Abstract
Pancreatic cancer (PC) has a very low average survival, but its prognosis is further reduced in the case of metastatic spread. Medical therapy in these cases is the only applicable methodology in the international guidelines. During anticancer treatments, common side effects are nausea, vomiting, arthralgia, neuropathy, and alopecia as well as a myelosuppressive effect. The toxicity of various drugs not only affects the quality of life of the patient, but often its severity requires a reduction in if not the termination of drug administration. Scientific studies have shown that a combined use of chemotherapy and certain natural substances, in the form of standardized extracts, can lead to an enhancement of the action of the chemotherapy. Here, we describe 2 cases of metastatic PC. The first case concerns the integrated treatment of a patient with cancer of the pancreas tail with metastatic involvement ab initio of peripancreatic lymph nodes and liver parenchyma, with numerous secondary lesions greater than 9.5 cm. The second case concerns the integrated treatment of a patient with cancer of the pancreatic body with metastatic involvement of the liver parenchyma, with a small secondary lesion. In both cases, an integrated cancer treatment approach, combining chemotherapy with natural remedies, extracts, and hyperthermia, induced a notable remission of primary and metastatic lesions.
Keywords: pancreatic cancer, metastasis, herbal medicine, hyperthermia, integrative therapy
Introduction
Pancreatic cancer (PC) is one of the most lethal solid tumors, and its prognosis is further reduced in the case of metastatic spread. In 2014, 337 872 patients were newly diagnosed with PC globally, and 330 391 patients died of the disease in the same year.1 The only curative approach for patients with pancreatic adenocarcinoma is surgical resection, but the vast majority of them (80% to 90%) have surgically inoperable disease, with 53% of patients carrying clinical or radiographic evidence of metastatic disease at diagnosis. Moreover, according to autopsy studies, approximately 90% of patients with PC, including resectable and unresectable forms, are metastatic at the time of manifestation.2-4 Chemotherapy remains the only applicable methodology for metastatic pancreatic adenocarcinoma, and the treatment varies according to the clinical and pathological stages of the disease, the patient’s age, and performance status. Based on these criteria, the following drugs are used: gemcitabine, taxanes, platinum derivatives, camptothecin, and fluorouracil and its derivatives, either alone or in combination.5-7 Unfortunately, during chemotherapy, common side effects arise, such as nausea, vomiting, arthralgia, neuropathy, and alopecia as well as a myelosuppressive effect.8,9 The toxicity of various drugs not only affects the quality of life (QoL) of the patient, but often its severity requires a reduction in if not a termination of drug administration.
Here, the term integrative oncology mostly refers to the use of a combination of conventional treatment with complementary and alternative medicine methods based on a certain level of evidence-based medicine. In cancer treatment, this often means adding to conventional treatment psycho-oncology interventions, movement therapies, art or mind body interventions, and natural remedies. The use of natural substances, such as herbal medicine or traditional Chinese medicine, and methods such as acupuncture, hyperthermia, and reflexology have been proved to be effective in the reduction of nausea, vomiting, asthenia, pain, neuropathy, myelosuppression, and other side effects.10-17 Scientific studies have shown that a combined use of chemotherapy and certain natural substances, in the form of standardized extracts, can lead to an enhancement of the action of the chemotherapy.18-21
Here, we report 2 cases of patients suffering from metastatic pancreatic tumor treated with integrative approaches, comprising conventional treatment, natural remedies, mind-body interventions (such as yoga and massage), hyperthermia, acupuncture, and movement therapy. Coadministration of chemotherapy, natural substances, and hyperthermia led in both cases to a complete remission of primary and metastatic lesions with a follow-up at 2 years.
It is important to underline the fact that the nature and purpose of our study were explained to all participants, who gave their informed written consent. These case studies were conducted in accordance with the principles outlined in the Declaration of Helsinki.
Case Report 1
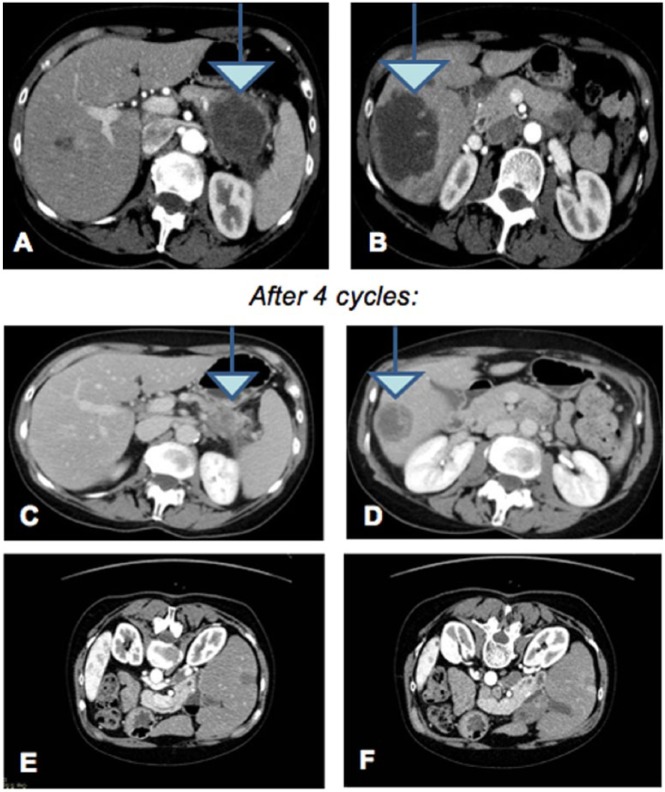
The first case concerns the integrated treatment of a 58-year-old woman affected by PC with metastatic involvement ab initio of peripancreatic lymph nodes and liver parenchyma, with numerous secondary lesions greater than 9.5 cm in size (Figures 1A and 1B) in March 2014. The disease was stage IV. A biopsy of the pancreatic lesion was done to define the histological features of the neoplastic lesion. The outcome was adenocarcinoma of pancreatic origin, moderately differentiated and parenchyma infiltrating. Because of the young age of the patient and her good health, chemotherapy based on 3 drugs was recommended: fluorouracil, irinotecan, and oxaliplatin in a 1-8-21 day regimen (FOLFIRINOX: oxaliplatin 85 mg/m2, intravenous [IV]; irinotecan 180 mg/m2, IV; 5-fluorouracil 400 mg/m2, IV; 5-fluorouracil 2400 mg/m2, IV over 46 hours). Side effects, such as severe nausea, vomiting, marked fatigue, and lack of appetite with a net impaired QoL, appeared at the beginning of the treatment. Because of such side effects, the second cycle of chemotherapy was given along with some natural substances known for their antitumor effects: curcumin for its antiangiogenic and antiproliferative properties18-20; polydatin for proapoptotic properties22-26; lactoferrin for antiproliferative and antimetastatic properties24,25; Active Hexose Correlated Compound (AHCC), a derivative of shitake mushroom cultured in rice bran extract, for its immunomodulating properties27; melatonin for immunomodulation28; and mistletoe for QoL and stimulation of bone marrow.29-31 Treatments were administered during and after chemotherapy, and the dose and daily time of treatment for each natural substance are reported in Table 1. Moreover, the patient underwent acupuncture treatment for nausea, vomiting, and pain, using the standard points (PC6, CV12, SP4, ST36).16,32,33 During chemotherapy, starting from the second cycle, the patient underwent capacitive regional deep hyperthermia treatment by means of an ONCOTHERM 3000, using the standard frequency of 13.6 MHz with 50-minute sessions. Every cycle of chemotherapy, the patient was monitored with blood counts (white and red blood cells as well as platelets) and tumor markers (CA 125, CA 19-9; CEA, all elevated) every 2 months, with liver function tests every month (alanine aminotransferase, gamma-glutamyltransferase, glutamic–pyruvic transaminase, lactate dehydrogenase, bilirubin total and fractionated, increased at the beginning). Initially, the number of blood cells was reduced, whereas tumor and liver markers were found to be upregulated. Throughout the treatment, all the parameters progressively improved toward normalization. At the end of the fourth cycle, the patient underwent an audit computed tomography (CT) that showed a net reduction of pancreatic injury and secondary liver lesions (Figures 1C and 1D). Although we did not use health-related QoL questionnaires, the patient’s general clinical status during the period of treatment was notable for side effects being almost absent, with only a mild asthenia the day after the administration of chemotherapy. Alopecia became complete only at the end of cycle III. The patient received massage therapy once a week during the entire period of chemotherapy. Once good clinical response and the well-being of the patient were established, we decided to administer 2 more cycles of the same chemotherapy to consolidate the results obtained. Oxaliplatin was withdrawn from the protocol because of minor sensory hand neuropathy referred by the patient.
Figure 1.
Computed tomography scan of March 2014 showing peripancreatic lymph nodes and liver parenchyma with numerous secondary lesions (A and B). Computed tomography scan of July 2014 after 4 cycles of integrated therapy that showed a net reduction in pancreatic injury and secondary liver lesions (C and D). After 2 more cycles, removing oxaliplatin, the result was the disappearance of the nuanced liver (E and F).
Table 1.
Natural substances administrated during and after chemotherapy.
| Compound | Concentration | Dosage |
|---|---|---|
| Curcumin (Norflo-Eye-pharma Meriva-patented) | 100 mg | 1 Capsule, twice a day |
| Polydatin (Polidal-Ghimas) | 40 mg | 2 Capsules, thrice a day |
| Lactoferrin (Oncophyt 10-Biogroup) | 300 mg | 1 Capsule, 4 times/d |
| AHCC (AHCC-International Biolife) | 500 mg | 2 Capsules, 3 times/d |
| Melatonin (Melatonmed-Natur) | 20 mg | 20 mg/d |
| Mistletoe (Viscum album Fermentatum “Qu”—quercus, Weleda Italia) | 10 mg | 1 Vial, 3 times/wk sc |
At the end of the sixth cycle, we observed the complete disappearance of the liver metastases, as evidenced by the diagnostic examination (Figure 1E and 1F). Currently, the patient has no late side effect, and blood tests are good in the absence of residual tumor. At the end of March 2017, 36 months after the diagnosis, the patient is still alive.
Case Report 2
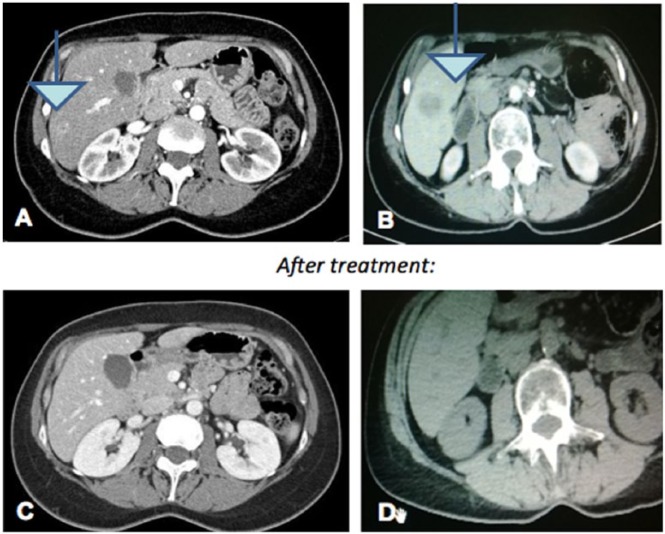
The second case concerns the integrated treatment of a patient bearing PC with metastatic involvement of the liver parenchyma with small secondary lesions. She was a 55-year-old woman operated for pancreatic pseudocyst in 2013. Following an abdominal ultrasound and a CT scan in September 2014, she was diagnosed with a lesion of the pancreatic body (Figures 2A and 2B). A biopsy of the lesion demonstrated a pancreatic histology of “moderately differentiated adenocarcinoma, G2, the pancreatic parenchyma.” The patient was characterized by young age and good compliance; thus, a treatment with FOLFIRINOX: fluorouracil + irinotecan + oxaliplatin (in a 1/8/21 regimen) was chosen (oxaliplatin 85 mg/m2, IV; irinotecan 180 mg/m2, IV; 5-fluorouracil 400 mg/m2, IV; 5-fluorouracil 2400 mg/m2, IV over 46 hours), with the addition of capacitive regional deep hyperthermia treatments (ONCOTHERM 3000 using the standard frequency of 13.6 MHz, with 50-minute sessions). Simultaneously with chemotherapy, the patient started taking some natural substances (used for their antitumor effects, as reported in case 1), such as curcumin,18-20 polydatin,22-26 lactoferrin,24,25 AHCC,27 melatonin,28mistletoe,29-31 and artemisinin,34 the last added for its proapoptotic properties. Dose and time of treatment for each natural substance are reported in Table 2. Moreover, the patient underwent acupuncture treatment for nausea, vomiting, and pain, using the points previously mentioned.16,32,33
Figure 2.
Computed tomography scan of September 2014 showing a lesion of the pancreatic body (A and B). At the end of the third cycle, a computed tomography scan showed a clear reduction of the pancreatic lesion (C and D).
Table 2.
Natural substances administrated during and after chemotherapy.
| Compound | Concentration | Dosage |
|---|---|---|
| Curcumin (Norflo-Eye-pharma Meriva-patented) | 100 mg | 1 Capsule, twice a day |
| Polydatin (Polidal-Ghimas) | 40 mg | 2 Capsules, thrice a day |
| Lactoferrin (Oncophyt 10-Biogroup) | 300 mg | 1 Capsule, 4 times/d |
| AHCC (AHCC-International Biolife) | 500 mg | 2 Capsules, 3 times/d |
| Melatonin (Melatonmed-Natur) | 20 mg | 20 mg/d |
| Mistletoe (Viscum album Fermentatum “Qu”–quercus, Weleda Italia) | 10 mg | 1 Vial, 3 times/wk sc |
| Artemisinin (Nutricology) | 100 mg | 1 Capsule, twice a day for 2 weeks |
The patient was monitored with instrumental examination, and it was observed that tumor markers as well as liver enzymes were normalized. At the end of the third cycle, a CT scan showed a clear reduction (60%) of pancreatic lesions (Figures 2C and 2D). She continued both treatments for 6 cycles. A new TC scan, after the sixth cycle of combined therapy, showed the quite complete disappearance of the pancreatic injury and of all the hepatic lesions (data not shown). Once a week during the period of chemotherapy, the patient received massage therapy and attended a yoga session. At the end of March 2017, 30 months after the diagnosis, the patient is still alive.
Discussion and Future Directions
Our work presents 2 cases in which an integrated treatment concept showed a remarkable outcome, with complete tumor response and tumor reduction as well as an improvement of the clinical conditions under chemotherapy in addition to a prolonged survival. PC with metastatic spread has a poor prognosis.35 Chemotherapy can often lead to the reduction of the primary and metastatic lesions in sensitive patients, but rarely leads to disappearance of the disease. Furthermore, the reduction is not lasting, with relapse and loss of sensitivity to anticancer drugs. The prognosis for cases of PC with metastases ab initio is very low and varies between 2.5 months and 12.8 months from diagnosis.22,23,35,36
The use of natural substances alongside chemotherapy has been reported to lead to a reduction in side effects (nausea, vomiting, neuropathy),29,30,37 which represent a severely detrimental aspect of chemotherapy. Interestingly, the effects of mistletoe in advanced PC patients have been reported in a randomized clinical trial, showing a prolongation of overall survival and a reduction of disease-related symptoms.31 Besides the widely used natural substances, we also used polydatin, a resveratrol glycoside isolated from Polygonum cuspidatum, whose conformational change provides resistance to enzymatic oxidation and a more efficient absorption by the intestine. Polydatin has been reported to possess a strong antimutagenic and antitumor action, involving pathways related to cell cycle, apoptosis, angiogenesis, and metastatic progression in vitro, in vivo, and in cancer patients.22,36 The clinical cases reported here, bearing liver and lymph node metastatic spread ab initio, were treated with a very aggressive chemotherapy protocol. The use of an integrated therapy combining chemotherapy with natural substances, such as curcumin, polydatin, modified shiitake, melatonin, mistletoe (see Tables 1 and 2), and other therapies, including hyperthermia, not only led to an almost complete reduction of side effects, but also caused the disappearance of both primary and secondary lesions. Such an unexpected result suggests supportive anticancer activity of the above-mentioned natural products when used in combination with chemotherapy.
These cases furnish a further demonstration that the integration of herbal medicine, acupuncture, nutrition, and capacitive regional deep hyperthermia in combination with anticancer treatment can be a way to proceed even in cases of metastatic malignant disease with poor prognosis.
Footnotes
Authors’ Note: Carla Fiorentini and Alessia Fabbri contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. Cancer facts and figures 2014. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. Accessed April 25, 2015.
- 3. Fortner JG, Kim DK, Cubilla A, et al. Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg. 1977;186:42-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [DOI] [PubMed] [Google Scholar]
- 5. Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088-2096. [DOI] [PubMed] [Google Scholar]
- 6. Lee JL, Kim SC, Kim JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery. 2012;152:851-862. [DOI] [PubMed] [Google Scholar]
- 7. Okada KI, Hirono S, Kawai M, et al. Phase I study of nab-paclitaxel plus gemcitabine as neoadjuvant therapy for borderline resectable pancreatic cancer. Anticancer Res. 2017;37:853-858. [DOI] [PubMed] [Google Scholar]
- 8. Otake A, Tsuji D, Taku K, et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with metastatic pancreatic cancer treated with gemcitabine. Eur J Clin Pharmacol. 2017;73:1033-1039. doi: 10.1007/s00228-017-2260-0 [DOI] [PubMed] [Google Scholar]
- 9. Nitta H, Baba H, Sugimori K, et al. Chemotherapy-induced nausea and vomiting in patients with hepatobiliary and pancreatic cancer treated with chemotherapy: a prospective observational study by the CINV study group of Japan. Anticancer Res. 2016;36:1929-1935. [PubMed] [Google Scholar]
- 10. Liu SH, Cheng YC. Old formula, new Rx: the journey of PHY906 as cancer adjuvant therapy. J Ethnopharmacol. 2012;140:614-623. [DOI] [PubMed] [Google Scholar]
- 11. Lee AM, Shandala T, Soo PP, et al. Effects of resveratrol supplementation on methotrexate chemotherapy-induced bone loss. Nutrients. 2017;9(3):pii: E255. doi: 10.3390/nu9030255 [DOI] [Google Scholar]
- 12. Yang JH. The effects of foot reflexology on nausea, vomiting and fatigue of breast cancer patients undergoing chemotherapy. Taehan Kanho Hakhoe Chi. 2005;35:177-185. [DOI] [PubMed] [Google Scholar]
- 13. Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655-663. [DOI] [PubMed] [Google Scholar]
- 14. He XR, Wang Q, Li PP. Acupuncture and moxibustion for cancer-related fatigue: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2013;14:3067-3074. [DOI] [PubMed] [Google Scholar]
- 15. Beuth J. Evidence-based complementary oncology: innovative approaches to optimise standard therapy strategies. Anticancer Res. 2010;30:1767-1771. [PubMed] [Google Scholar]
- 16. Towler P, Molassiotis A, Brearley SG. What is the evidence for the use of acupuncture as an intervention for symptom management in cancer supportive and palliative care: an integrative overview of reviews. Support Care Cancer. 2013;21:2913-2923. [DOI] [PubMed] [Google Scholar]
- 17. Palazzi M, Maluta S, Dall’Oglio S, Romano M. The role of hyperthermia against cancer. Tumori. 2010;96:902-910. [PubMed] [Google Scholar]
- 18. Wei Y, Pu X, Zhao L. Preclinical studies for the combination of paclitaxel and curcumin in cancer therapy (review). Oncol Rep. 2017;37:3159-3166. [DOI] [PubMed] [Google Scholar]
- 19. Kumar P, Barua CC, Sulakhiya K, Sharma RK. Curcumin ameliorates cisplatin-induced nephrotoxicity and potentiates its anticancer activity in SD rats: potential role of curcumin in breast cancer chemotherapy. Front Pharmacol. 2017;8:132. doi: 10.3389/fphar.2017.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bimonte S, Barbieri A, Leongito M, et al. Curcumin anti cancer studies in pancreatic cancer. Nutrients. 2016;8(7):pii: E433. doi: 10.3390/nu8070433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yue Q, Gao G, Zou G, et al. Natural products as adjunctive treatment for pancreatic cancer: recent trends and advancements. Biomed Res Int. 2017;2017:8412508. doi: 10.1155/2017/8412508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel KR, Brown VA, Jones DJ, et al. Clinical pharmacology of resveratrol and its metabites in colorectal cancer patients. Cancer Res. 2010;70:7392-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62:2540-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kozu T, Iinuma G, Ohashi Y, et al. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev Res. 2009;2:975-983. [DOI] [PubMed] [Google Scholar]
- 26. Boukes GJ, Van de Venter M. The apoptotic and autophagic properties of two natural occurring prodrugs, hyperoside and hypoxoside, against pancreatic cancer cell lines. Biomed Pharmacother. 2016;83:617-626. [DOI] [PubMed] [Google Scholar]
- 27. Kulkarni AD, Calder P, Ito T, eds. Clinician’s Guide to AHCC: Evidence-Based Nutritional Immunotherapy. 2016. Published by International Congress on Nutrition and Integrative Medicine. [Google Scholar]
- 28. Lissoni P, Rovelli F. Principles of psychoneuroendocrinoimmunotherapy of cancer. Immunotherapy. 2012;4:77-86. [DOI] [PubMed] [Google Scholar]
- 29. Matthes H, Friedel WE, Bock PR, Zänker KS. Molecular mistletoe therapy: friend or foe in established antitumor protocols? A multicenter, controlled, retrospective pharmaco-epidemiological study in pancreas cancer. Curr Mol Med. 2010;10:430-439. [DOI] [PubMed] [Google Scholar]
- 30. Cassady JM, Baird WM, Chang CJ. Natural products as a source of potential cancer chemotherapeutic and chemopreventive agents. J Nat Prod. 1990;53:23-41. [DOI] [PubMed] [Google Scholar]
- 31. Tröger W, Galun D, Reif M, et al. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: a randomised clinical trial on overall survival. Eur J Cancer. 2013;49:3788-3797. [DOI] [PubMed] [Google Scholar]
- 32. Deng G, Cassileth B. Acupuncture in cancer care. Oncology (Williston Park). 2011;25(7, suppl nurse ed):21-23, 30-31. [PubMed] [Google Scholar]
- 33. Johnstone PA, Polston GR, Niemtzow RC, Martin PJ. Integration of acupuncture into the oncology clinic. Palliat Med. 2002;16:235-239. [DOI] [PubMed] [Google Scholar]
- 34. Slezakova S, Ruda-Kucerova J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017;37:5995-6003. [DOI] [PubMed] [Google Scholar]
- 35. DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 10th ed. Alphen aan den Rijn, Netherlands: Wolters Kluwer; 2014. [Google Scholar]
- 36. Fuggetta MP, Lanzilli G, Tricarico M, et al. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J Exp Clin Cancer Res. 2006;25:189-193. [PubMed] [Google Scholar]
- 37. Saif MW, Lansigan F, Ruta S, et al. Phase I study of the botanical formulation PHY906 with capecitabine in advanced pancreatic and other gastrointestinal malignancies. Phytomedicine. 2010;17:161-169. [DOI] [PubMed] [Google Scholar]