Abstract
The aim of the present study was to investigate whether fraction from Lycium barbarum polysaccharide (LBP) could reduce immunotoxicity and enhance antitumor activity of doxorubicin (Dox) in mice. A water-soluble LBP fraction, designated LBP3, was isolated from edible Chinese herbal Lycium barbarum and used in this study. To investigate the effect of LBP3 on Dox-induced immunotoxicity, tumor-free mice were used and treated with either normal saline, Dox, or Dox plus LBP3. To investigate the effect of LBP3 on antitumor activity of Dox, H22 tumor-bearing mice were used and treated with either normal saline, Dox, LBP3, or Dox plus LBP3. The results showed that LBP3 did not protect against the body weight loss caused by Dox, but it promoted the recovery of body weight starting at day 5 after Dox treatment in tumor-free mice. LBP3 also improved peripheral blood lymphocyte counts, promoted cell cycle recovery in bone marrow cells, and restored the cytotoxicity of natural killer cells. Furthermore, in H22 tumor-bearing mice, LBP3 enhanced antitumor activity of Dox and improved peripheral blood lymphocyte counts and the cytotoxicity of splenocytes. In brief, our results demonstrated that LBP3 could reduce the immunotoxicity and enhance antitumor activity of Dox.
Keywords: Lycium barbarum polysaccharides, doxorubicin, immunotoxicity, antitumor activity, chemotherapy
Introduction
Cancer is a complex collection of distinct genetic diseases.1 Data from the World Health Organization show that cancer, which accounted for 8.8 million deaths in 2015, is the second leading cause of death globally. Chemotherapy is one of the conventional treatments for cancer.2 The anticancer drugs used in chemotherapy act by killing rapidly dividing cells in a cytotoxic manner.3 However, the drugs are not selective for cancer cells, killing healthy cells as well.3 It is known that chemotherapy often causes side effects, such as immunosuppression, nausea and vomiting, fatigue, and hair loss. The immunosuppression often leads to the development of opportunistic infections.4 As a result, the clinical application of chemotherapy may be hampered by the toxic side effects. New therapeutic strategies are urgently needed for chemotherapy to overcome side effects and enhance therapeutic efficacy.
Recent studies have shown that some Chinese herbals could reduce side effects and enhance the efficacy of anticancer drugs. For example, the traditional Chinese medical compound rocaglamide protected nonmalignant primary cells from DNA damage-induced toxicity by inhibiting p53 expression.4 The 4-herb Chinese formula PHY906 could reduce gastrointestinal toxicity induced by CPT-11 (irinotecan) and enhance its antitumor efficacy.5 Shenqi Fuzheng Injection, composed of Radix Codonopsis and Radix Astragali, could accelerate recovery of immunosuppression in patients with hematologic malignancies undergoing chemotherapy.6 Lentinan and pachymaran have been shown to have efficacy for accelerating recovery of immunosuppression and are used as therapeutic agents for cancer, accompanying chemotherapy.7-9
Doxorubicin (Dox) is one of the cytotoxic drugs that is widely used for treatment of solid and hematopoietic tumors. Dox kills the cancer cells by interacting with DNA and blocking the process of replication.10 However, it can kill healthy cells as well and cause toxic side effects. Cardiotoxicity and immunotoxicity are common toxic side effects caused by Dox. It has been demonstrated that polysaccharides from the edible Chinese herbal Lycium barbarum could alleviate Dox-induced cardiotoxicity,11 but its effect on immunotoxicity is unclear. Our previous study indicated that a medium-sized molecular weight fraction of Lycium barbarum polysaccharide (LBP), named LBP3, had the highest antitumor activity in LBP. In the present study, we further investigated whether LBP3 could reduce immunotoxicity and enhance antitumor activity of Dox in mice.
Materials and Methods
Materials
Doxorubicin for injection was purchased from Shenzhen Main Luck Pharmaceuticals Inc (Shenzhen, China). Propidium iodide (PI) was purchased from Sigma-Aldrich (St Louis, MO). 5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from eBioscience (Santa Clara, CA). FITC-anti-mouse CD3 and PE/CY5-anti-mouse CD8 were purchased from BioLegend (San Diego, CA). Fetal bovine serum (FBS) was purchased from Biological Industries (Kibbutz Beit-Haemek, Israel).
Preparation of LBP Fraction
The crude extract of LBP was prepared with hot water as described previously.12 The content of polysaccharide was 70.13% as determined by phenol-sulfuric acid method. A water-soluble LBP fraction, designated LBP3, was isolated by ultrafiltration membranes with molecular weight cutoff 40 kDa and 350 kDa. The fraction was collected and ground into powder. The powdered fraction was dissolved in normal saline for experiments in mice.
Cell Line and Animals
Murine hepatoma H22 cells were cultured in RMPI-1640 medium (containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin) and maintained at 37°C with 5% CO2 in a cell incubator. The cells were subcultured and used until reaching logarithmic growth phase.
Specific pathogen-free male BALB/c mice (weighing 20 ± 2 g) were purchased from the Guangdong Medical Laboratory Animal Center (Foshan, China) and kept under specific pathogen-free conditions. All mice were used in accordance with the National Institutes of Health guidelines, and all experimental procedures were approved by the Animal Care and Use Committee of Guangzhou University of Chinese Medicine, Guangzhou, the People’s Republic of China.
Animal Experiments and Treatment Protocol
It had been reported that LBP could alleviate the acute Dox-induced cardiotoxicity at dose of 200 mg/kg in rats.11 Furthermore, in our previous study, we found that LBP3 had the highest antitumor activity at the dose of 250 mg/kg in H22 tumor-bearing mice. Thus, LBP3 at a dose of 250 mg/kg was used in this study.
To investigate the effect of LBP3 on immunotoxicity caused by Dox, tumor-free mice were divided into a normal control group, a Dox group, and a Dox plus LBP3 group. Mice were orally administered with normal saline in the control group, intraperitoneally injected with Dox (5 mg/kg) in the Dox group, and orally administered with LBP3 (250 mg/kg) and intraperitoneally injected with Dox (5 mg/kg) in the Dox plus LBP3 group. Normal saline and LBP3 were given once daily for 10 days. Dox was injected on day 1. Body weight loss, peripheral blood white blood cell counts, cell cycles of bone marrow cells (BMCs), and cytotoxicity of splenocytes were assayed in this experiment.
To investigate the effect of LBP3 on the antitumor activity of Dox, mice were divided into a model group, a Dox group, a LBP3 group, and a Dox plus LBP3 group. All mice were injected with H22 cells (2 × 106 cells/mouse) in the right armpit subcutaneously to prepare tumor-bearing mice. In the model group, mice were orally administered with normal saline for 10 days. In the Dox group, mice were intraperitoneally injected with Dox (5 mg/kg) on day 1 and day 6. In the LBP3 group, mice were orally administered with LBP3 (250 mg/kg) once daily for 10 days. In the Dox plus LBP3 group, mice were orally administered with LBP3 (250 mg/kg) once daily for 10 days and intraperitoneally injected with Dox (5 mg/kg) on day 1 and day 6. Tumor inhibition rate, peripheral blood lymphocyte counts, and cytotoxicity of splenocytes were assayed in this experiment.
Determination of Body Weights and Tumor Inhibition Rate
Body weight was recorded daily. After mice were killed by cervical dislocation following the last administration, the tumor was immediately dissected and weighed. The antitumor activity of treatment was evaluated by tumor weight and tumor inhibition rate, as described previously.13 Briefly, tumor inhibition rate was calculated using the following equation: Tumor inhibition rate (%) = (the tumor weight of model group − the tumor weight of treatment group)/the tumor weight of model group × 100%.
Determination of White Blood Cell Counts in Peripheral Blood
Blood from the orbital venous plexus of the mice was collected into heparin tubes and the erythrocytes from blood of 50 µL were lysed in Red Blood Cell Lysis Buffer (NH4Cl 82.9 g, Na2EDTA 3.7 g, and KHCO3 10.0 g in 1 L buffer) for 5 minutes using water bath at 37°C. White blood cells were collected by centrifugation at 200× g for 5 minutes at 4°C and then washed twice with phosphate buffered saline (PBS). For calculating the relative white blood cell counts, cells were resuspended in 1 mL of PBS. The samples were analyzed on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) at medium speed for 1 minute. The relative cell counts of lymphocytes, monocytes, and granulocytes were determined. To calculate the relative cell counts of T-cell subsets, the cells were stained with PE/cy5-conjugated anti-mouse CD8 and FITC-conjugated anti-mouse CD3 antibodies at room temperature for 20 minutes in the dark. The samples were washed twice with PBS and resuspended in 1 mL of PBS, and then analyzed by flow cytometry (FCM).
Cell Cycle Assay
BMC suspensions were prepared from femurs of the mice. The femurs were flushed with 10 mL of PBS several times through syringe needles, and the erythrocytes were lysed in Red Blood Cell Lysis Buffer for 5 minutes at room temperature. After twice washing with PBS, the BMC was fixed with 70% ethyl alcohol for 24 hours at −20°C. Cells were then washed twice with PBS and labeled with 10 µg/mL PI staining solution for 10 minutes at room temperature in the dark. The cell cycle was determined by FCM and analyzed with ModFit software.
Cytotoxicity Assay
Splenocytes were prepared by gently pressing the spleen through a sterile 200-gauge steel mesh with the plunger of a syringe.14 The cytotoxicity of natural killer (NK) cells was assayed by FCM as described previously.15 Briefly, splenocytes were collected and washed twice with precold PBS at 200× g for 5 minutes at 4°C. The erythrocytes were lysed in Red Blood Cell Lysis Buffer for 5 minutes at room temperature, and the cells were washed twice with pre-cold PBS. The cells were resuspended in RPMI-1640 complete medium (containing 10% FBS, 100 µg/mL streptomycin, and 100 U/mL penicillin). Splenocytes were taken as the effector cells and H22 cells were used as the target cells. The H22 cells were labeled with CFSE solution according to the instruction and washed twice with PBS, and then resuspended in RPMI-1640 complete medium. The splenocytes (5 × 105 cells/well) were cocultured with H22 cells (1 × 104 cells/well) in 96-well round-bottom microplate for 24 hours in a cell incubator. The cells were collected and washed twice with PBS, and then labeled with 5 µg/mL PI staining solution for 10 minutes in the dark. Cytotoxicity of splenocytes against H22 cells was evaluated by calculating the percentage of PI-positive H22 cells with FCM.
Statistical Analysis
All values were expressed as mean ± standard error of the mean. Student t test was used to assess the statistical significance of differences between experimental groups. P <.05 was considered to be significant.
Results
LBP3 Restores Body Weight of Dox-Treated Tumor-Free Mice
To investigate the effect of LBP3 on immunotoxicity caused by Dox, tumor-free mice were used. All the mice were alive at the end of the treatment period. LBP3 did not reduce the body weight loss caused by Dox, but it promoted body weight recovery starting at day 5, and there was significant difference between Dox plus LBP3 group and Dox group in body weight recovery at the end of the treatment period (P < .05; Figure 1).
Figure 1.
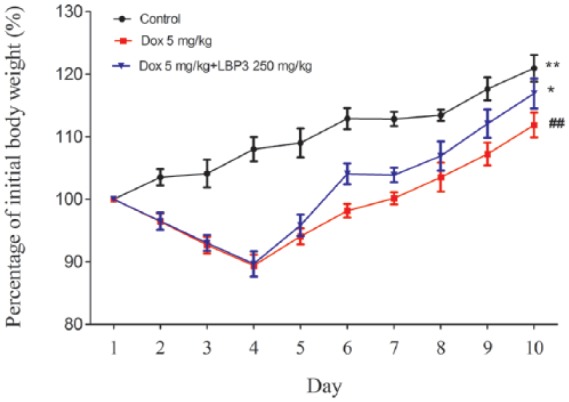
LBP3 restored body weight in Dox-treated tumor-free mice. Data were shown as the mean ± SEM (n = 6).
##P < .01 versus control group. *P < .05 and **P < .01 versus Dox group.
LBP3 Improves Peripheral Blood Lymphocyte Counts and Promotes Cell Cycle Recovery of BMC in Dox-Treated Tumor-Free Mice
Relative cell counts of lymphocytes, monocytes, and granulocytes in peripheral blood were taken to evaluate the effect of LBP3 on white blood cell counts in Dox-treated tumor-free mice. Dox treatment caused significant decrease in relative lymphocyte counts (P < .01; Table 1) compared with the control group, but not in monocyte and granulocyte counts. Compared with the Dox group, LBP3 significantly improved the relative lymphocyte counts of Dox-treated tumor-free mice (P < .05), and there was no difference when the Dox plus LBP3 group was compared with the control group.
Table 1.
Effect of LBP3 on Relative Peripheral White Blood Cell Counts in Dox-Treated Tumor-Free Micea.
| Group | Lymphocytes | Monocytes | Granulocytes |
|---|---|---|---|
| Control | 7785 ± 1112## | 905 ± 346 | 2974 ± 966 |
| Dox | 5879 ± 669** | 1085 ± 312 | 3831 ± 830 |
| Dox + LBP3 | 6810 ± 583# | 1178 ± 161 | 3343 ± 404 |
Abbreviations: LBP, Lycium barbarum polysaccharide; Dox, doxorubicin.
Data were shown as mean ± SEM (n = 6).
P < .01 versus the control group. #P < .05 and ##P < .01 versus the Dox group.
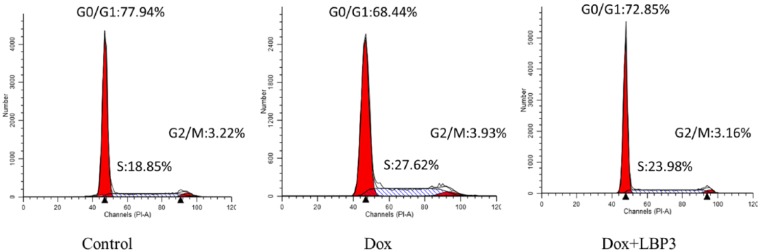
Cell cycle in BMC was analyzed to evaluate the effect of LBP3 on BMC in Dox-treated tumor-free mice. Dox treatment caused significant change in cell cycle of BMC leading to an obvious decrease in the number of cells in the G0/G1 phase with a significant increase in the S phase (P < .05; Figure 2 and Table 2) compared with the control group. Compared with the Dox group, LBP3 significantly increased the percentage of cells in the G0/G1 phase and decreased the number of cells in the S phase (both P < .05). There was no difference between the Dox plus LBP3 group and the control group in cell cycle of BMC. These results indicated that LBP3 promoted recovery of cell cycle of BMC in Dox-treated tumor-free mice.
Figure 2.
LBP3 promoted cell cycle recovery of BMC in Dox-treated tumor-free mice. The cell cycle was assayed by flow cytometry and analyzed with ModFit software. Data were shown as the mean ± SEM (n = 6).
Table 2.
Effect of LBP3 on Cell Cycle Progression of Bone Marrow Cells in Dox-Treated Tumor-Free Micea.
| Group | G0/G1 Phase (%) | S Phase (%) | G2/M Phase (%) |
|---|---|---|---|
| Control | 73.46 ± 3.52# | 22.75 ± 2.86# | 3.79 ± 0.77 |
| Dox | 68.23 ± 3.14* | 27.4 ± 2.93* | 4.37 ± 0.43 |
| Dox + LBP3 | 71.24 ± 1.54 | 24.71 ± 1.20 | 4.05 ± 0.66 |
Abbreviations: LBP, Lycium barbarum polysaccharide; Dox, doxorubicin.
Data were shown as mean ± SEM (n = 6).
P < .05 versus the control group. #P < .05 versus the Dox group.
LBP3 Restores the Cytotoxicity of NK Cells in Splenocytes of Dox-Treated Tumor-Free Mice
To investigate the effect of LBP3 on NK cells activity in Dox-treated tumor-free mice, splenocytes were prepared and cocultured with H22 cells. As shown in Figure 3, Dox treatment significantly inhibited the cytotoxicity of NK cells compared with the control group (P < .05). Compared with the Dox group, the cytotoxicity of NK cells was evidently improved in the Dox plus LBP3 group (P < .05), while there was no difference between the Dox plus LBP3 group and the control group in NK cell activity. These results indicated that LBP3 restored the cytotoxicity of NK cells in the Dox-treated tumor-free mice.
Figure 3.
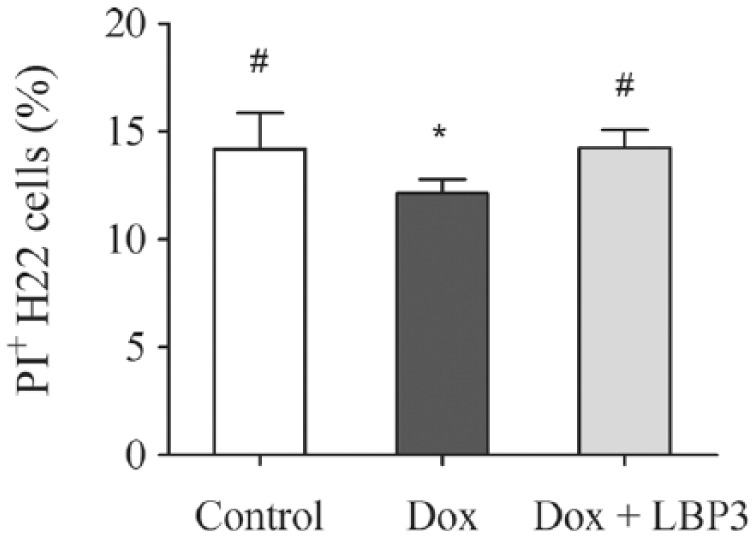
LBP3 restored the cytotoxicity of NK cells in Dox-treated tumor-free mice. H22 cells as target cells were labeled with CFSE before incubation with splenocytes. The effector cells (splenocytes) and target cells (H22 cells) were incubated for 24 hours at ratios of 50:1. After staining of PI, flow cytometry was performed to calculate the percentage of PI+ H22 cells. Data were shown as the mean ± SEM (n = 6).
*P < .05 versus control group. #P < .05 versus Dox group.
LBP3 Enhances the Antitumor Activity of Dox in H22 Tumor-Bearing Mice
To investigate the effect of LBP3 on antitumor activity of Dox, H22 tumor-bearing mice were used. Two mice were dead in the model group at the end of the treatment period, while all the mice were alive in the Dox group, the LBP3 group, and the Dox plus LBP3 group. As shown in Figure 4, Dox, LBP3, and Dox plus LBP3 significantly inhibited tumor growth in H22 tumor-bearing mice by 51.99%, 32.49%, and 79.06%, respectively. LBP3 significantly enhanced the antitumor activity of Dox in the Dox plus LBP3 group (compared with Dox group, P < .01).
Figure 4.
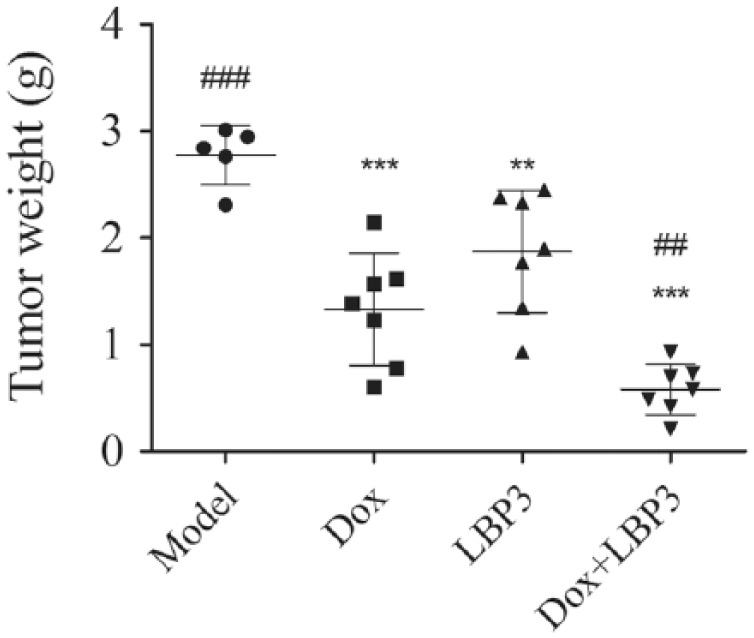
LBP3 enhanced the antitumor activity of Dox in H22 tumor-bearing mice. H22 tumor-bearing mice were used in this study. Once mice were killed by cervical dislocation at the end of the treatment period, the tumor was immediately dissected and weighed. Data were shown as the mean ± SEM (n = 7, n = 5 in model group).
**P < .01 and ***P < .001 versus model group. ##P < .01 and ###P < .001 versus Dox group.
LBP3 Improves Cell Counts of T-Cell Subsets in Dox-Treated H22 Tumor-Bearing Mice
Previous results had shown that LBP3 improved peripheral blood lymphocyte counts in Dox-treated tumor-free mice. We thus further investigated whether LBP3 could also improve cell counts of lymphocyte subsets in Dox-treated tumor-bearing mice. As shown in Table 3, Dox treatment caused significant decrease in relative cell counts of lymphocytes, T cells, CD8+ T cells, and CD8− T cells in tumor-bearing mice (all P < .01). LBP3 by itself did not affect cell counts of lymphocyte subsets in non–Dox-treated tumor-bearing mice. But it could improve cell counts of lymphocytes, T cells, CD8+ T cells, and CD8− T cells in Dox-treated tumor-bearing mice (P < .05 or .01).
Table 3.
Effect of LBP3 on Relative Peripheral Blood Lymphocyte Counts in Tumor-Bearing Micea.
| Group | Lymphocytes | T Cells | CD8+ T Cells | CD8− T Cells |
|---|---|---|---|---|
| Model | 5675 ± 689### | 3571 ± 644## | 712 ± 176## | 2886 ± 477## |
| Dox | 2601 ± 533*** | 2072 ± 566** | 348 ± 141** | 1724 ± 427** |
| LBP3 | 6530 ± 1844### | 3645 ± 977## | 636 ± 186## | 3050 ± 833## |
| Dox + LBP3 | 4326 ± 623# | 3613 ± 601## | 582 ± 118# | 3036 ± 495## |
Abbreviations: LBP, Lycium barbarum polysaccharide; Dox, doxorubicin.
Data were shown as mean ± SEM (n = 7, n = 5 in model group).
P < .05, **P < .01, and ***P < .001 versus the model group. #P < .05, ##P < .01, and ###P < .001 versus the Dox group.
LBP3 Restores the Cytotoxicity of Splenocytes in Dox-Treated H22 Tumor-Bearing Mice
Both NK cells and cytotoxic T lymphocytes in the splenocytes of tumor-bearing mice can kill tumor cells in a cytotoxic manner. To further investigate the effect of LBP3 on immune response in Dox-treated H22 tumor-bearing mice, the cytotoxicity of splenocytes was investigated. Dox treatment caused a significant decrease in the cytotoxicity of splenocytes in tumor-bearing mice compared with the control mice (P < .01; Figure 5). LBP3 by itself could enhance the cytotoxicity of splenocytes both in non–Dox-treated and Dox-treated tumor-bearing mice significantly (P < .01 and P < .05, respectively).
Figure 5.

LBP3 restored the cytotoxicity of splenocytes in Dox-treated H22 tumor-bearing mice. The splenocytes and H22 cells were incubated for 24 hours at ratios of 50:1. After being stained with PI, flow cytometry was performed to calculate the percentage of PI+ H22 cells. Data were shown as the means ± SEM (n = 7, n = 5 in model group).
*P < .05 and **P < .01 versus model group. #P < .05 and ##P < .01 versus Dox group.
Discussion
Dox is widely used for treatment of solid and hematopoietic tumors. However, its toxic side effects are a large obstacle in cancer treatment. Reducing side effects may help enhance the treatment efficacy of Dox in cancer chemotherapy. A previous study showed that LBP alleviated the acute cardiotoxicity caused by Dox in rats.11 In the present study, we found that a water-soluble LBP fraction, designated LBP3, could reduce immunotoxicity and enhance antitumor activity of Dox in mice.
First, we used tumor-free mice to investigate the effect of LBP3 on immunotoxicity induced by Dox. The results showed that LBP3 alleviated Dox-induced immunosuppression by promoting cell cycle recovery of BMC and improving peripheral blood lymphocyte counts and cytotoxicity of NK cells in splenocytes. It is known that bone marrow is the organ most affected during any immunosuppression therapy.16 One possible reason is that the anticancer cytotoxic drugs kill rapidly dividing cells including cancer cells and bone marrow stem cells by preventing DNA from replicating properly.3 And this interpretation is supported by our result that Dox arrested the BMC cell cycle in the S-phase. Since bone marrow is a primary lymphoid organ that regulates the development of immune cells from immature precursors,17 the BMC suppression would make it unable to regenerate new blood cells resulting in thrombocytopenia and leukopenia.16 Additionally, chemotherapy drugs, such as Dox and cisplatin, also cause DNA damage to lymphocytes and induce cell apoptosis.18 As is known, these might lead to significant morbidity and mortality. Thus, it is important to protect BMC and lymphocytes from damage in Dox treatment. In our study, as expected, we found that cell counts of lymphocytes, but not monocytes or granulocytes, were significantly decreased after Dox treatment. LBP3 promoted cell cycle recovery of BMC and improved the peripheral blood lymphocyte counts in Dox-induced mice, but had little effect on monocytes or granulocytes. These results indicated that LBP3 could reduce Dox-induced immunotoxicity in mice. And this is also supported by another finding in our study that LBP3 could restore NK cell activity, which plays an important role in antitumor immune response via producing cytokines and chemokines and exerting direct cytolytic activity.19 There are several normal methods for NK cell cytolytic activity assay, such as 51Cr-release assay,20 MTT assay,16 and FCM.15 FCM can detect the status of target cells that are killed by NK cells in a single-cell level, and it was used in our experiment. The results showed that LBP3 restored the cytolytic activity of NK cells to H22 cells in Dox-treated mice. These results demonstrate that LBP3 can reduce immunotoxicity caused by Dox.
Second, we further investigated the effect of LBP3 on antitumor activity of Dox. Dox is used effectively in therapy of liver cancer.21 Previous studies had shown that LBP by itself could inhibit tumor growth in mice.22-24 Our previous study demonstrated that LBP3 is the major component of LBP in inhibiting H22 tumor growth. Thus, we supposed LBP3 might enhance the antitumor activity of Dox in H22 tumor-bearing mice. As expected, results showed that LBP3 by itself could inhibit H22 tumor growth as well as enhance the antitumor activity of Dox.
Third, we continued investigating the effect of LBP3 on immunity in Dox-treated tumor-bearing mice. In the first part of our study, it was demonstrated that LBP3 could reduce Dox-induced immunotoxicity in tumor-free mice, but whether it also works in tumor-bearing mice is unknown. In this section, we further investigated the effect of LBP3 on lymphocyte counts and cytotoxicity of splenocytes in Dox-treated tumor-bearing mice. Since the T cell is the backbone of cell-mediated immunity and postchemotherapy T-cell recovery is a marker of improved survival in cancer patients,25 we not only investigated the lymphocyte counts but also the T-cell subsets in the mice. The results showed that Dox caused significant decrease in cell counts of lymphocytes, T cells, CD8+ T cells, and CD8− T cells in tumor-bearing mice. LBP3 by itself did not affect the cell counts of either lymphocytes or T-cell subsets, but it improved such cell counts in Dox-treated tumor-bearing mice. Furthermore, LBP3 could improve cytotoxicity of splenocytes by itself or in Dox-treated tumor-bearing mice. In fact, NK cells and cytotoxic T lymphocytes are 2 kinds of immune cells in splenocytes that can kill the cancer cells via a cytotoxic manner. The results indicate that LBP3 enhances the antitumor immune response in Dox-treated tumor-bearing mice.
In summary, we find that LBP3 not only reduces Dox-induced immunotoxicity in tumor-free mice and tumor-bearing mice but also enhances antitumor activity of Dox in H22 tumor-bearing mice. Since LBP3 is extracted from edible Chinese herbal Lycium barbarum, it may be a safe and attractive reagent for adjuvant therapy in Dox treatment.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a project funded by China Postdoctoral Science Foundation (Grant No. 2016M602548); the Special Funds from Central Finance of China in Support of the Development of Local Colleges and University (Educational Finance Grant No. 338 [2013] and No. 276 [2014]); and the national students’ project for innovation and entrepreneurship training program of China (Grant No. 201510572013).
References
- 1. Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nars MS, Kaneno R. Immunomodulatory effects of low dose chemotherapy and perspectives of its combination with immunotherapy. Int J Cancer. 2013;132:2471-2478. [DOI] [PubMed] [Google Scholar]
- 3. Levesque JP, Winkler IG. It takes nerves to recover from chemotherapy. Nat Med. 2013;19:669-671. [DOI] [PubMed] [Google Scholar]
- 4. Becker MS, Schmezer P, Breuer R, et al. The traditional Chinese medical compound rocaglamide protects nonmalignant primary cells from DNA damage-induced toxicity by inhibition of p53 expression. Cell Death Dis. 2014;5:e1000 http://www.nature.com/cddis/journal/v5/n1/full/cddis2013528a.html. Accessed January 2, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lam W, Bussom S, Guan F, et al. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2010;2:45ra59. [DOI] [PubMed] [Google Scholar]
- 6. Zhu XY, Zhang XZ, Zhong XY. Effect of Shenqi Fuzheng injection for hemopoietic and immune function reconstruction in patients with hematologic malignancies undergoing chemotherapy [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:205-207. [PubMed] [Google Scholar]
- 7. Kataoka H, Shimura T, Mizoshita T, et al. Lentinan with S-1 and paclitaxel for gastric cancer chemotherapy improve patient quality of life. Hepatogastroenterology. 2009;56:547-550. [PubMed] [Google Scholar]
- 8. Ina K, Furuta R, Kataoka T, et al. Lentinan prolonged survival in patients with gastric cancer receiving S-1-based chemotherapy. World J Clin Oncol. 2011;2:339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canhong W, Xiaowei H, Xiaoshan H, Dongyu L, Liyong L, Li C. Effect of carboxymethyl pachymaran on life extension and attenuation of cyclophosphamide-induced toxicity in CT26 tumor-bearing mice. Food Sci. 2016;37:229-233. [Google Scholar]
- 10. Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34:106-135. [DOI] [PubMed] [Google Scholar]
- 11. Xin YF, Wan LL, Peng JL, Guo C. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem Toxicol. 2011;49:259-264. [DOI] [PubMed] [Google Scholar]
- 12. Wang L, Yan L, Wang Q, Yang Y. Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. Biomed Res Int. 2014;2014:196198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of Astragalus membranaceus, polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2013;62:287-290. [DOI] [PubMed] [Google Scholar]
- 14. Luo X, Zheng Y, Wen R, Deng X, Zhou L, Liao H. Effects of ceftriaxone induced intestinal dysbacteriosis on lymphocytes in different tissues in mice. Immunobiology. 2016;221:994-1000. [DOI] [PubMed] [Google Scholar]
- 15. Kang TW, Kim HS, Lee BC, et al. Mica nanoparticle, STB-HO eliminates the human breast carcinoma cells by regulating the interaction of tumor with its immune microenvironment. Sci Rep. 2015;5:17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Tong X, Li P, Cao H, Su W. Immuno-enhancement effects of Shenqi Fuzheng injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J Ethnopharmacology. 2012;139:788-795. [DOI] [PubMed] [Google Scholar]
- 17. Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol. 2007;8:131-135. [DOI] [PubMed] [Google Scholar]
- 18. Nadin SB, Vargas-Roig LM, Drago G, Ibarra J, Ciocca DR. DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: a pilot study on the implications in the clinical response to chemotherapy. Cancer Lett. 2006;239:84-97. [DOI] [PubMed] [Google Scholar]
- 19. Kwon HJ, Kim N, Kim HS. Molecular checkpoints controlling natural killer cell activation and their modulation for cancer immunotherapy. Exp Mol Med. 2017;49:e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maher KJ, Klimas NG, Hurwitz B, Schiff R, Fletcher MA. Quantitative fluorescence measures for determination of intracellular perforin content. Clin Diagn Lab Immunol. 2002;9:1248-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154-2160. [DOI] [PubMed] [Google Scholar]
- 22. Chen S, Liang L, Wang Y, et al. Synergistic immunotherapeutic effects of Lycium barbarum polysaccharide and interferon-α2b on the murine Renca renal cell carcinoma cell line in vitro and in vivo. Mol Med Rep. 2015;12:6727-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang MH, Wang XY, Wang XM, Liu Q, Yang ML. Inhibition of Lycium barbarum polysaccharide on transplanted liver cancer in mice. Chin Tradit Herbal Drug. 2012;43:1142-1146. [Google Scholar]
- 24. Gan L, Zhang SH, Yang XL, Xu HB. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int Immunopharmacol. 2014;4:563-569. [DOI] [PubMed] [Google Scholar]
- 25. McCoy MJ, Lake RA, van der Most RG, Dick IM, Nowak AK. Post-chemotherapy T-cell recovery is a marker of improved survival in patients with advanced thoracic malignancies. Br J Cancer. 2012;107:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]