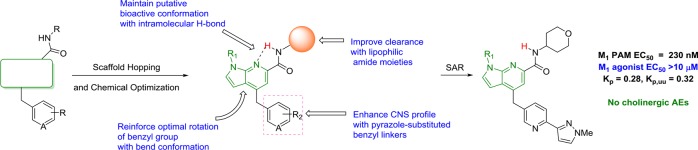
Abstract
Herein, we report the chemical optimization of a new series of M1 positive allosteric modulators (PAMs) based on a novel pyrrolo[2,3-b]pyridine core, developed via scaffold hopping and iterative parallel synthesis. The vast majority of analogs in this series proved to display robust cholinergic seizure activity. However, by removal of the secondary hydroxyl group, VU6007477 resulted with good rat M1 PAM potency (EC50 = 230 nM, 93% ACh max), minimal M1 agonist activity (agonist EC50 > 10 μM), good CNS penetration (rat brain/plasma Kp = 0.28, Kp,uu = 0.32; mouse Kp = 0.16, Kp,uu = 0.18), and no cholinergic adverse events (AEs, e.g., seizures). This work demonstrates that within a chemical series prone to robust M1 ago-PAM activity, SAR can result, which affords pure M1 PAMs, devoid of cholinergic toxicity/seizure liability.
Keywords: Positive allosteric modulator (PAM), muscarinic acetylcholine receptor 1 (M1), cholinergic toxicity, VU6007477
Muscarinic acetylcholine receptor subtype 1 (M1) positive allosteric modulators (PAMs) hold great promise for the treatment of cognitive impairment, schizophrenia, and Alzheimer’s disease.1−7 Originally, the clinical promise of M1 as a target was derailed by M1 agonists lacking true M1 selectivity,1,2,8 and later, by robust M1 ago-PAMs that overstimulated the M1 receptor, resulting in cholinergic toxicity and adverse events (AEs).9−18 While these ago-PAMs, represented by 1–4 (Figure 1), dampened enthusiasm for the translational utility of selective M1 activation, next generation M1 PAMs 5 and 6, devoid of agonism in cells and native systems (and without cholinergic toxicity/seizures), provided a new path to the clinic.14,19 However, due to the voluminous literature with ago-PAMs 1–4, multiple new M1 PAMs are required for the community to evaluate the safety and efficacy of the “pure” M1 PAM mechanism and independently embrace the therapeutic potential of M1 PAMs. Herein, a novel series of M1 PAMs featuring a pyrrolo[2,3-b]pyridine carboxamide core is described, including the design, SAR, DMPK properties, pharmacology, and cholinergic adverse effect profiles to provide the community with another new, pure M1 PAM tool for in vivo efficacy and tolerability studies.
Figure 1.
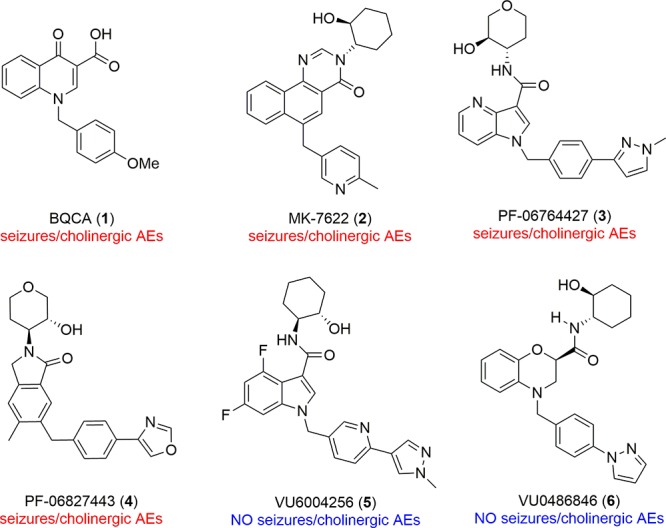
Structures of representative M1 PAMs 1–6. Of these, 1–4 are robust ago-PAMs in high expressing cell lines and native systems, resulting in severe seizures/cholinergic AEs due to overstimulation of M1. M1 PAMs 5 and 6 are devoid of seizures/cholinergic AEs and show little to no M1 agonism in high expressing cell lines and native systems.
Scaffold-hopping has been a major strategy in the M1 PAM arena, and this approach led to the discovery of the benzomorpholine-based pure M1 PAM 6.19 We once again held the key carboxamide and heterobiaryl tail moieties of 3, 5, and 6 constant while surveying new heterobiaryl cores, as in 7, that might engender pure M1 PAM pharmacology (Figure 2). From this exercise, a novel pyrrolo[2,3-b]pyridine carboxamide core, represented generically by 8, resulted, with a spectrum of M1 pharmacology from potent ago-PAMs to pure PAMs.
Figure 2.
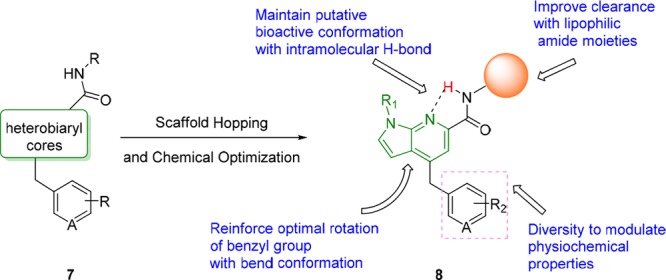
Scaffold hopping approach based on M1 PAMs 3, 5, and 6, which led to the discovery of a novel pyrrolo[2,3-b]pyridine carboxamide-based series of M1 ago-PAMs and pure PAMs, 8.
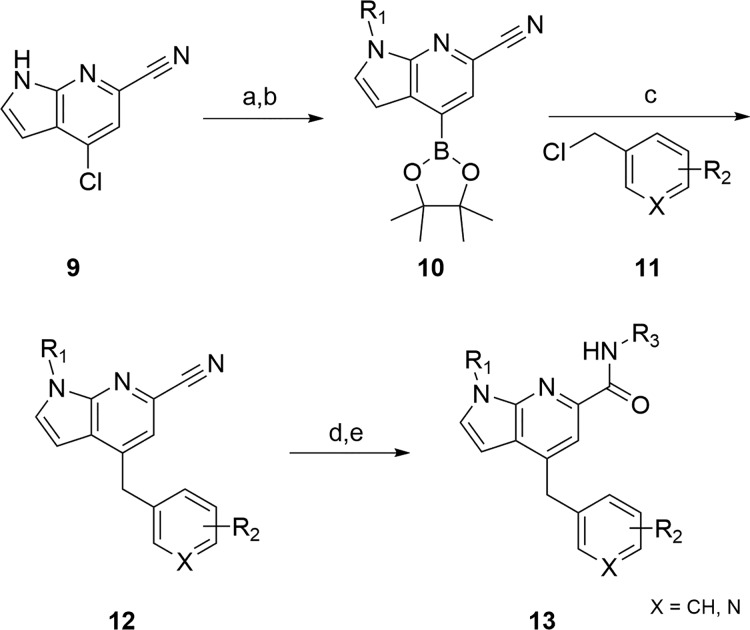
The synthesis of diverse analogs 13 (Scheme 1) proved straightforward with readily available starting materials from commercial sources.20 4-Chloro-1H-pyrrolo[2,3-b]pyridine-6-carbonitrile 9 could be alkylated with various R1 moieties, but we first explored methyl. Conversion to the boronate ester under standard conditions provided 10 in >90% yield for the two steps. Suzuki coupling with either benzyl halides or heteroaryl methyl halides 11 generated derivatives 12 in 50–84% yield. Finally, hydrolysis of the nitrile and standard HATU amide coupling reactions delivered the putative M1 PAMs 13 in 35–66% yield.20
Scheme 1. Synthesis of M1 PAM Analogs 13.
Reagents and conditions: (a) R1I, NaH, DMF, 0 °C–rt, 85–90%; (b) bis(pinacolato)diboron, KOAc, Pd(dppf)Cl2, 1,4-dioxane, 100 °C, 16 h, >98%; (c) benzyl chloride, Cs2CO3, Pd(dppf)Cl2, THF/H2O, 90 °C, 16 h; 50–84%; (d) conc. HCl, reflux, 2 h, >98%; (e) amine, HATU, DIEA, DMF, rt, 20 min, 35–66%.
We then surveyed this new core with a variety of heterobiaryl tails and amide congeners, grouped based on internal knowledge regarding SAR to engender robust M1 ago-PAM versus PAM activity. The first amide moiety surveyed was the (3S,4R)-3-hydroxy-4 amino tetrahydropyranyl (THP) amide, well documented to engender potent M1 PAMs, but with undesired potent M1 agonist activity.11,13−17,19 As we previously reported, M1 PAM potencies below 100 nM, coupled with M1 agonist activity, generally lead to robust cholinergic AEs.19 As anticipated, analogs 14 (Table 1) with diverse southern heterobiaryl tails provided potent M1 ago-PAMs. Analog 14b stands out as a 14 nM M1 PAM (86% ACh max), with an agonist EC50 of 575 nM (67% ACh max) and a direction not worthy of further pursuit, as overstimulation of M1 would definitely result.
Table 1. Structures and M1 Pharmacology for Analogs 14a.
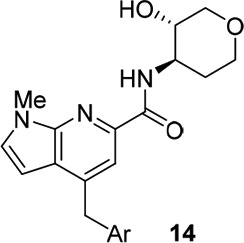
Calcium mobilization assays with rat M1-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine for PAM, and no exogenous ACh for the agonist EC50; values represent means from three (n = 3) independent experiments performed in triplicate.
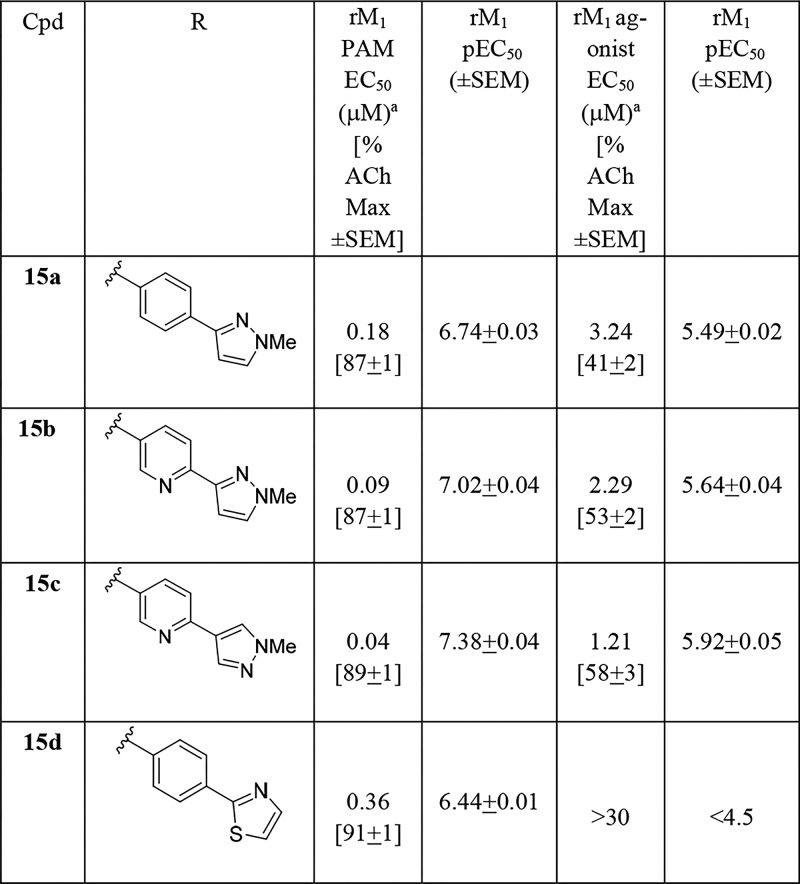
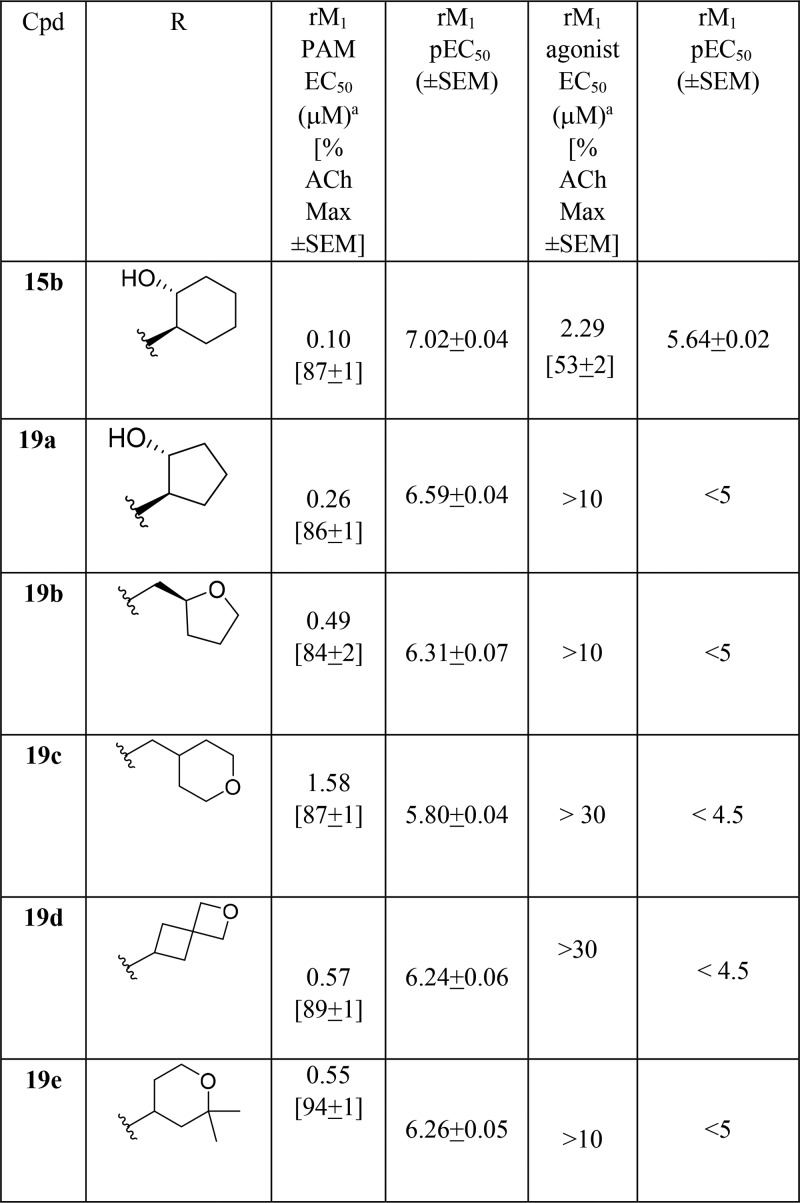
As the analogous cyclohexyl congener 15 of THP 14 generally afforded a lower degree of M1 agonism, we next evaluated such analogs. As shown in Table 2, analogs 15 were also potent M1 ago-PAMs (15a–c), but with reduced M1 agonism relative to 14,11,13−17,19 as well as a moderately potent M1 PAM (15d). Interestingly, both 15b and 15c were not CNS penetrant in rat, with Kp values below level of quantitation (BLQ), despite favorable CNS MPO scores (4–5).21 Previously, we have shown that the secondary hydroxyl moiety engenders poor CNS penetration in rodents, and future analogs would avoid this moiety.19
Table 2. Structures and M1 Pharmacology for Analogs 15a.
Calcium mobilization assays with rM1-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine for PAM, and no exogenous ACh for the agonist EC50; values represent means from three (n = 3) independent experiments performed in triplicate.
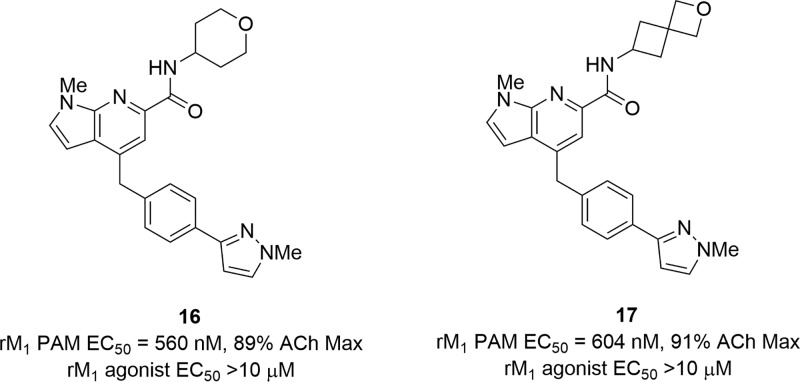
Thus, we evaluated the profile of simple, unsubstituted pyranyl amides, as we hoped this would engender a balance between M1 PAM potency (and diminished M1 agonism) and CNS penetration. Our initial evaluation produced pyranyl amide 16 and the spiro-cyclic congener 17 (Figure 3); importantly, both proved to be pure M1 PAMs (agonist EC50s > 10 μM). However, M1 PAM potency was modest (16: rM1 PAM EC50 = 560 nM, pEC50 = 6.25 ± 0.05, ACh max = 89 ± 1; 17: rM1 PAM EC50 = 604 nM, pEC50 = 6.22 ± 0.04, ACh max = 91 ± 1). Still, similar pyranyl amides (lacking the secondary hydroxyl moiety) in other series (such as 5 and 6) were inactive or weak, suggesting this is a unique core. To further optimize M1 PAM potency with the pyranyl amide, we next explored alternative heterobiaryl tails.
Figure 3.
SAR evaluation of nonhydroxylated amides 16 and 17.
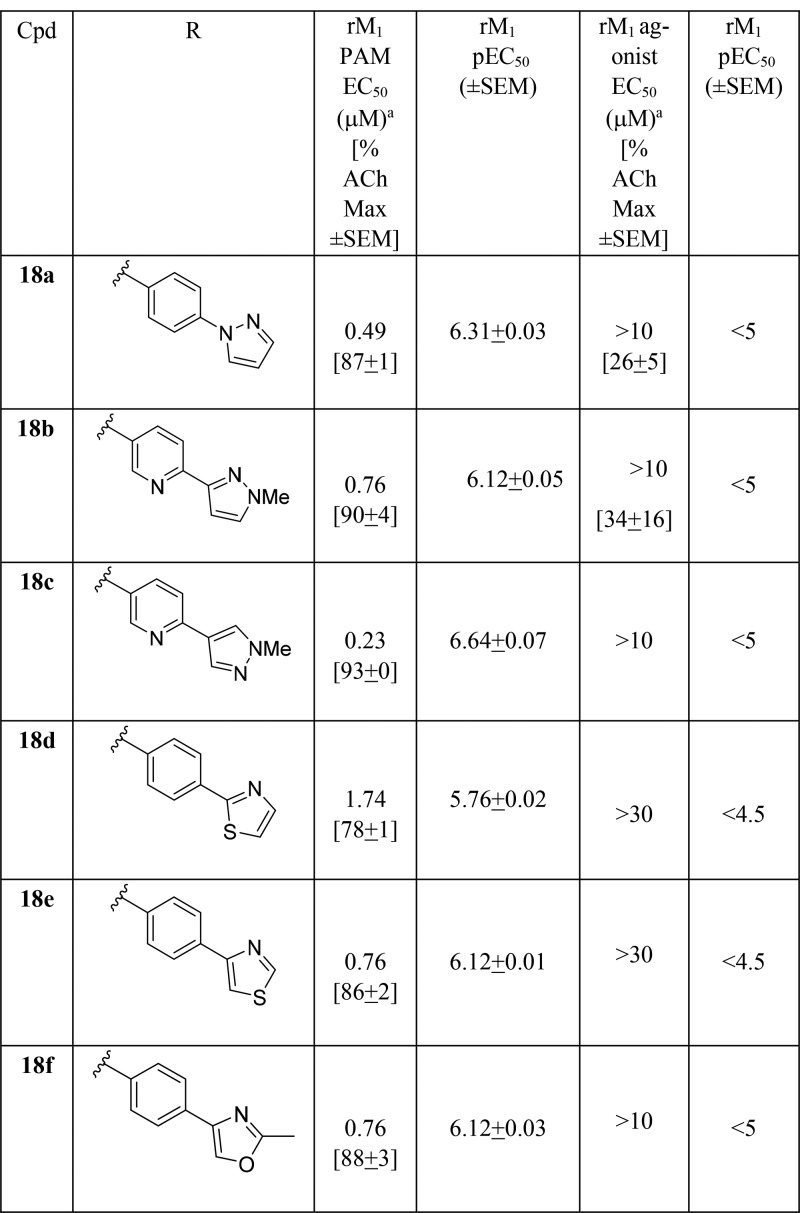
Surprisingly, while holding the pyranyl amide moiety constant in analogs 18 and varying the heterobiaryl tail (Table 3), the lack of M1 agonism in these des-hydroxy congeners proved to be a generally conserved pharmacological property. Of these, 18c emerged as an attractive M1 PAM (EC50 = 230 nM, 93% ACh max; log Kb = −4.82; log αβ = 2.6) with minimal to no agonism in our high expressing cell line (M1 agonist EC50 > 10 μM).20 Replacement of the N-Me pyrazole in 16 for an oxazole analog (e.g., 18f) provided an M1 PAM of comparable potency (EC50 = 760 nM, 88% ACh max) to 16 and still devoid of M1 agonism. Regioisomeric pyridyl pyrazole heterobiaryls 18b and 18c showed similar M1 PAM activity, but 18b displayed weak M1 agonism (∼34% @ 10 μM). An N-linked pyrazole congener, 18a, displayed similar PAM potency and weak M1 agonism (∼26% at 10 μM) as well, highlighting the sensitivity of this series for M1 agonist activity.
Table 3. Structures and M1 Pharmacology for Analogs 18a.
Calcium mobilization assays with rM1-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine for PAM, and no exogenous ACh for the agonist EC50; values represent means from three (n = 3) independent experiments performed in triplicate.
A larger amide scan of the pyrazole regioisomers 19 based on the 18b heterobiaryl tail motif (Table 4) similarly identified a number of pure M1 PAMs and ago-PAMs (with novel amides), but none showed advantages over analogs highlighted in Tables 1–3. However, unlike predecessor series 1–4, this scaffold afforded pure M1 PAMs, devoid of agonist activity in high expressing M1 cell lines.
Table 4. Structures and M1 Pharmacology for Analogs 19a.
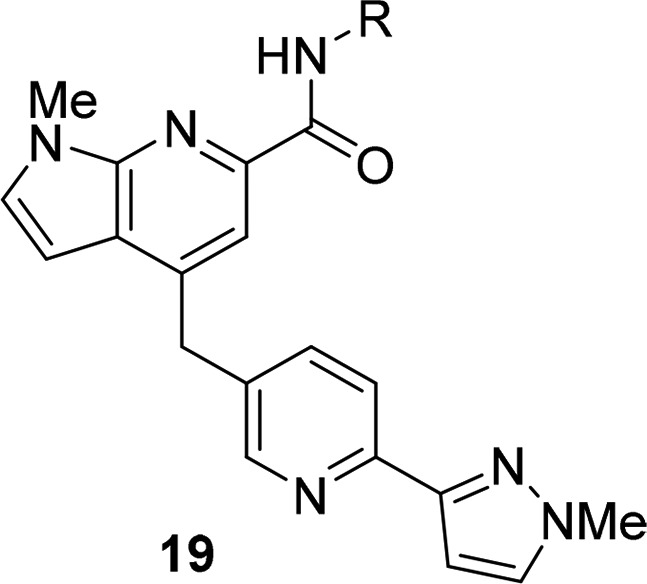
Calcium mobilization assays with rM1-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine for PAM, and no exogenous ACh for the agonist EC50; values represent means from three (n = 3) independent experiments performed in triplicate.
Prior to assessing physiochemical and DMPK properties, we wanted to ensure good species alignment for key M1 PAMs at both human and rat M1. Gratifyingly, there was high species conservation (Table 5), indicating translational potential for this novel series of M1 PAMs.
Table 5. Human and Rat M1 PAM Activities for Select Analogs 14–18.
| Cpd | rat M1 PAM EC50 (μM)a [% ach Max ± SEM] | rat M1 pEC50 (±SEM) | hum M1 PAM EC50 (μM)a [% ACh max ± SEM] | hum M1 pEC50 (±SEM) |
|---|---|---|---|---|
| 14a | 0.04, [87 ± 0] | 7.41 ± 0.06 | 0.06, [83 ± 3] | 7.22 ± 0.10 |
| 14c | 0.10, [90 ± 1] | 7.00 ± 0.03 | 0.09, [80 ± 8] | 7.05 ± 0.20 |
| 15a | 0.18, [87 ± 1] | 6.74 ± 0.03 | 0.18, [84 ± 2] | 6.74 ± 0.10 |
| 15b | 0.10, [87 ± 1] | 7.02 ± 0.04 | 0.12, [79 ± 4] | 6.91 ± 0.10 |
| 16 | 0.56, [89 ± 1] | 6.25 ± 0.05 | 0.66, [87 ± 3] | 6.18 ± 0.10 |
| 18c | 0.23, [93 ± 0] | 6.64 ± 0.07 | 0.32, [84 ± 7] | 6.49 ± 0.05 |
Calcium mobilization assays with rM1-CHO or hM1-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine for PAM; values represent means from three (n = 3) independent experiments performed in triplicate.
The M1 PAMs highlighted in Table 5 displayed favorable physiochemical properties (MWs < 450, cLogPs between 1.2 and 4.0, TPSAs 89–102 Å2, CNS MPO scores between 3.2 and 4.3) and acceptable in vitro DMPK profiles (Table 6).20 The majority show modest predicted hepatic clearance in human and rat, good free fraction (human, rat, and rat brain homogenate binding), but low to modest CNS penetration in rat (rat brain/plasma Kps and Kp,uus ≤ 0.3). Moreover, as we have seen significant variations between brain penetration in mice and rats, we also assessed mouse Kp at 100 mg/kg ip. Despite low but measurable Kp in mice, brain levels were high (both total and unbound). The low Kps were driven by very high plasma concentrations (Supplemental Figure 1).20
Table 6. In Vitro DMPK Data and PBL Data for Select M1 PAMs 14–18a.
| property | 14a | 14c | 15b | 15c | 16 | 18c |
|---|---|---|---|---|---|---|
| MW | 445 | 446 | 443 | 444 | 427 | 430 |
| cLogP | 2.40 | 1.22 | 4.02 | 2.84 | 5.21 | 1.77 |
| TPSA | 89.7 | 102.1 | 80.5 | 92.9 | 60.3 | 81.9 |
| In Vitro PK Parameters | ||||||
| rat CLHEP (mL/min/kg) | 39.1 | 42.0 | 49.6 | 27.3 | 47.9 | 44.2 |
| human CLHEP (mL/min/kg), | 6.97 | 10.6 | 17.8 | 9.3 | 10.7 | 6.78 |
| rat fu (plasma) | 0.03 | 0.02 | 0.03 | 0.06 | 0.04 | 0.06 |
| human fu (plasma) | 0.02 | 0.02 | 0.12 | 0.04 | 0.02 | 0.04 |
| rat fu (brain) | 0.01 | 0.01 | 0.04 | 0.07 | 0.01 | 0.08 |
| Rat PBL (IV, 0.2 mg/kg) | ||||||
| Kp | 0.11 | 0.21 | BLQ | BLQ | BLQ | 0.28 |
| Kp,uu | 0.05 | 0.13 | BLQ | BLQ | BLQ | 0.32 |
| Mouse PBL (IP, 100 mg/kg) | ||||||
| Kp | 0.09 | ND | 0.28 | 0.03 | ND | 0.16 |
| Kp,uu | 0.04 | ND | 0.17 | 0.04 | ND | 0.18 |
BLQ: brain concentration is below limit of quantitation (BLQ) in low dose rat cassette (0.2 mg/kg). ND = not determined.
As we have documented previously,11,13−17,19 robust ago-PAM activity can lead to over stimulation of the M1 receptor and/or induce M1 agonist-dependent long-term depression in the prefrontal cortex leading to cognitive dysfunction and, in mice (the most susceptible species to excessive M1 activation), Racine scale 4 to 5 seizures.11,14,17,19 Thus, a quick phenotypic screen in mice (at 100 mg/kg IP) to assess seizure liability has become a proven method of choice to eliminate compounds from the lead progression flow-chart and deem them nondevelopable. Interestingly, this pyrrolo[2,3-b]pyridine carboxamide-based series showed a pronounced tendency for cholinergic adverse effect liability in the mouse seizure model (Figure 4), with all analogs showing robust Racine scale seizures within 30 min of IP administration (100 mg/kg), save 18c (VU6007477). These data further highlight the range of clean versus adverse event (AE) in vivo pharmacology that can occur within a highly conserved chemical series of M1 PAMs (and the value of an informative phenotypic triage screen).14,19
Figure 4.
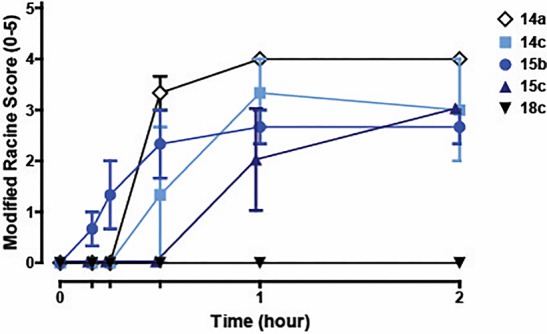
Phenotypic mouse seizure assay with compounds dosed at 100 mg/kg i.p. VU6007477 (18c) was devoid of seizure liability (mouse brain levels: 7 μM total, 546 nM unbound), whereas 14a, 14c, 15b, and 15c elicited robust Racine scale 4/5 seizures.20
Due to the significant AE liability within this series, we were hesitant to further advance this series down the lead optimization flow-chart; however, additional characterization quickly led to a no-go decision for both 18c and this series. While 18c was highly selective for M1 (M2–M5 EC50s > 30 μM, Supplemental Figure 2),20 it proved to be a human P-gp substrate (ER = 4.5), with only moderate permeability (Papp = 1.2 × 10–5 cm/s). Thus, 18c is a new in vitro/in vivo rodent M1 “pure” PAM tool compound but is not suitable for translation to the clinic.
In summary, a scaffold-hopping exercise identified a novel pyrrolo[2,3-b]pyridine carboxamide-based series of M1 PAMs that displayed a range of potent ago-PAM and pure PAM pharmacology. While congeners possessing the prototypical (3S,4R)-3-hydroxy-4 amino tetrahydropyranyl (THP) amide moiety (or the cylcohexyl analog) engendered a range of M1 ago-PAM activity, they also resulted in severe Racine scale 4/5 seizures that correlated with the presence of M1 agonist activity (which is predicted to result in overstimulation of M1). In contrast, a simple pyranyl amide derivative, 18c (VU6007477), was a pure M1 PAM in high expressing cell lines (M1 agonist EC50 > 10 μM), displayed improved CNS penetration over the hydroxylated congeners, and was devoid of seizure liability in mice. While 18c was not advanceable as a clinical candidate, it remains an important new rodent tool compound to study selective M1 activation without concern for cholinergic toxicity and related AEs. The optimization of other pure M1 PAMs, devoid of cholinergic toxicity and adverse effect liability, with translational potential, will be reported in due course.
Acknowledgments
The authors would also like to thank William K. Warren, Jr. and the William K. Warren Foundation who funded the William K. Warren, Jr. Chair in Medicine (to C.W.L.). Studies were supported by the NIH (NIMH, MH082867 and MH108498).
Glossary
ABBREVIATIONS
- PAM
positive allosteric modulator
- PBL
plasma/brain level
- AE
adverse event
- dppf
1′-ferrocenediyl-bis(diphenylphosphine)
- HATU
1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate
- DIEA
diisopropylethyl amine
- DMPK
drug metabolism and pharmacokinetics
- MPO
multiparameter optimization
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00261.
General methods for the synthesis and characterization of all compounds, and methods for the in vitro and in vivo DMPK protocols and supplemental figures (PDF)
Author Contributions
C.W.L., C.M.N., P.J.C., H.P.C., and J.M.R. drafted/corrected the manuscript. J.L.E., E.S.C., M.F.L., R.A.C., and D.W.E. performed the chemical synthesis. C.W.L., P.J.C., C.M.N., J.K.R., and H.P.C. oversaw the medicinal chemistry and target selection and interpreted the biological data. V.B.L. and H.P.C. performed the in vitro molecular pharmacology studies. A.L.B. performed the in vitro and in vivo DMPK studies. J.M.R. oversaw the in vivo experiments. J.W.D. performed the in vivo studies. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): Hold IP on M1 PAMs.
Supplementary Material
References
- Melancon B. J.; Tarr J. C.; Panarese J. D.; Wood M. R.; Lindsley C. W. Allosteric modulation of the M1 muscarinic acetylcholine receptor: improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discovery Today 2013, 18, 1185–1199. 10.1016/j.drudis.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. M.; LeBois E. P.; Hopkins C. R.; Wood M. R.; Jones J. K.; Conn P. J.; Lindsley C. W. Antipsychotic potential of muscarinic allosteric modulation’. Drug News Perspect. 2010, 23, 229–240. 10.1358/dnp.2010.23.4.1416977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A. I.; Edmunds S. M.; Koliatsos V.; Wiley R. G.; Heilman C. J. Expression of M1-M4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervations. J. Neurosci. 1995, 15, 4077–4092. 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A. I. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 13541–13546. 10.1073/pnas.93.24.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C.; Porter A. C.; Skillman T. L.; Zhang L.; Bymaster F. P.; Nathanson N. M.; Hamilton S. E.; Gomeza J.; Wess J.; McKinzie D. L. Elucidating the role of muscarinic receptors in psychosis. Life Sci. 2001, 68, 2605–2613. 10.1016/S0024-3205(01)01059-1. [DOI] [PubMed] [Google Scholar]
- Caccamo A.; Oddo S.; Billings L. M.; Green K. N.; Martinez-Coria H.; Fisher A.; LaFerla F. M. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 2006, 49, 671–682. 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Conn P. J.; Lindsley C. W.; Meiler J.; Niswender C. M. Opportunities and challenges in the discovery of allosteric modulators of GPCRs for the treatment of CNS disorders. Nat. Rev. Drug Discovery 2014, 13, 692–708. 10.1038/nrd4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A. M.; Jones C. K.; Lindsley C. W. Classics in Chemical Neuroscience: Xanomeline. ACS Chem. Neurosci. 2017, 8, 435–443. 10.1021/acschemneuro.7b00001. [DOI] [PubMed] [Google Scholar]
- Ma L.; Seager M.; Wittman M.; Bickel N.; Burno M.; Jones K.; Graufelds V. K.; Xu G.; Pearson M.; McCampbell A.; Gaspar R.; Shughrue P.; Danzinger A.; Regan C.; Garson S.; Doran S.; Kreatsoulas C.; Veng L.; Lindsley C. W.; Shipe W.; Kuduk S.; Jacobson M.; Sur C.; Kinney G.; Seabrook G. R.; Ray W. J. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 15950–15955. 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey J. K.; Brady A. E.; Jones P. J.; Davis A. A.; Bridges T. M.; Jadhav S. B.; Menon U.; Christain E. P.; Doherty J. J.; Quirk M. C.; Snyder D. H.; Levey A. I.; Watson M. L.; Nicolle M. M.; Lindsley C. W.; Conn P. J. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and can restore impairments of reversal learning. J. Neurosci. 2009, 29, 14271–14286. 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran S. P.; Dickerson J. W.; Plumley H. C.; Xiang Z.; Maksymetz J.; Remke D. H.; Doyle C. A.; Niswender C. M.; Engers D. W.; Lindsley C. W.; Rook J. M.; Conn P. J. M1 positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition. Neuropsychopharmacology 2018, 43, 1763. 10.1038/s41386-018-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal A.; Rook J.; Dickerson J.; Roop G.; Morrison R.; Jalan-Sakrikar N.; Lamsal A.; Noetzel M.; Poslunsey M.; Stauffer S. R.; Xiang Z.; Daniels J. S.; Niswender C. M.; Jones C. K.; Lindsley C. W.; Conn P. J. Selective potentiation of M1 muscarinic receptors reverses deficits in plasticity and negative and cognitive symptoms in repeated phencyclidine treated mouse model of schizophrenia. Neuropsychopharmacology 2016, 41, 598–610. 10.1038/npp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grannan M. D.; Mielnik C. A.; Moran S. P.; Gould R. W.; Ball J.; Bubser M.; Ramsey A. J.; Abe M.; Cho H. P.; Nance K. D.; Blobaum A. L.; Niswender C. M.; Conn P. J.; Lindsley C. W.; Jones C. K. Prefrontal cortex-mediated impairments in a genetic model of NMDA receptor hypofunction are reversed by the novel M1 PAM VU6004256. ACS Chem. Neurosci. 2016, 7, 1706–1716. 10.1021/acschemneuro.6b00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook J. M.; Abe M.; Cho H. P.; Nance K. D.; Luscombe V. B.; Adams J. J.; Dickerson J. W.; Remke D. H.; Garcia-Barrantes P. M.; Engers D. W.; Engers J. L.; Chang S.; Foster J. J.; Blobaum A. L.; Niswender C. M.; Jones C. K.; Conn P. J.; Lindsley C. W. Diverse effects on M1 signaling and adverse effect liability within a series of M1 Ago-PAMs. ACS Chem. Neurosci. 2017, 8, 866–883. 10.1021/acschemneuro.6b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoren J. E.; O’Neil S. V.; Anderson D. P.; Brodeny M. A.; Chenard L.; Dlugolenski K.; Edgerton J. R.; Green M.; Garnsey M.; Grimwood S.; Harris A. R.; Kauffman G. W.; LaChapelle E.; Lazzaro J. T.; Lee C.-W.; Lotarski S. M.; Nason D. M.; Obach R. S.; Reinhart V.; Salomon-Ferrer R.; Steyn S. J.; Webb D.; Yan J.; Zhang L. Design and optimization of selective azaindole amides M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2016, 26, 650–655. 10.1016/j.bmcl.2015.11.053. [DOI] [PubMed] [Google Scholar]
- Davoren J. E.; Lee C.-W.; Garnsey M.; Brodney M. A.; Cordes J.; Dlugolenski K.; Edgerton J. R.; Harris A. R.; Helal C. J.; Jenkinson S.; Kauffman G. W.; Kenakin T. P.; Lazzaro J. T.; Lotarski S. M.; Mao Y.; Nason D. M.; Northcott C.; Nottebaum L.; O’Neil S. V.; Pettersen B.; Popiolek M.; Reinhart V.; Salomon-Ferrer R.; Steyn S. J.; Webb D.; Zhang L.; Grimwood S. Discovery of the potent and selective M1 PAM-agonist N-[(3R,4S)-3-hydroxytetrahydro-2H-pyran-4-yl]-5-methyl-4-[4-(1,3-thiazol-4-yl)benzyl]pyridine-2-carboxamide (PF-06767832): Evaluation of efficacy and cholinergic side effects. J. Med. Chem. 2016, 59, 6313–6328. 10.1021/acs.jmedchem.6b00544. [DOI] [PubMed] [Google Scholar]
- Moran S. P.; Cho H. P.; Maksymetz J.; Remke D.; Hanson R.; Niswender C. M.; Lindsley C. W.; Rook J. M.; Conn P. J. PF-06827443 displays robust allosteric agonist and positive allosteric modulator activity in high receptor reserve and native systems. ACS Chem. Neurosci. 2018, 10.1021/acschemneuro.8b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H. S.; Cannon C. E.; Drott J. T.; Kuduk S. D.; Uslaner J. M. The M1 muscarinic positive allosteric modulator PQCA improves performance on translatable tests of memory and attention in rhesus monkeys. J. Pharmacol. Exp. Ther. 2015, 355, 442–450. 10.1124/jpet.115.226712. [DOI] [PubMed] [Google Scholar]
- Rook J. M.; Berton J. L.; Cho H. P.; Gracia-Barrantes P. M.; Moran S. P.; Maksymetz J. T.; Nance K. D.; Dickerosn J. W.; Remke D. H.; Chang S.; Harp J. M.; Blobaum A. L.; Niswender C. M.; Jones C. K.; Stauffer S. R.; Conn P. J.; Lindsley C. W. A novel M1 PAM VU0486846 exerts efficacy in cognition models without displaying agonist activity or cholinergic toxicity. ACS Chem. Neurosci. 2018, 10.1021/acschemneuro.8b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See Supporting Information for full details.
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. Central nervous system multiparameter optimization desirability: application in drug discovery. ACS Chem. Neurosci. 2016, 7, 767–775. 10.1021/acschemneuro.6b00029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.