Important Compound Classes
Title
Spiro Bicyclic Inhibitors of Menin-MLL Interaction
Patent Application Number
WO 2018/050686 A1
Publication Date
March 22, 2018
Priority Application
US 62/394,295; EP 16192431.1
Priority Date
14 September 2016; 05 October 2016
Inventors
Angibaud, P. R.; Pande, V.; Herkert, B.; Krosky, D. J.; Querolle, O. A. G.; Pilatte, I. N. C.; Patrick, A. N.
Assignee Company
Janssen Pharmaceutica NV [BE/BE]; Turnhoutseweg 30, 2340 Beerse (BE).
Disease Area
Leukemia and different forms of cancer
Biological Target
Menin/MLL protein/protein interaction inhibition
Summary
The invention in this patent application relates to spiro bicyclic compounds represented generally by formula I. These compounds are menin/MLL protein/protein interaction inhibitors and may potentially be useful for treating leukemias [such as acute myeloid leukemia (AML)] and solid tumor cancers (such as prostate cancer).
Mixed lineage leukemia gene 1 (MLL1 or MLL), also known as histone-lysine N-methyltransferase 2A (KMT2A), is an enzyme that methylates histone H3 on lysine 4 (H3K4) and functions in multiprotein complexes. Studies have shown that while MLL1 is important in sustaining hematopoietic stem cells (HSCs) and developing B cells, its histone methyl transferase activity is not essential for hematopoiesis. MLL1 gene is known to undergo chromosomal rearrangements (or translocations). Chromosomal translocations are chromosomal abnormalities in which a segment from one chromosome is transferred to another chromosome or to a new site of the same chromosome. Studies have also shown that chromosomal rearrangements in MLL1 are linked to the pathogenesis of acute leukemias across all age groups. Leukemia is a form of cancer that starts in blood-forming tissue such as bone marrow and causes the production of large numbers of abnormal white blood cells in the bloodstream. Leukemia can be either chronic or acute. Acute leukemia is an aggressive form of the disease that progresses faster than chronic leukemia and requires immediate treatment. Acute leukemias harboring chromosomal translocations of MLL1 are classified as lymphoid, myeloid, or biphenotypic disease and constitute 5 to 10% of acute leukemias in adults and approximately 70% in infants. Acute leukemia has remained mostly an incurable disease. Thus, there is an urgent need for novel therapeutic approaches to treat acute leukemia.
The chromosomal translocations involving MLL1 cause the generation of novel chimeric proteins that still contain the amino-terminus of MLL. The amino-terminus can be fused with one of many possible partner proteins. To date, researchers have identified more than 60 protein partners that can form fusion complexes with MLL, and this fusion has been associated with the formation and progression of leukemia. Interestingly, the SET domain [Su(var.)3–9, Enhancer of zeste, and Trithorax) domain] of MLL is not retained in chimeric proteins but is replaced by the fusion partner. The fusion partners use chromatin modifying enzymes including Dot1L and/or the pTEFb complex to enhance transcription and transcriptional elongation of MLL target genes. The main MLL target genes include the HOX gene cluster A (HOXA) (e.g., HOXA9) and the HOX cofactor MEISJ.
Menin is a tumor suppressor protein that is encoded by the Multiple Endocrine Neoplasia type 1 (MEN1) gene. Menin is expressed ubiquitously in the nuclei of many types of cells and appears to be active in all stages of human development. It interacts with numerous proteins, and it is believed to be involved in a variety of cellular processes. The best understood function of menin is its role as an oncogenic cofactor of MLL fusion proteins. Menin interacts with two motifs within the N-terminal fragment of MLL, that is retained in all fusion proteins, namely, menin-binding motifs 1 and 2 (MBM1 and MBM2). The menin/MLL interaction leads to a structural modification that allows the binding of the lens epithelium-derived growth factor (LEDGF). LEDGF is a transcriptional coactivator believed to play a role in cancer, autoimmunity, and HIV proviral integration. While MLL can directly bind LEDGF, menin is needed to stabilize the interaction between MLL and LEDGF and the gene specific chromatin recruitment of the MLL complex via the PWWP domain of LEDGF. There is a clear evidence that menin performs a critical role in oncogenic transformation by MLL fusion proteins. Studies have also shown that menin is required for the maintenance of HOX gene expression by MLL fusion proteins. These findings have led to the conclusion that inhibition of the menin/MLL interaction is an attractive therapeutic target for the treatment of several forms of cancer. Thus, the development and testing of several small molecule inhibitors of menin/MLL interaction have demonstrated the efficacy of these inhibitors in preclinical models of acute myeloid leukemia (AML). The above data, together with the observation that menin is not a requisite cofactor of MLL1 during normal hematopoiesis, validate the potential of disruption of menin/MLL interaction as a promising new therapeutic approach for the treatment of leukemia and other cancers with an active HOX/MEISJ gene signature.
Related studies have revealed an internal partial tandem duplication (PTD) within the 5′ region of the MLL gene as another major abnormality that is found predominantly in de novo and secondary AML as well as myeloid dysplasia syndromes. While the molecular mechanism and the biological function of MLL-PTD is not yet well understood, the inhibition of the menin/MLL interaction may potentially prove effective in the treatment of MLL-PTD-related leukemias. Another potential benefit is a possible treatment for the castration-resistant prostate cancer, which has been shown to be dependent on the menin/MLL interaction.
Key Structures
The inventors described the structures
of 324 compounds of formula I including the following representative
examples: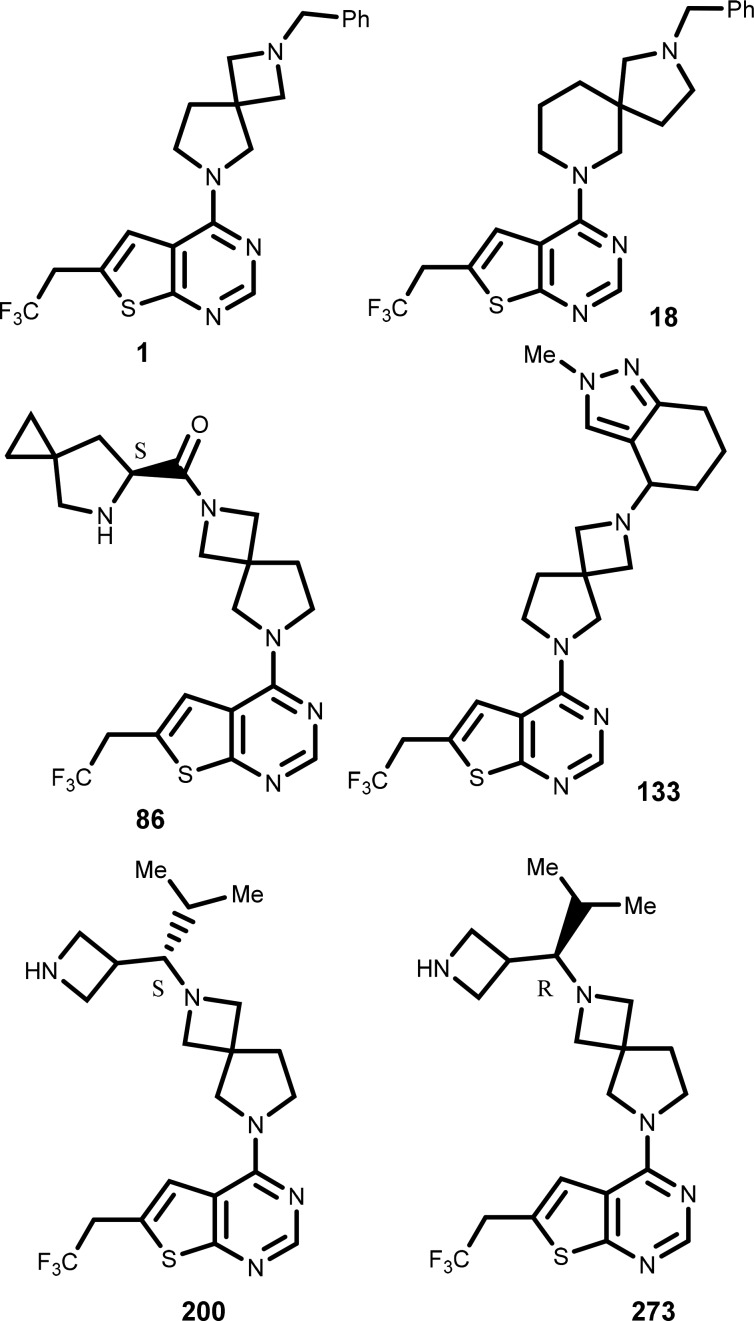
Biological Assay
The following biological assays were described:
-
1.
Menin/MLL fluorescence polarization (FP) assay
-
2.
Proliferation assay
-
3.
Menin/MLL homogeneous time-resolved fluorescence (HTRF) assay
Biological Data
The biological data obtained by performing
these assays on the above representative examples are listed in the
following table: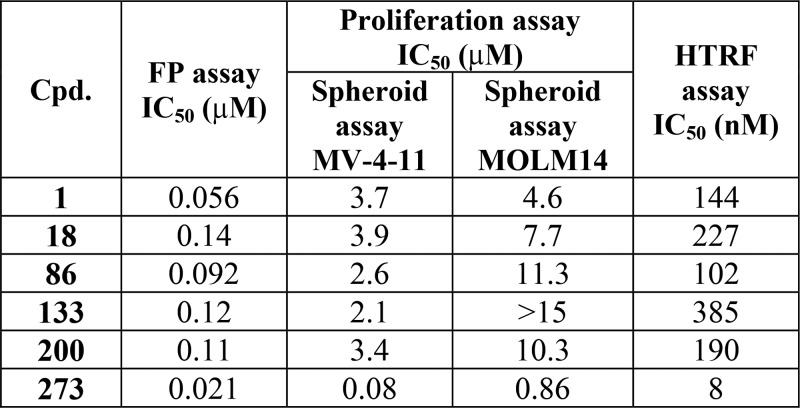
Recent Review Articles
-
1.
Kuhn M. W. M.; Armstrong S. A.. Cancer Cell 2015, 27 ( (4), ), 431–433.
-
2.
Cierpicki T.; Grembecka J.. Future Med. Chem. 2014, 6 ( (4), ), 447–462.
-
3.
Thiel A. T.; Huang J.; Lei M.; Hua X.. BioEssays 2012, 34 ( (9), ), 771–780.
The author declares no competing financial interest.
This paper was published ASAP on August 6, 2018, with minor errors in the biological data table. The corrected version was republished ASAP on August 7, 2018.


