Abstract
Glycans attached to the IgG Fc domain affect strongly biological activities such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of therapeutic antibodies. However, molecular mechanism in the glycoform-dependent functional modulation of the IgGs remains elusive. The present study communicates that selected reaction monitoring (SRM)-based assay of tryptic IgG Fc glycopeptides is a promising approach for the characterization of antibodies when combined with structure-defined synthetic Fc peptides having a focused N-glycoform as a calibration standard. We describe a novel synthetic approach to the human IgG1 Fc peptide having a bisected decasaccharide and its nonbisected counterpart compound, the signatures of antibodies involving Fc domain with rare N-glycans expected to show much higher ADCC/CDC than abundant IgG N-glycans, and their application to the SRM-based quantitative glycoproteomics. Use of a key intermediate, phenyl (2-O-benzyl-4,6-O-benzylidine-β-d-mannopyranosyl)-(1 → 4)-3,6-di-O-benzyl-2-azido-2-deoxy-1-thio-β-d-glucopyranoside, derived from locust bean gum galactomannan, facilitated greatly the synthesis of a bisected nonasaccharide as a stable precursor of oxazoline derivative needed for the enzymatic trans-glycosylation with Fc nonapeptide carrying a GlcNAc at Asn297 residue, while the coupling reaction catalyzed by mutant endo-M-N175Q proceeded very slowly. Strikingly, SRM assay using the synthetic Fc glycopeptides as calibration standards uncovered the occurrence of the targeted IgG1 Fc fragment carrying a nonfucosylated and bisected (315 fmol, 0.20%) and its nonbisected counterpart (1154 fmol, 0.73%) in the tryptic digests from 158 pmol of anticancer antibody Herceptin (trastuzumab). The results suggest that aberrantly glycosylated IgG Fc variants may contribute to the total biological activities of the therapeutic antibodies.
Keywords: therapeutic antibodies, synthetic glycopeptides, selected reaction monitoring, glycoproteomics
The effector functions and efficacy of therapeutic monoclonal antibodies depend critically on post-translational glycosylation at Asn297 of human IgG Fc domain.1 The distribution of the N-glycan population of therapeutic antibodies is thus considered to be an important critical quality attribute (CQA), attracting particular interest because of its impact on antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of antibodies.2,3 The deletion of core fucose results in the IgG antibody having increased affinity for the FcγRIIIa receptor, with distinctly enhanced efficacy of NK cell-mediated ADCC.4,5 Recombinant human IgGs produced from engineered CHO cell lines to express high-level N-acetyl-d-glucosaminyl transferase III (GnTIII) that adds bisecting GlcNAc exhibited an improved ADCC by two or more orders of magnitude when compared with the original antibody.6 However, removal of terminal galactose from antibody reduced CDC without any effect on ADCC.7 Therefore, a promising method for the production of human IgGs with homogeneous glycoforms is required for the quality control of the therapeutic antibodies.8,9 Importantly, understanding the significance of heterogeneity in Fc N-glycans is a major challenge for the manufacture to provide an optimal efficacy and safety of the therapeutic antibodies.10−16
Our attention was first directed to N-glycan profile of the therapeutic antibodies produced by nonhumoral CHO cell lines. A preliminary analysis based on the glycoblotting-assisted MALDI-TOFMS18 identified nine major N-glycoforms (a–i) in Herceptin (trastuzumab) as an example of the approved anticancer therapeutic antibodies, in which six glycoforms are found to be core fucosylated N-glycans (Figure 1 and Table S1). This observation and the results reported by others8,10−16 suggest that nonfucosylated N-glycan structures (a), (b), and (d) may enhance ADCC, whereas other glycoforms including the bisected and fucosylated N-glycans (g) and (i) may not influence positively ADCC. Although N-glycan profiles of Herceptin might depend on the expression levels of various glycosyltransferases, the levels of sugar nucleotides in ER/Golgi compartments, and underlying mechanism of the metabolic/anabolic pathways in CHO cells, we assumed that some scarce nonfucosylated N-glycoforms listed in Figure 1 can also contribute significantly to the functions of antibodies. Especially, it seems likely that bisected and nonfucosylated N-glycans could have strong impact on CQA by the synergistic effects on the enhanced ADCC,4−6 even though their expression levels are extremely low levels when compared with the above major N-glycoforms identified in Herceptin. It was thought that the synthetic IgG Fc glycopeptides allow for the absolute quantitation of the tryptic Fc fragments bearing even such extremely rare N-glycoforms when used as calibration standards for selected/multiple reaction monitoring (SRM/MRM) channel setting.19,20
Figure 1.
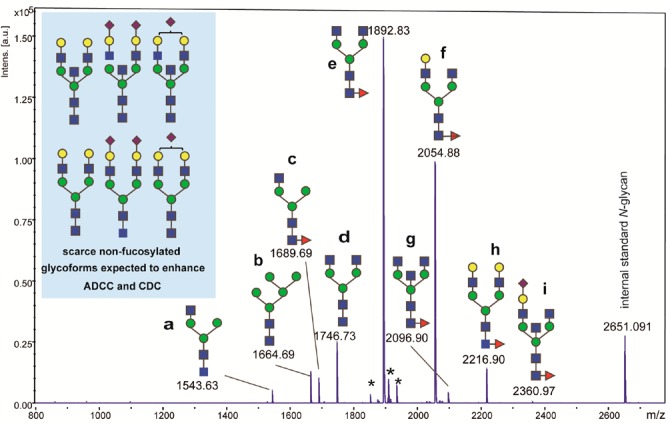
Major N-glycans released from Herceptin (trastuzumab) identified by glycoblotting-assisted MALDI-TOFMS, and some scarce nonfucosylated N-glycoforms supposed to show distinct ADCC could not be detected in this experiment. The m/z values indicate molecular mass of N-glycans tagged with an aminooxy-Trp-Arg reagent for enhancing the ionization potentials of free N-glycans, and asterisks represent peaks of unknown compounds or byproducts generated during on-beads chemical manipulations.17,18
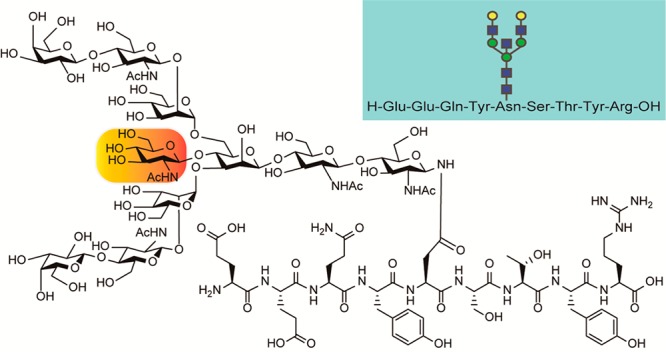
To test this hypothesis, we synthesized two of the scarce IgG1 Fc fragments that could be made by tryptic digestion of human IgG1 antibodies, notably nonfucosylated IgG1 Fc nonapeptide 1 carrying a bisected decasaccharide (a bisected G2) and its nonbisected counterpart 2 having a biantennary nonasaccharide (a biantennary G2), as the tentative targets (Figure 2). As shown in Scheme 1A, Fc fragment 2 having a simple biantennary G2 was prepared readily by using an established procedure as follows: (a) recombinant endo-β-N-acetyl-d-glycosaminidase (endo-M from Mucor hiemalis)-catalyzed trans-glycosylation between egg yolk sialoglycopeptide (SGP)21,22 and synthetic Fc GlcNAc-nonapeptide 3, and (b) subsequent sialidase treatment to trim the terminal sialic acids (Supporting Information). However, given that SGP provides only simple biantennary N-glycans, advent of an alternative approach that enables to construct glycopeptides having highly complicated N-glycans such multiantennary and bisecting glycoforms has been strongly required. In the previous study, we developed an efficient method for the synthesis of multiantennary N-glycans on the basis of a disaccharide intermediate 4, phenyl (2-O-benzyl-4,6-O-benzylidine-β-D-mannopyranosyl)-(1 → 4)-3,6-di-O-benzyl-2-azido-2-deoxy-1-thio-β-d-glucopyranoside, derived from abundant locust bean gum polysaccharide.23 It was demonstrated that oxazoline derivatives of tri- and tetra-antennary oligosaccharides can become substrates of an engineered endoglycosidase (endo-M-N175Q)24−27 and allow for the synthesis of erythropoietin peptides having tri- and tetra-antennary N-glycans.23 Importantly, the formation of β-glycoside found ubiquitously in the Manβ(1 → 4)GlcNAc unit is one of the most difficult steps in the glycoside synthesis because both the anomeric effect and neighboring-group participation of d-mannosyl donors are not beneficial for the stereoselective synthesis of β-mannosides.28−30 Efficiency of synthesis depends on the flexibility of the strategy in the multistep couplings between a central Manβ(1 → 4)GlcNAc moiety and glycosyl donors with different reactivity and steric effects of the protective groups.31,32
Figure 2.
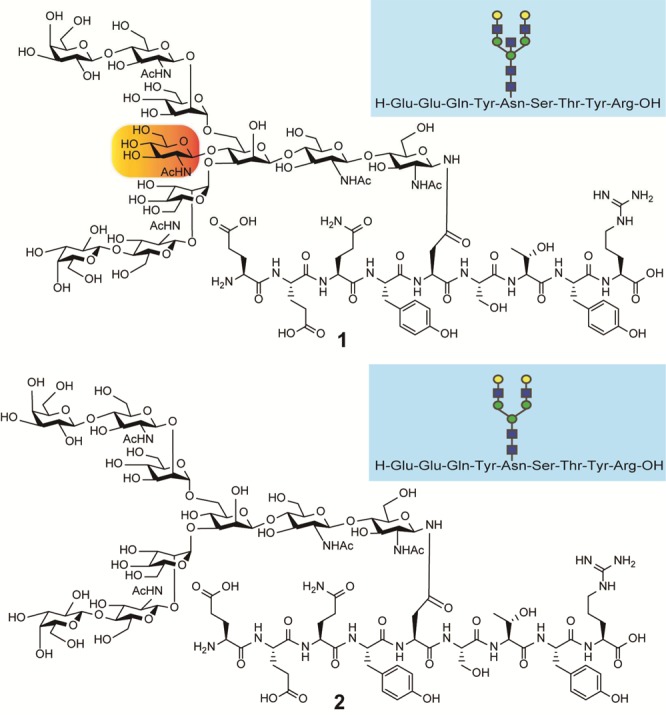
Chemical structures of the targeted tryptic Fc fragments from Herceptin (human IgG1 antibody) representing IgG1 Fc glycopeptide 1 modified with one of the scarce nonfucosylated and bisected decasaccharide (a bisected G2), and its nonbisected counterpart 2 having a biantennary nonasaccharide (a biantennary G2) listed in Figure 1.
Scheme 1. Synthetic Strategy of IgG1 Fc Glycopeptides.
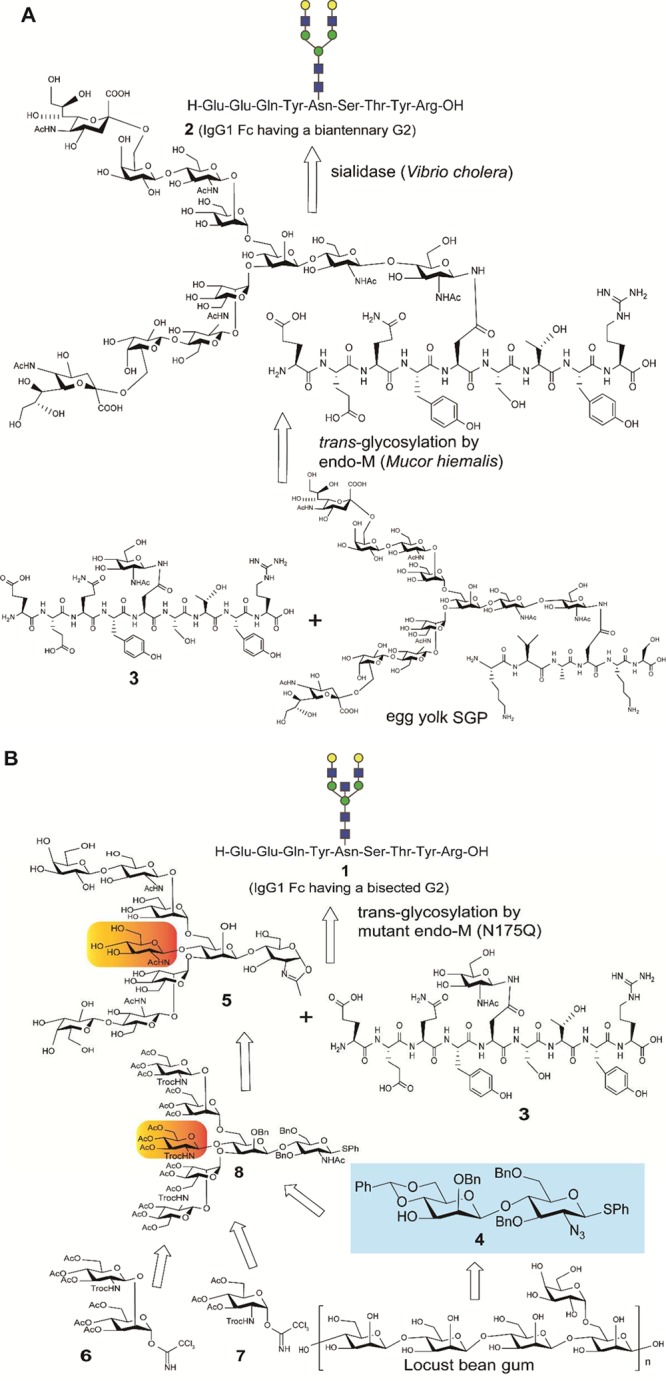
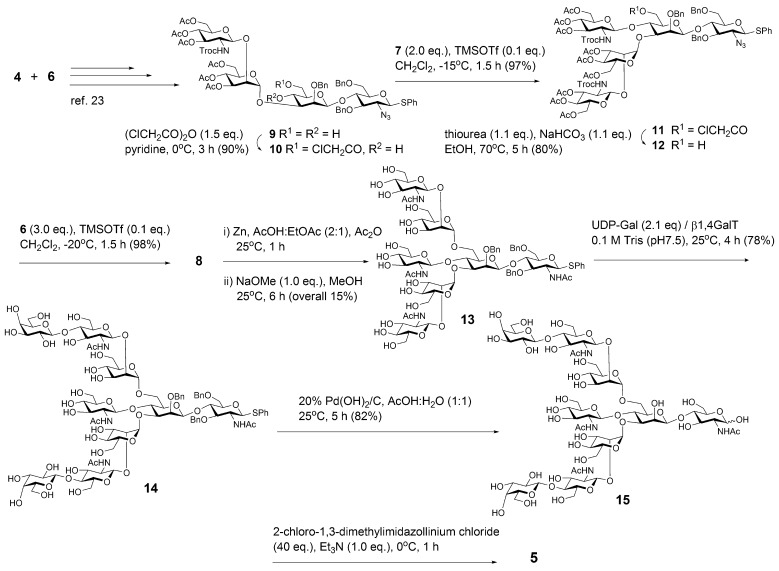
To establish an efficient synthesis of the Fc peptide carrying a bisected decasaccharide (1), we decided to use compound 4 as a key intermediate for the construction of versatile bisected synthons. It was considered that nonasaccharide oxazoline 5 from the synthetic blocks 4, 6, and 7 can be coupled directly with an acceptor 3 by using a mutant endo-M-N175Q to yield the target compound 1 (Scheme 1B). Advantage of the use of an intermediate 4 is evident because (a) core Manβ(1 → 4)GlcNAc unit 4 can be prepared in a large-scale manner,23 and (b) conversion of 4 into many glycosyl acceptors may permit rational synthesis of various multiantennary and complicated N-glycans including the bisected glycoforms.
To achieve rapid and efficient synthesis of an oxazoline 5, our interest was focused on the feasibility of an approach based on an early installation of a bisecting GlcNAc to the core N-glycan moiety (Scheme 2). Use of the tetrasaccharide diol 9(23) derived by coupling 4 with 6 may permit further seamless synthesis of the bisected N-glycans. Reaction of GlcNHTroc derivative 7 with compound 10 derived by the selective chloroacetylation of 9 proceeded smoothly to give the pentasaccharide 11 in 97% yield. Surprisingly, glycosylation of 12 having 6-OH with 6 afforded the bisected heptasaccharide 8 in 98% yield. These results clearly indicate that the combination of 2,2,2-trichloroethoxycarbonylamino (Troc)-protection of GlcNAc moiety and trichloroacetimidates 6 and 7 contributes greatly to the efficient installation of a bisecting GlcNAc and mannosyl branches into 3-OH, 4-OH, and 6-OH liberated sequentially from 4. Partially O-benzylated derivative 13 converted from 8 was modified by using recombinant human β1,4-galactosyl transferase (β1,4-GalT) in the presence of uridine diphosphoryl-α-d-galactose (UDP-Gal) to afford nonasaccharide 14 in 78% yield. Deprotection of benzyl and thio phenyl groups gave free bisected nonasaccharide 15 in 82% yield. Unstable oxazoline 5 formed by treating 15 with 2-chloro-1,3-dimethylimidazolinium chloride/triethylamine23,33 subjected directly to the glycosylation with an acceptor 3 by using a mutant endo-M-N175Q.
Scheme 2. Synthesis of Bisected N-Glycoforms from a Key Intermediate 4.
Coupling between oxazoline 5 and 3 catalyzed by endo-M-N175Q was found to proceed very slowly when compared with previous results,23−27 while IgG1 Fc glycopeptide 1 was identified as a peak at m/z 3036.48 (m/z 3037.15 [C120H187N19O70+Na]+) of the MALDI-TOFMS, in which the yield at 10 h after incubation was estimated to be only 4.7% (67.1 pmol/μL) based on the HPLC (Figure 3). Although this result indicates the limitation of endo-M-N175Q due to the donor substrate specificity, synthetic IgG1 glycopeptides 1 and 2 were employed preliminarily for the feasibility test of the SRM-based quantitation of the targeted glycopeptide fragments in the tryptic digests generated from anticancer therapeutic antibody, Herceptin (see also Supporting Information).
Figure 3.
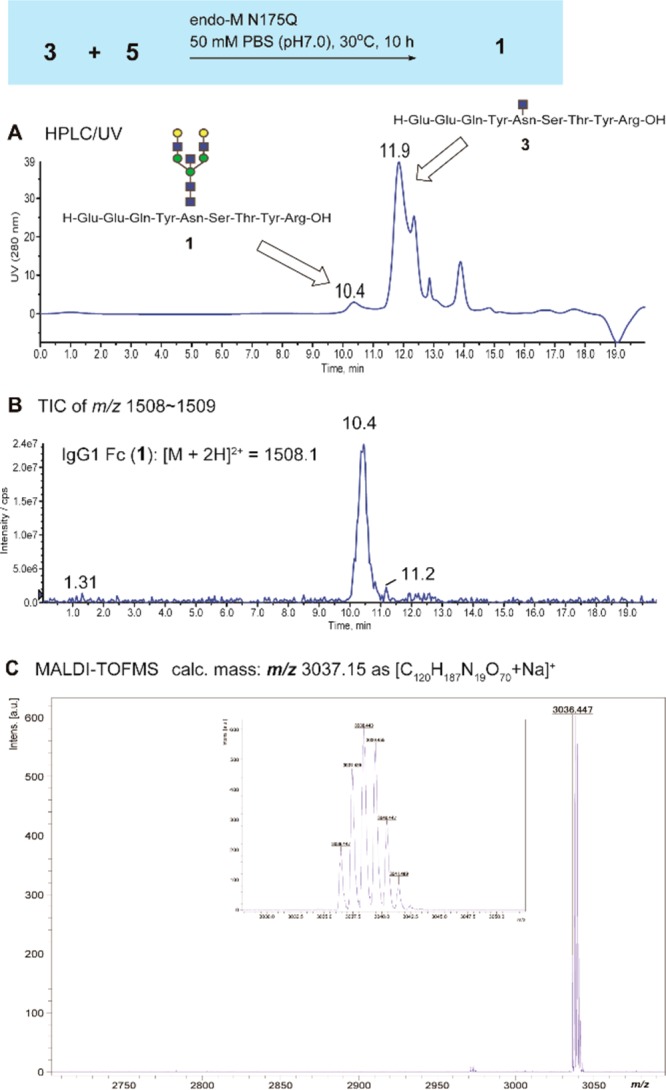
Glycosylation of oxazoline 5 with an acceptor peptide bearing GlcNAc residue 3 catalyzed by recombinant endo-M N175Q monitored by size-exclusion HPLC on Inertsil Diol detected by UV (A), TIC of a range of m/z 1508–1509 (B), and MALDI-TOFMS of the product eluted at 10.4 min (C).
SRM/MRM channel setting by using synthetic IgG1 Fc glycopeptides 1 and 2 was performed as follows: (i) precursor ion selection (Q1), (ii) collision induced dissociation (Q2), and (iii) product ion selection (Q3).19,20 Quadrupole works as mass filter and excludes other ions except the target ion, implying that fragmentations can be optimized by monitoring the actual measurement values obtained only from the ions due to the synthetic Fc glycopeptide 1 or 2. SRM/MRM parameters for the glycopeptides 1 and 2 were optimized using 4000 QTrap triple quadrupole mass spectrometer with UltiMate 3000 HPLC (Supporting Information). Q1 of compounds 1 and 2 were detected as proton adduct divalent positive 2+ ions at m/z 1508.3 [C120H187N19O70 + 2H]2+ and m/z 1407.2[C112H176N18O65 + 2H]2+, respectively. Fragmentation of the precursor ions by collision-induced dissociation (CID) was monitored with the enhanced product ion mode under gradual increase of collision energy (CE). Among the potential fragment ions generated, practically available Q3 and CE (Q2) were selected for setting the ideal SRM/MRM channels of compounds 1 and 2 with the best signal-to-noise ratio, Q3: m/z 366.2 [C14H24NO10]+ at 59 eV and m/z 2447.8 [C98H152N17O55]+ at 66 eV, respectively. However, it should be noted that N-acetyllactosamine oxonium ion selected as Q3 (m/z 366.2) can be generated from a glycopeptide having a triantennary isomer with the same Q1, while this glycoform has not been observed in human IgG1 Fc N-glycans.
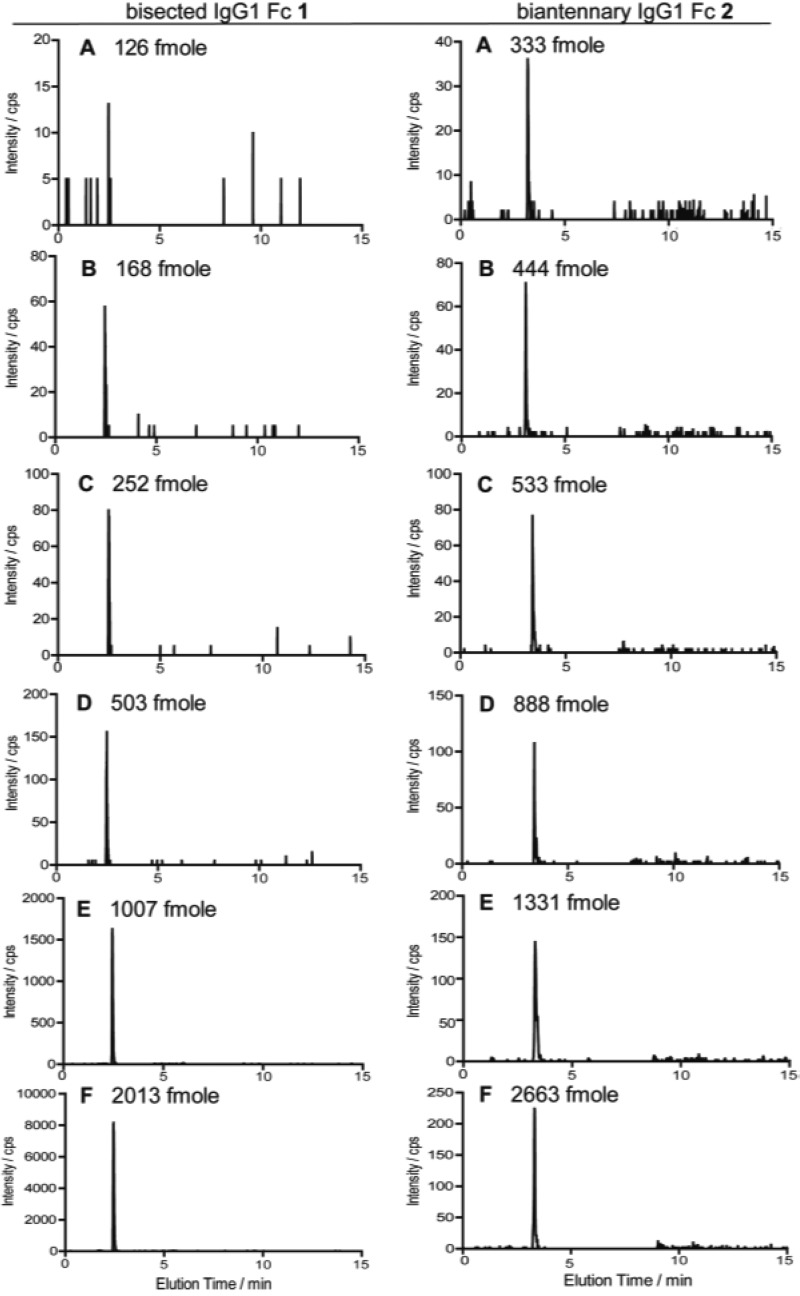
As shown in Figure 4, SRM total ion chromatograms (TICs) were measured by using the standard solutions with six different concentrations (A–F) for making the calibration curves. Calibration curves made by the mass peak area in the TICs corresponding to each Q3 (m/z 366.2 at 2.46 min for 1 and m/z 2447.8 at 3.30 min for 2) were shown in Figure 5 and employed for the glycoform-focused quantitative analysis of therapeutic antibodies.
Figure 4.
Total ion chromatograms of a series of standard solutions (A–F) in SRM analysis using synthetic IgG1 Fc glycopeptide 1 (left) with Q3 (m/z 366.2) and 2 (right) with Q3 (m/z 2447.8), respectively. Original solutions prepared from pure 1 and 2 were diluted, and 1.5 μL of each solutions were employed for the measurements.
Figure 5.
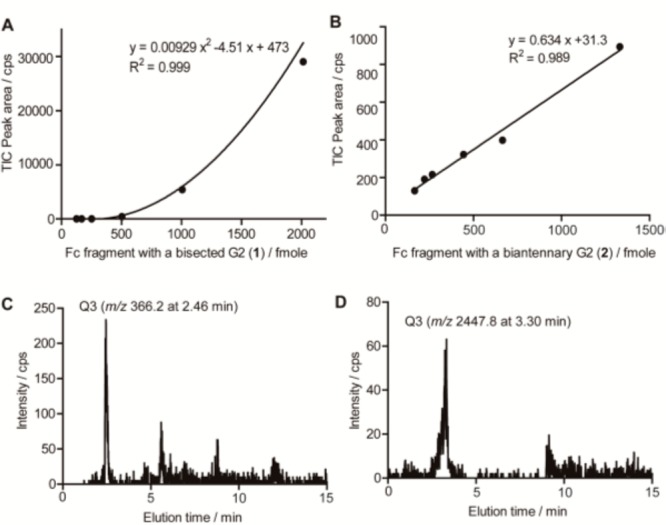
Glycoform-focused quantitative analysis of therapeutic antibody based on the SRM channels made by using synthetic human IgG1 Fc fragments having a bisected G2 (1) and a biantennary G2 (2). (A,B) Calibration curves for glycopeptides 1 and 2 were made by plotting the results of TICs (Figure 4). (C,D) TICs in the SRM assay using 1.5 μL of tryptic digests of Herceptin (158 pmole) uncovered the peaks of Q3 (m/z 366.2) at 2.46 min and Q3 (m/z 2447.8) at 3.30 min representing the occurrence of the targeted fragments corresponding to the Fc glycopeptides 1 (left) and 2 (right), respectively. Because of the detection limit, calibration curve for 1 did not give an ideal linearity below 200 fmol, indicating that accuracy on analyte quantities should be assessed in the assayed concentration range.
Finally, our interest was centered on the verification of the targeted quantitation of nonfucosylated IgG1 Fc fragments 1 and 2 in the tryptic digests of an anticancer antibody (Herceptin) by using the designated SRM channels. Strikingly, SRM assay revealed for the first time the occurrence of nonfucosylated and bisected IgG1 Fc fragment 1 (315.3 fmol, 0.2%) and its nonbisected counterpart 2 (1154 fmole, 0.7%) when the tryptic digests derived from 158 pmol of Herceptin were tested (Figure 5C,D; see also Supporting Information).
The results indicate that expression levels of the Fc domain having such extremely rare N-glycoforms are estimated to be only below 1% of whole N-glycans attached to Herceptin. However, given accumulated evidence that Fc N-glycoforms such as a bisected G2 and biantennary G2 may enhance dramatically ADCC without loss of CDC of the antibody drugs,2−8 the distribution of such low abundance nonfucosylated N-glycoforms at Asn297 residue is an important CQA of the antibodies. Notably, targeted quantitation of glycopeptide can avoid the influence of N-glycans of contaminated glycoproteins and/or even IgGs involving N-glycans at other glycosylation sites than Asn297 residue often existing in Fab domain.
In conclusion, we demonstrated for the first-time the occurrence of scarce N-glycoforms, bisected G2 and biantennary G2 structures, in Herceptin by means of synthetic IgG1 Fc glycopeptides as calibration standards for SRM-based targeted glycoproteomics. Notably, we established an efficient synthetic approach to the bisected N-glycoforms by using a key intermediate 4 derived from locust bean gum. Combined use of the synthetic oligosaccharide oxazolines and endoglycosidase (mutant endo-M-N175Q) facilitated construction of the human IgG1 Fc peptide carrying nonfucosylated bisecting N-glycan (1), while improvement of the substrate specificities of enzymes would expand the feasibility of this synthetic strategy. Given that monoclonal antibodies modified with rare nonfucosylated N-glycoforms could have remarkably strong impact on ADCC,2−8 the present results indicate that the designated SRM channels made by using synthetic glycopeptides will be nice tools for the reliable product assessment in terms of the distribution of the Fc N-glycan population, one of the most important CQAs of the therapeutic antibodies.10−16 It seems likely that an engineered antibody having such scarce Fc N-glycans will provide a new class of therapeutic antibody showing an improved efficacy. Our extensive efforts to construct a robust library of such human Fc glycopeptides are under way, and the results of comprehensive SRM-based analysis of various therapeutic antibodies will be reported as soon as possible.
Acknowledgments
This work was partly supported by a grant from the Ministry of Education, Culture, Science and Technology of Japan and JSPS (KAKENHI Grant 25220206). The authors appreciate the technical assistance in the preparation of the manuscript by Ms. Maki Morita and Ms. Tomoko Takahashi.
Glossary
ABBREVIATIONS
- ADCC
antibody-dependent cellular cytotoxicity
- SRM
selected reaction monitoring
- CQA
critical quality attribute
- MRM
multiple reaction monitoring
- CID
collision-induced dissociation
- CE
collision energy
- TIC
total ion chromatogram
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00127.
General procedure of experiments, synthesis, characterization of new compounds, and details for SRM/MRM channel setting and assay (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gornik O.; Pavic T.; Lauc G. Alternative glycosylation modulates function of IgG and other proteins - Implications on evolution and disease. Biochim. Biophys. Acta, Gen. Subj. 2012, 1820, 1318–1326. 10.1016/j.bbagen.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Arnold J. N.; Wormald M. R.; Sim R. B.; Rudd P. M.; Dwek R. A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- Jiang X. R.; Song A.; Bergelson S.; Arroll T.; Parekh B.; May K.; Chung S.; Strouse R.; Mire-Sluis A.; Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discovery 2011, 10, 101–111. 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- Shinkawa T.; Nakamura K.; Yamane N.; Shoji-Hosaka E.; Kanda Y.; Sakurada M.; Uchida K.; Anazawa H.; Satoh M.; Yamasaki M.; Hanai N.; Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- Satoh M.; Iida S.; Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin. Biol. Ther. 2006, 6, 1161–1173. 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]
- Umaña P.; Jean-Mairet J.; Moudry R.; Amstutz H.; Bailey J. E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 1999, 17, 176–180. 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- Boyd P. N.; Lines A. C.; Patel A. K. The effect of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol. Immunol. 1995, 32, 1311–1316. 10.1016/0161-5890(95)00118-2. [DOI] [PubMed] [Google Scholar]
- Hodoniczky J.; Zheng Y. Z.; James D. C. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog. 2005, 21, 1644–1652. 10.1021/bp050228w. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discovery 2009, 8, 226–234. 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Guideline on development, production, characterisation and specification for monoclonal antibodies and related products (EMA/CHMP/BWP/532517/2008). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/08/WC500211640.pdf
- Stadlmann J.; Pabst M.; Kolarich D.; Kunert R.; Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics 2008, 8, 2858–2871. 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- Damen C. W. N.; Chen W.; Chakraborty A. B.; van Oosterhout M.; Mazzeo J. R.; Gebler J. C.; Schellens J. H. M.; Rosing H.; Beijnen J. H. Electrospray ionization quadrupole ion-mobility time-of-flight mass spectrometry as a tool to distinguish the lot-to-lot heterogeneity in N-glycosylation profile of the therapeutic monoclonal antibody trastuzumab. J. Am. Soc. Mass Spectrom. 2009, 20, 2021–2033. 10.1016/j.jasms.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Toyama A.; Nakagawa H.; Matsuda K.; Sato T.-A.; Nakamura Y.; Ueda K. Quantitative structural characterization of local N-glycan microheterogeneity in therapeutic antibodies by energy-resolved oxonium ion monitoring. Anal. Chem. 2012, 84, 9655–9662. 10.1021/ac3023372. [DOI] [PubMed] [Google Scholar]
- Nwosu C.; Yau H. K.; Becht S. Assignment of core versus antenna fucosylation types in protein N-glycosylation via procainamide labeling and tandem mass spectrometry. Anal. Chem. 2015, 87, 5905–5913. 10.1021/ac5040743. [DOI] [PubMed] [Google Scholar]
- Upton R.; Bell L.; Guy C.; Caldwell P.; Estdale S.; Barran P. E.; Firth D. Orthogonal assessment of biotherapeutic glycosylation: A case study correlating N-glycan core afucosylation of Herceptin with mechanism of action. Anal. Chem. 2016, 88, 10259–10265. 10.1021/acs.analchem.6b02994. [DOI] [PubMed] [Google Scholar]
- Mimura Y.; Kelly R. M.; Unwin L.; Albrecht S.; Jefferis R.; Goodall M.; Mizukami Y.; Mimura-Kimura Y.; Matsumoto T.; Ueoka H.; Rudd P. M. Enhanced sialylation of a human chimeric IgG1 variant produced in human and rodent cell lines. J. Immunol. Methods 2016, 428, 30–36. 10.1016/j.jim.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Nishimura S.-I.; Niikura K.; Kurogochi M.; Matsushita T.; Fumoto M.; Hinou H.; Kamitani M.; Nakagawa H.; Deguchi K.; Miura N.; Monde K.; Kondo H. High-throughput protein glycomics: Combined use of chemoselective glycoblotting and MALDI-TOF/TOF mass spectrometry. Angew. Chem., Int. Ed. 2005, 44, 91–96. 10.1002/anie.200461685. [DOI] [PubMed] [Google Scholar]
- Nishimura S.-I. Toward automated glycan analysis. Adv. Carbohydr. Chem. Biochem. 2011, 65, 219–271. 10.1016/B978-0-12-385520-6.00005-4. [DOI] [PubMed] [Google Scholar]
- Anderson L.; Hunter C. L. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics 2006, 5, 573–588. 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- Kurogochi M.; Matsushista T.; Amano M.; Furukawa J.; Shinohara Y.; Aoshima M.; Nishimura S.-I. Sialic acid-focused quantitative mouse serum glycoproteomics by multiple reaction monitoring assay. Mol. Cell. Proteomics 2010, 9, 2354–2368. 10.1074/mcp.M110.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko A.; Koketsu M.; Nishizono M.; Enoki Y.; Ibrahim H. R.; Juneja L. R.; Kim M.; Yamamoto T. Occurrence of a sialylglycopeptide and free sialylglycans in hen’s egg yolk. Biochim. Biophys. Acta, Gen. Subj. 1997, 1335, 23–32. 10.1016/S0304-4165(96)00118-3. [DOI] [PubMed] [Google Scholar]
- Fujita M.; Shoda S.-I.; Haneda K.; Inazu T.; Takegawa K.; Yamamoto K. A novel disaccharide substrate having 1,2-oxazoline moiety for detection of transglycosylating activity of endoglycosidases. Biochim. Biophys. Acta, Gen. Subj. 2001, 1528, 9–14. 10.1016/S0304-4165(01)00164-7. [DOI] [PubMed] [Google Scholar]
- Kumar H. V. R.; Naruchi K.; Miyoshi R.; Hinou H.; Nishimura S.-I. A new approach for the synthesis of hyperbranched N-glycan core structures from locust bean gum. Org. Lett. 2013, 15, 6278–6281. 10.1021/ol403140h. [DOI] [PubMed] [Google Scholar]
- Umekawa M.; Higashiyama T.; Koga Y.; Tanaka T.; Noguchi M.; Kobayashi A.; Shoda S.-I.; Huang W.; Wang L.-X.; Ashida K. Efficient transfer of sialo-oligosaccharide onto proteins by combined use of a glycosynthase-like mutant of Mucor hiemalis endoglycosidase and synthetic sialo-complex-type sugar oxazoline. Biochim. Biophys. Acta, Gen. Subj. 2010, 1800, 1203–1209. 10.1016/j.bbagen.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Goodfellow J. J.; Baruah K.; Yamamoto K.; Bonomelli C.; Krishna B.; Harvey D. J.; Crispin M.; Scanlan C. N.; Davis B. An endoglycosidase with alternative glycan specificity allows broadened glycoprotein remodeling. J. Am. Chem. Soc. 2012, 134, 8030–8033. 10.1021/ja301334b. [DOI] [PubMed] [Google Scholar]
- Huang W.; Giddens J.; Fan S.-Q.; Toonstra C.; Wang L.-X. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J. Am. Chem. Soc. 2012, 134, 12308–12318. 10.1021/ja3051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Jiang K.; Zhu H.; Ma C.; Yu Z.; Li L.; Guan W.; Liu Y.; Zhu H.; Chen Y.; Wang L.-X. Site-directed glycosylation of peptide/protein with homogeneous O-linked eukaryotic N-glycans. Bioconjugate Chem. 2016, 27, 1972–1975. 10.1021/acs.bioconjchem.6b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H.; Unverzagt C. Highly branched oligosaccharides: A general strategy for the synthesis of multiantennary N-glycans with a bisected motif. Angew. Chem., Int. Ed. 2003, 42, 4261–4263. 10.1002/anie.200351625. [DOI] [PubMed] [Google Scholar]
- Wang G.; Zhang W.; Lu Z.; Wang P.; Zhang X.; Li Y.; Wang G.; Zhang W.; Lu Z.; Wang P.; Zhang X.; Li Y. Convenient synthesis of an N-glycan octasaccharide of the bisecting type. J. Org. Chem. 2009, 74, 2508–2515. 10.1021/jo900016j. [DOI] [PubMed] [Google Scholar]
- Wang P.; Zhu J.; Yuan Y.; Danishefsky S. J. Total synthesis of the 2,6-sialylated immunoglobulin G glycopeptide fragment in homogeneous form. J. Am. Chem. Soc. 2009, 131, 16669–16671. 10.1021/ja907136d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mönnich M.; Eller S.; Karagiannis T.; Perkams L.; Luber T.; Ott D.; Niemietz M.; Hoffman J.; Walcher J.; Berger L.; Pischl M.; Weishaupt M.; Wirkner C.; Lichtenstein R. G.; Unverzagt C. Highly efficient synthesis of multiantennary bisected N-glycans based on imidates. Angew. Chem., Int. Ed. 2016, 55, 10487–10492. 10.1002/anie.201604190. [DOI] [PubMed] [Google Scholar]
- Boltje T. J.; Buskas T.; Boons G.-J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 2009, 1, 611–622. 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M.; Tanaka T.; Gyakushi H.; Kobayashi A.; Shoda S.-I. Efficient synthesis of sugar oxazolines from unprotected N-acetyl-2-amino sugars by using chloroformamidinium reagent in water. J. Org. Chem. 2009, 74, 2210–2212. 10.1021/jo8024708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.