview.: University College London. [Google Scholar]
- Grasso DJ, Moser JS, Dozier M, & Simons R (2009). ERP correlates of attention allocation in mothers processing faces of their children. Biological Psychology, 81(2), 95–102. doi: 10.1016/j.biopsycho.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. doi: 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Grienenberger JF, Kelly K, & Slade A (2005). Maternal reflective functioning, mother-infant affective communication, and infant attachment: Exploring the link between mental states and observed caregiving behavior in the intergenerational transmission of attachment. Attachment and Human Development, 7(3), 299–311. doi: 10.1080/14616730500245963 [DOI] [PubMed] [Google Scholar]
- Gustafson GE, & Green JA (1989). On the importance of fundamental frequency and other acoustic features in cry perception and infant development. Child Development, 60(4), 772–780. doi: 10.2307/1131017 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, … Stein A (2008). A Specific and Rapid Neural Signature for Parental Instinct: Public Library of Science. [DOI] [PMC free article] [PubMed]
- Landi N, Crowley MJ, Wu J, Bailey CA, & Mayes LC (2012). Deviant ERP response to spoken non-words among adolescents exposed to cocaine in utero. Brain and Language, 120(3), 209–216. doi: 10.1016/j.bandl.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJV, Mencl E, Worhunsky P, … Mayes LC (2011). Maternal neural responses to infant cries and faces: Relationships with substance use. Frontiers in Psychiatry, 2(32). doi: 10.3389/fpsyt.2011.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2005). An introduction to the event-related potential technique Cambridge, MA: MIT Press. [Google Scholar]
- Luyten P, Fonagy P, Lowyck B, & Vermote R (2012). The assessment of mentalization In Bateman A, Fonagy W&P (Eds.), Handbook of Mentalizing in Mental Health Practice (pp. 43–65). Washington DC: American Psychiatric Association. [Google Scholar]
- Luyten P, Mayes LC, Nijssens L, & Fonagy P (under review). The parental reflective functioning questionnaire: Development and preliminary validation. [DOI] [PMC free article] [PubMed]
- Malak SM, Crowley MJ, Mayes LC, & Rutherford HJ (2015). Maternal anxiety and neural responses to infant faces. Journal of Affective Disorders, 172, 324–330. doi: 10.1016/j.jad.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Maupin A, Hayes N, Mayes L, & Rutherford HJV (2015). The application of electroencephalography to investigate the neural basis of parenting. Parenting: Science and Practice, 15(1), 9–23. doi: 10.1080/15295192.2015.992735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes L, & Bornstein MH (1995). Infant information-processing performance and maternal education. Early Development and Parenting, 4(2), 91–96. doi: 10.1002/edp.2430040206 [DOI] [Google Scholar]
- Näätänen R, & Picton T (1987). The N1 Wave of the Human Electric and Magnetic Response to Sound: A Review and an Analysis of the Component Structure. Psychophysiology, 24(4), 375–425. [DOI] [PubMed] [Google Scholar]
- Noll LK, Mayes LC, & Rutherford HJV (2012). Investigating the impact of parental status and depression symptoms on the early perceptual coding of infant faces: An event- related potential study. Social Neuroscience, 1–12. doi: 10.1080/17470919.2012.672457 [DOI] [PubMed] [Google Scholar]
- Papousek H (Ed.). (2000). Intuitive Parenting (Vol. 3). New York: John Wiley & Sons, Inc. [Google Scholar]
- Proverbio AM, Brignone V, Matarazzo S, Del Zotto M, & Zani A (2006). Gender and parental status affect the visual cortical response to infant facial expression. Neuropsychologia, 44(14), 2987–2999. doi: 10.1016/j.neuropsychologia.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Purhonen M, Kilpeläinen-Lees R, Pääkkönen A, Yppärilä H, Lehtonen J, & Karhu J (2001). Effects of maternity on auditory event-related potentials to human sound. Neuroreport, 12(13), 2975–2979. doi: 10.1097/00001756-200109170-00044 [DOI] [PubMed] [Google Scholar]
- Purhonen M, Pääkkönen A, Yppärilä H, Lehtonen J, & Karhu J (2001). Dynamic behavior of the auditory N100 elicited by a baby’s cry. International Journal of Psychophysiology, 41(3), 271–278. doi: 10.1016/S0167-8760(01)00139-8 [DOI] [PubMed] [Google Scholar]
- Purhonen M, Valkonen-Korhonen M, & Lehtonen J (2008). The impact of stimulus type and early motherhood on attentional processing. Developmental Psychobiology, 50(6), 600–607. doi: 10.1002/dev.20321 [DOI] [PubMed] [Google Scholar]
- Ritter W, & Ruchkin DS (1992). A Review of Event - Related Potential Components Discovered in the Context of Studying P3a. Annals of the New York Academy of Sciences, 658(1), 1–32. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, León I, Quiñones I, Lage A, Byrne S, & Bobes MA (2011). Brain and personality bases of insensitivity to infant cues in neglectful mothers: An event-related potential study. Development and Psychopathology, 23(01), 163–176. doi: 10.1017/S0954579410000714 [DOI] [PubMed] [Google Scholar]
- Rossion B, & Jacques C (2011). The N170: Understanding the time-course of face perception in the human brain. The Oxford Handbook of ERP Components, 115–142. [Google Scholar]
- Rutherford HJV, Goldberg B, Luyten P, Bridgett DJ, & Mayes LC (2013). Parental reflective functioning is associated with tolerance of infant distress but not general distress: Evidence for a specific relationship using a simulated baby paradigm. Infant Behavior and Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, & Mayes LC (2011). Primary maternal preoccupation: Using neuroimaging techniques to explore the parental brain. Psyche(65), 973–988. doi: Retrieved from: http://pep.gvpi.net/toc.php?journal=psyche&volume=65-p0973 [Google Scholar]
- Sadler LS, Slade A, Close N, Webb DL, Simpson T, Fennie K, & Mayes LC (2013). Minding the Baby: Enhancing reflectiveness to improve early health and relationship outcomes in an interdisciplinary home-visiting program. Infant Mental Health Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, & Zuccolotto A (2002). E-prime user’s guide. Pittsburg, Psychology Software Tools Inc. [Google Scholar]
- Slade A (2005). Parental reflective functioning: An introduction. Attach Hum Dev, 7(3), 269–281. doi: 10.1080/14616730500245906 [DOI] [PubMed] [Google Scholar]
- Slade A (2007). Reflective Parenting Programs: Theory and Development. Psychoanalytic Inquiry, 26(4), 640–657. doi: 10.1080/07351690701310698 [DOI] [Google Scholar]
- Slade A, Bernbach E, Grienenberger JF, Levy D, & Locker A (2002). Addendum to Reflective Functioning Scoring Manual: For Use with the Parent Development Interview. The City College and Graduate Center of the City University of New York: Unpublished manuscript. [Google Scholar]
- Slade A, Grienenberger J, Bernbach E, Levy D, & Locker A (2005). Maternal reflective functioning, attachment, and the transmission gap: A preliminary study. Attachment and Human Development, 7(3), 283–298. [DOI] [PubMed] [Google Scholar]
- Strathearn L, & McClure SM (2002). A functional MRI study of maternal responses of infant facial cues Paper presented at the Annual ScientificMeeting of the Society forNeuroscience, Washington DC. [Google Scholar]
- Suchman NE, Decoste C, Castiglioni N, Legow N, & Mayes L (2008). The Mothers and Toddlers Program: Preliminary Findings From an Attachment-Based Parenting Intervention for Substance-Abusing Mothers. Psychoanalytic psychology : the official journal of the Division of Psychoanalysis, American Psychological Association, Division 39, 25(3), 499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman NE, DeCoste C, Leigh D, & Borelli J (2010). Reflective functioning in mothers with drug use disorders: Implications for dyadic interactions with infants and toddlers. Attach Hum Dev, 12(6), 567–585. doi: 10.1080/14616734.2010.501988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE (2011). The human parental brain: In vivo neuroimaging. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(5), 1242–1254. doi: 10.1016/j.pnpbp.2010.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]