Abstract
Introduction: Stomach cancer, historically, has a low survival rate advances in curative resection procedures. Objectives: To assess the potential benefits of traditional herbal medicines in conjunction with chemotherapy in postoperative gastric cancer patients in terms of overall survival and disease-free survival. Data Sources: We searched the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, a Chinese database (CNKI), a Korean database, a Japanese database, AMED, and CINAHL up to September 2016. We summarized survival data from all RCTs. Study Selection: All RCTs of oral traditional medicines for resectable gastric cancer compared with chemotherapy alone were eligible. Data Extraction: Thirteen eligible trials with survival data (1075 patients) were deemed eligible for inclusion. Results: There were 217 documented deaths of the 574 patients assigned to adjuvant traditional medicines groups and 319 documented deaths of the 501 patients assigned to the chemotherapy-only groups. Adjuvant traditional medicines were associated with a statistically significant benefit in terms of overall survival rate (hazard ratio = 0.56; 95% confidence interval = 0.47-0.66; P < .00001) and disease-free survival (hazard ratio = 0.54; 95% confidence interval = 0.43-0.66; P < .00001). Conclusion: Among the RCTs included, the inclusion of postoperative adjuvant traditional medicines was associated with reduced risk of death in gastric cancer patients, when survival rates were compared with the group of patients who received chemotherapy alone. However, most of the included studies utilized are thought to be of low quality, so it would certainly appear that more trials are both advisable and necessary to arrive at correct and convincing conclusions.
Keywords: traditional herbal medicine, gastric cancer, randomized controlled trials, systematic review, meta-analysis
Introduction
Gastric cancer is the fifth most common cancer diagnosed and continues to represent the most common cause of gastrointestinal cancer–related death (causing more than 800 000 fatalities annually).1
Gastric cancer diagnosed at an early stage will often respond to surgical intervention with curative intent. However, the high rate of recurrence and poor survival after surgery would suggest a firm therapeutic basis for implementation of other, adjuvant treatments.2,3
Postoperative adjuvant chemotherapy has been associated with reduced risk of death in gastric cancer patients.3 However, adjuvant chemotherapy or adjuvant radiotherapy, when used alone, do not seem to significantly improve the survival rate following surgery.2,4
In recent years, the effect of natural products (when incorporated into traditional medicine) has demonstrated material and beneficial effects in improving available cancer therapeutics.5 Traditional medicine has been used and has been shown to increase the survival rate of gastric cancer patients (when combining therapy with conventional medicines) in East Asia.6 Also, traditional medicines have been often used to treat patients suffering from anemia or chronic exhaustive symptoms.7 It has been documented, in this regard, that traditional medicines have served to increase intestinal motility and decrease the negative postoperative symptoms of gastrectomy patients,8 and traditional medicines have been regularly prescribed to improve the postoperative quality of life of patients.9
Numerous studies have indicated that the combination of traditional medicine with chemotherapy can be used to enhance the efficacy of and diminish the side effects and complications of medical procedures.10 Yang et al reported that limited, weak evidence showed that traditional medicine improved leukopenia and decreased adverse events of chemotherapy in the short-term remission of advanced or late gastric cancer.11
However, there has been no comprehensive review of the randomized controlled trials (RCTs) on the benefit of adjuvant traditional medicine for resectable gastric cancer. In view of the foregoing, it was deemed important to assess the overall survival (OS) rate and overall disease-free survival (DFS) of adjuvant traditional medicine with chemotherapy quantitatively by means of a meta-analysis from all relevant trials.
Methods
Information Sources and Search Strategy
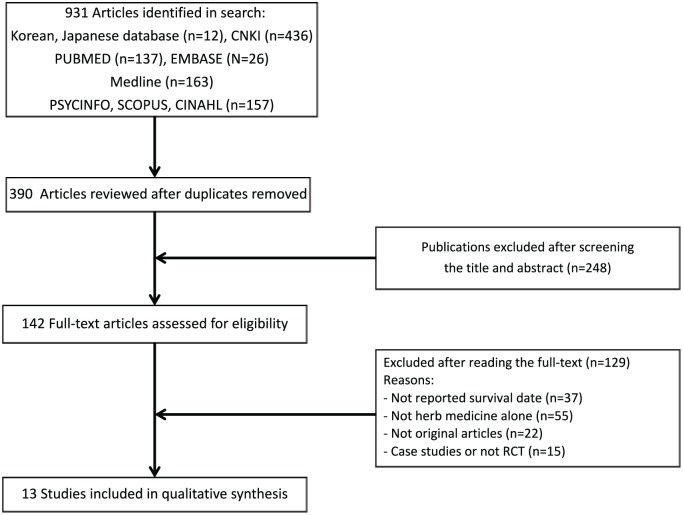
For this systematic literature review, several health care databases including Medline, CINAHL, a Chinese database (CNKI), a Korean database (Oriental Medicine Advanced Searching Integrated System), and a Japanese database (J-STAGE) were explored following the procedure suggested in PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Figure 1).12 Language differences were no constraint, and foreign-language articles were translated.
Figure 1.
Flow diagram for the study selection on resectable gastric cancer.
Searches were conducted up to September 2016, using the following search terms (“stomach cancer” or “stomach neoplasm” or “gastric cancer” or “gastric tumor” and “herb” or “traditional medicine” or “Traditional Chinese medicine” or “Chinese medicine” or “Kampo” or “plants”).
The results of these literature were investigated, and then the titles and abstracts of all articles were reviewed. The irrelevant articles and duplicates were eliminated. Finally, the references of all eligible full-text articles were examined for potentially relevant RCTs.
Eligibility Criteria and Study Selection
Two reviewers evaluated the inclusion criteria independently. Disagreements between reviewers about inclusion and exclusion were solved by discussion. Identified abstracts and citations were evaluated for the following inclusion criteria.
Types of Studies
Data from RCTs were sought electronically. Quasi-randomized trials were also included. The RCTs comparing the inclusion of adjuvant tradition medicine in conjunction with chemotherapy alone after surgery for resectable gastric cancers were included.
Types of Participants
Patients undergoing partial or total gastrectomy for gastric cancer with any overall TNM (tumor, node, and metastasis) stage, overall stage grouping system, or performance status (World Health Organization or Karnofsky Index). Patients of any age were included.
Types of Interventions
Studies that examined oral traditional medications with postsurgical chemotherapy versus postsurgical chemotherapy alone were included. We defined traditional medicines as any orally ingested product of any type (such as decoctions, capsules, and tablets) from plants, animal parts, and mineral medicines.
Drugs requiring intravenous administration were excluded. Combination of presurgical chemotherapy in patients were also excluded. Trials with radiotherapy were excluded. Chemotherapy included any protocol such as monochemotherapy, fluorouracil, motomycin C, or any other polychemotherapy regimens.
Types of Outcome Measures
We included studies that reported survival data as mortality, OS, and DFS. OS was defined as the survival rate from trials to the documented date of death form any cause or the last follow-up that was used as a censoring date. DFS was defined as the rate to relapse, metastasis, second cancer, or death form any other cause. OS and DFS were analyzed though hazard ratios (HRs). Secondary outcomes were adverse events.
Assessment of Risk of Bias
The authors individually evaluated risk of bias with the Cochrane Collaboration’s Tool for evaluating risk of bias.13 This tool supports consideration of “how the sequence was generated, how allocation was concealed, the integrity of blinding, the completeness of outcome data, selective reporting, and other biases.” If the raters disagreed, the final rating was made by consensus. The risk of bias in each domain was evaluated and categorized into 3 groups: (1) “low risk of bias,” “plausible bias unlikely to seriously alter the results”; (2) “high risk of bias,” “plausible risk of bias that seriously weakens confidence in the results”; and (3) “unclear risk of bias,” “plausible bias that raises some doubt about the results.”
Data Collection and Synthesis of Results
Data from the included studies in the review were individually recorded by each reviewer. Disagreements between reviewers were resolved by consensus. Trials were coded by 2 raters for reliability, and results were compared confirm accuracy. Trials were coded for the following characteristics (sample size, UICC [Union for International Cancer Control] stage of patients with gastric cancer, regimens of chemotherapy, schedule of interventions, and main components of traditional treatment).
OS and DFS were analyzed through the software package RevMan 5 provided by the Cochrane Collaboration. These statistics were entered directly into RevMan using the “O-E and Variance” outcome type. To calculate “O-E” and “V” statistics, a log-rank approach was applied for a HR. Fixed-effect meta-analysis methods were used in RevMan for “O-E and Variance” outcomes. Forest plots were used to display HRs with trials and overall. Subgroup analyses were examined according to the main components of traditional treatment. Heterogeneity between trials and subgroups of trials with different regimens of chemotherapy was calculated using χ2 statistics and analyzed with the I2 statistics. Sensitivity analysis was conducted. The treatment effects were examined according to high-/medium-quality studies, versus low-quality studies.
Results
Study Characteristics
The search of several health care databases including Medline, CINAHL, a Chinese database (CNKI), a Korean database, and a Japanese database provided a total of 931 citations. After extracting results that were missed in the initial vetting of duplicates, 390 articles remained. Of these, 248 studies were excluded because after reviewing the titles and the abstracts, they did not meet the criteria. Thus, 142 studies remained, which were then assessed more attentively for their eligibility to be included in the present review. The full texts of the remaining references were examined in more detail. The 37 studies without survival date were excluded. Finally, 13 trials14-26 with 1075 patients were identified for inclusion in the review (Figure 1).
The characteristics of the 1075 patients, traditional medicines used, and chemotherapy regimen are listed in Table 1. The main components of tradition medicines were identified as Hedyotis diffusa, Scutellaria barbata, Semen Coicis, Poria, Atractylodes macrocephala, and Radix Pseudostellariae. However, a wide variation was found between trials as for the traditional medicines regimens in all the trials. The methods of preparation of herb formulas were decoctions, pills, or tablets. In all studies, the primary outcome assessed was survival rate. Seven studies reported DFS. None of studies mentioned side effects or adverse events.
Table 1.
Characteristics of Included Randomized Trials.
| Source | Traditional Treatment (Main Components) | Chemotherapy, Dosage | Schedule | UICC Stage (No. of Patients) |
|---|---|---|---|---|
| Hedyotis diffusa + Scutellaria barbata | ||||
| Meng 201525 | Decoction (Scutellaria barbata 30 g, Hedyotis diffusa 10 g, etc); 300 mL/day for 3 months | Fluorouracil 500 mg/m2 IV | Every 4 weeks (6 cycles) for 2 years | I, 8; II, 36; III, 42; IV, 14 |
| Calcium folinate 100 mg/m2 IV, 5 days | ||||
| Oxaliplatin 130 mg/m2 IV, 1 day | ||||
| Yue 200919 | Pill (Scutellaria barbata 30 g, Hedyotis diffusa 30 g, etc) 45 g/day for 3 months | Fluorouracil 600 mg/m2 IV, 2-6 days | Every 3-4 weeks (3-4 cycles) | II, 101; III, 57 |
| Adriamycin 30 mg/m2 IV, 1-8 days | ||||
| Mitomycin 10 mg/m2 IV, 1 day | ||||
| Shi 200818 | Decoction (Scutellaria barbata 10-20 g, Hedyotis diffusa 15-30 g, etc); 20 days every year for 3 years | Tegafur 0.6 g/day orally | Every week for 2 months, 1 month for next year | NA 67 |
| Mitomycin 6-8 mg/m2 IV | ||||
| Tegafur 0.6 g/day orally | ||||
| Hedyotis diffusa + Semen Coicis | ||||
| He 201424 | Decoction (Semen Coicis 30 g, Hedyotis diffusa 30 g, etc); 300 mL/day for 6 months | Docetaxel 75-85 mg/m2 IV, 1 day | Every month (6 cycles) | II, 46; III, 53 |
| Cisplatin 75 mg/m2 IV, 1 day | ||||
| Fluorouracil 750 mg/m2 IV, 1-2 days | ||||
| Cui 200614 | Decoction (Semen Coicis 30 g, Hedyotis diffusa 24 g, etc); 500 mL/day for 6 months | Oxaliplatin 150 mg/m2 IV, 1 day | Every 3 weeks (6 cycles) | II, 15; III, 27 |
| Calcium folinate 200 mg/m2 IV, 1-3 days | ||||
| Fluorouracil 400 mg/m2 IV then 600 mg/m2 IV, 1-3 days | ||||
| Poria + Semen Coicis | ||||
| Fu 201626 | Decoction (Semen Coicis 30 g, Poria 20 g, etc), 200 mL/day | Fluorouracil 500 mg/m2 IV | Every 4 weeks (6 cycles) for 2 years | I, 7; II, 38; III, 40; IV, 15 |
| Calcium folinate 200 mg/m2 IV, 5 days | ||||
| Cisplatin 20 mg/m2 IV, 3 days | ||||
| Chen 200817 | Decoction (Semen Coicis 30 g, Poria 12 g, etc) | (Fluorouracil, Calcium folinate, Cisplatin) OR (Epirubicin, Fluorouracil, Cisplatin) OR (Oxaliplatin, Calcium folinate, Fluorouracil) | Every month (4-6 cycles) | III, 90 |
| Poria + Atractylodes macrocephala | ||||
| Fang 201322 | Ba Zhen Jiao Nang (Atractylodes macrocephala, Poria, etc), 12 pills/day for 4 months | Paclitaxel 175 mg/m2 IV, 1 day | Every 3 weeks (4 cycles) | II-III, 40 |
| Cisplatin 80 mg/m2 IV, 3 days | ||||
| Jiang 201120 | Decoction (Atractylodes macrocephala 30 g, Poria 15 g, etc) for 3 months | Fluorouracil 500 mg/m2 IV, 1-5 days | Every 4 weeks (6 cycles) | II-III, 96 |
| Calcium folinate 200 mg/m2 IV, 1-5 days | ||||
| Cisplatin 20 mg/m2 IV, 3-5 days | ||||
| Poria + Atractylodes macrocephala + Radix Pseudostellariae | ||||
| Zhou 200716 | Decoction (Radix Pseudostellariae 12 g, Atractylodes macrocephala 12 g, Poria 15 g, etc), every day for 3 months | Calcium folinate 200 mg/m2 IV, 5 days | Every 3 weeks (6 cycles) | NA 59 |
| Fluorouracil 500 mg/m2 IV, 5 days | ||||
| Cisplatin 20 mg/m2 IV, 5 days | ||||
| OR | ||||
| Calcium folinate 200 mg/m2 IV, 5 days | ||||
| Fluorouracil 500 mg/m2 IV, 5 days | ||||
| Epirubicin-Adriamycin 50 mg/m2 IV, 1 days | ||||
| Cisplatin 20 mg/m2 IV, 5 days | ||||
| Yang 200315 | Decoction (Radix Pseudostellariae 12 g, Atractylodes macrocephala 12 g, Poria 30 g, etc), every day for 6 months | Fluorouracil 500 mg/m2 IV, 1-5 days | Every 3 weeks (6 cycles) | II, 4; III, 81; IV, 4 |
| Epirubicin 50 mg/m2 IV, 1 day | ||||
| Mitomycin 4 mg/m2 IV, 1 day | ||||
| Other components | ||||
| Cai 201321 | Biejiajian Wan pill, 9 g/day for 6 months | Oxaliplatin 85 mg/m2 IV, 1 day | Every 2 weeks (12 cycles) | II-IV, 56 |
| Calcium folinate 200 mg/m2 IV | ||||
| Fluorouracil 400 mg/m2 IV, then 600 mg/m2 IV, 1-2 days | ||||
| Zhang 201323 | Norcantharidin 9 tablets/day for 6 months | Cisplatin 20 mg/m2 IV, 3 days | Every 4 weeks (6 cycles) for 2 years | II-III, 82 |
| Calcium folinate 200 mg/m2 IV, 5 days | ||||
| Fluorouracil 500 mg/m2 IV, 5 days | ||||
Abbreviations: UICC, Union for International Cancer Control; IV, intravenous; NA, not available.
Risk of Bias in Included Studies
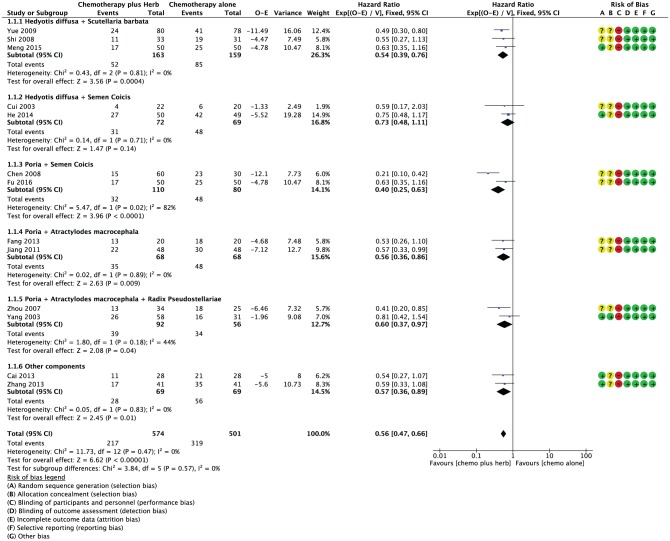
The methodological quality of the 13 included studies was assessed (Figure 2). Five studies described the method used to generate the allocation sequences or allocation concealment.15,21,23-25 Two studies used a computer-generated random table.23,24 Cai and Meng mentioned the random number table for generating a random allocation sequence.21,25 Two studied reported the use of sealed envelopes.15,16 None of the studies mentioned blinding of participants and personnel. All studies were silent regarding the blinding of outcome assessment; however, it judged that the outcome measurements of survival data were not likely to be influenced by lack of blinding.
Figure 2.
Risk of bias, overall survival, and overall hazard ratio for overall survival when comparing traditional medicine versus chemotherapy alone, and risk of bias of 13 included studies.
Synthesis of Results
Figure 2 shows the HRs for OS. There was a significant benefit from any traditional medicine plus chemotherapy, when compared with chemotherapy alone with an overall HR of death equal to 0.56 (95% confidence interval [CI] = 0.47-0.66; P < .00001). No significant heterogeneity was apparent across the set of trials (P = .47).
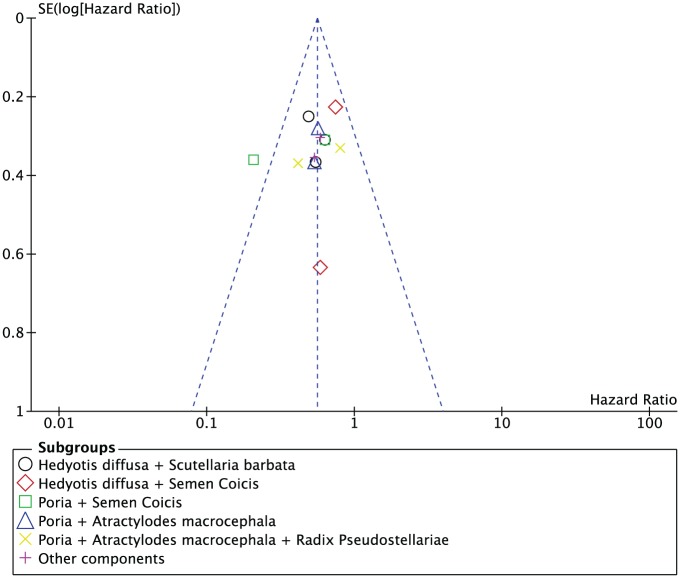
An interaction test between the type of regimen (Hedyotis diffusa + Scutellaria barbata, Hedyotis diffusa + Semen Coicis, Semen Coicis + Poria, Poria + Atractylodes macrocephala, Poria + Atractylodes macrocephala + Radix Pseudostellariae, and other component) and the treatment effect on OS were not significant (P = .57). Three trials involving 322 patients implemented herbal medicine including Hedyotis diffusa + Scutellaria barbata and a statistically significant benefit for OS was observed (HR = 0.54; 95% CI = 0.39-0.76; P = .0004).18,19,25 Two trials using traditional medicine including Hedyotis diffusa + Semen Coicis had 141 patients with OS data, but no significant effect was detected (HR = 0.73; 95% CI = 0.48-1.11;P = .14).14,24 The other regimens (Semen Coicis + Poria,17,26 Poria + Atractylodes macrocephala,20,22 Poria + Atractylodes macrocephala + Radix Pseudostellariae,15,16 and other component21,23) were explored comparing with chemotherapy alone, and they showed a statistically significant benefit of adjuvant traditional medicines over chemotherapy alone for resectable gastric cancer (HR = 0.40, 95% CI = 0.25-0.63, P < .0001; HR = 0.56, 95% CI = 0.36-0.86, P = .009; HR = 0.60, 95% CI = 0.37-0.97, P = .004; HR = 0.57, 95% CI = 0.36-0.89, P = .001, respectively). In sensitivity analyses, the summary overall HR remained statistically significant, including 5 trials with adequate sequence generation or allocation concealment (HR = 0.67, 95% CI = 0.52-0.87; P < .003). There was no perceptible asymmetry in funnel plots of these 13 studies (Figure 3).
Figure 3.
Funnel plots of 13 trials comparing traditional medicine versus chemotherapy alone.
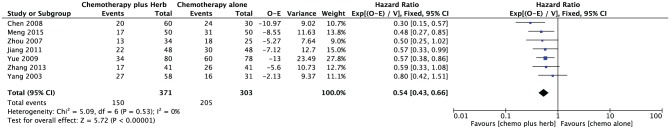
DFS was available on 7 trials with a total number of 674 patients from the 13 trials.15-17,19,20,23,25 The implementation of adjuvant traditional medicine improved DFS compared with chemotherapy alone with an overall HR of 0.54 (95% CI = 0.43-0.66; P < .00001). HRs for DFS in 7 trials and overall are shown in Figure 4. No significant heterogeneity was detected (P = .53).
Figure 4.
Disease-free survival and overall hazard ratio for disease-free survival when comparing traditional medicine versus chemotherapy alone.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis of adjuvant traditional herbal RCTs for resectable gastric cancer with OS. Overall, postoperative herbal treatments have not been the standard of care for gastric cancer, but the evidences from the meta-analysis showed the benefit of adjuvant herbal treatment with chemotherapy.
The 13 trials showed a statistically significant benefit associated with adjuvant herbal treatments after curative resection of gastric cancers. Seven trials reported statically significant difference for the improvement of rate of remission. Assessments of risk of bias showed potential biases in RCTs. The methods of allocation concealment for only 2 trials were clarified. Only 4 studies mentioned a random method. No blinding of outcome assessment was used; however, the assessment of total mortality may be considered unbiased. There was no perceptible asymmetry in funnel plots; however, the validity has been questioned because of the relatively small number of studies.27
Our review is limited by potential heterogeneity and bias from including methodological issues. The heterogeneity appeared at many fields in included studies, such as in the sample group, the herbal treatments, and the chemotherapies. Heterogeneity of study was considered a potential limitation in this systematic review. These trials had limited heterogeneity of studies making it difficult to draw definitive conclusions. Methodological heterogeneity included different components of herb, control interventions, and the stage of the cancer afflicting the particular patients.
Patient-level meta-analyses could not be carried out, so we were unable to address the estimates of the survival curve. Although there was no limitation of language, there were no non-Chinese articles included in the studies. Therefore, we could not reach a cogent conclusion. We suggest that future trials should focus on such methodological quality of the study with standard treatments (components of herb, treatment period, and treatment regimens) in multinational clinic centers.
Conclusion
In conclusion, our findings suggest that traditional herbal medicines are efficacious in the treatment of resectable gastric cancer. Among the RCTs included, postoperative adjuvant traditional medicines were associated with reduced risk of death (HR = 0.56) and disease (HR = 0.54) in gastric cancer compared with chemotherapy alone, and is recommended for patients with resectable gastric cancer. However, most of the included studies were of low quality with potential heterogeneity so that the conclusion is not convincing. More trials are needed to draw convincing conclusion about the benefits. Future studies based on more precise evaluation will estimate overall survival.
Footnotes
Authors’ Note: This systematic review was formatted according to PRISMA guidelines.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant of the Traditional Korean Medicine R&D Project, Ministry for Health & Welfare & Family Affairs, Republic of Korea (HI15C0006).
ORCID iD: Kyeore Bae  https://orcid.org/0000-0002-8596-8293
https://orcid.org/0000-0002-8596-8293
References
- 1. Kuipers EJ. Gastric cancer: synopsis and epidemiology of gastric cancer. In: Kim N. ed. Helicobacter pylori. Singapore: Springer; 2016:241-249. [Google Scholar]
- 2. Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(suppl 2):ii31-ii36. [DOI] [PubMed] [Google Scholar]
- 3. Foo M, Leong T. Adjuvant therapy for gastric cancer: current and future directions. World J Gastroenterol. 2014;20:13718-13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GASTRIC Group; Paoletti X, Oba K. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [DOI] [PubMed] [Google Scholar]
- 5. Efferth T, Li PC, Konkimalla VSB, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353-361. [DOI] [PubMed] [Google Scholar]
- 6. Jung JH, Seo JC, Kwak MA, Sohn KC. Literature review on traditional Chinese medicine treatment of gastric cancer. J Korean Oriental Intern Med. 2014;35:332-342. [Google Scholar]
- 7. Okamoto T, Motohasi H, Takemiya S, Sugimasa Y, Sairenji M, Kobayasi S. Clinical effects of Juzendaiho-to on immunologic and fatty metabolic states in postoperative patients with gastrointestinal cancer [in Japanese]. Gan To Kagaku Ryoho. 1989;16(4 pt 2-2):1533-1537. [PubMed] [Google Scholar]
- 8. Endo S, Nishida T, Nishikawa K, et al. Dai-kenchu-to, a Chinese herbal medicine, improves stasis of patients with total gastrectomy and jejunal pouch interposition. Am J Surg. 2006;192:9-13. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi T, Endo S, Nakajima K, Souma Y, Nishida T. Effect of rikkunshito, a Chinese herbal medicine, on stasis in patients after pylorus-preserving gastrectomy. World J Surg. 2009;33:296-302. [DOI] [PubMed] [Google Scholar]
- 10. Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo or radio-therapy for cancer. Biosci Trends. 2010;4:297-307. [PubMed] [Google Scholar]
- 11. Yang J, Zhu L, Wu Z, Wang Y. Chinese herbal medicines for induction of remission in advanced or late gastric cancer. Cochrane Database Syst Rev. 2013;(4):CD005096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, England: Cochrane Collaboration; 2011. [Google Scholar]
- 14. Cui L. Clinical Study on Integrated Traditional Chinese and Western Medicine in the Treatment of Postoperative Patients with Gastric Cancer. Shandong, China: Shandong University of Traditional Chinese Medicine; 2003. [Google Scholar]
- 15. Yang JK, Zhen J, Shen KP. Clinical study on post-operative metastasis prevention of progressive stage of gastric cancer by weichang’an [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:580-582. [PubMed] [Google Scholar]
- 16. Zhou H, Zheng J, Shen KP. Clinical study on Wei Chang: a recipe in the treatment of advanced gastric cancer. Tumor. 2007;27:487-489. [Google Scholar]
- 17. Chen GQ, Wang JZ, Fu XH, Xu YY, Huang ZB, Huang ZY. Clinical research on prevention of recurrence and metastasis of postoperative advanced gastric cancer by Fuzhuan decoction. Zhejiang J Tradit Chin Med. 2008;43:715. [Google Scholar]
- 18. Shi HW, Zhang BX. Clinical research of treating gastric cancer with Jianweiyixiaotang. J Binzhou Med Univ. 2008;31:395-396. [Google Scholar]
- 19. Yue JT, Yuan W, Wang CX. Effect of Sixiaoshejiawan and chemotherpy on quality of life and side effect with gastric cancer. Shaanxi J Tradit Chin Med. 2009;30:1110-1111. [Google Scholar]
- 20. Jiang HM, Jiang LX, Zhou JY, Hu X, Mao WD. Clinical analysis of treating gastric cancer in TCM combined with chemotherapy. Clin J Chin Med. 2011;3:21-22. [Google Scholar]
- 21. Cai X, Yan C, Wang G, Cui T, Lin Y. Clinical research on prevention of recurrence and metastasis of postoperative gastric cancer by Biejia decoction. J Liaoning Univ Tradit Chin Med. 2013;15:218-219. [Google Scholar]
- 22. Fang Y. Effect of Bazhenjiaonang and chemotherapy on gastric cancer. Practical Clin J Integr Tradit Chin West Med. 2013;13:65-66. [Google Scholar]
- 23. Zhang LT, Xing H. Clinical efficacy of Norcantharidin combined with conventional chemotherapy treating postoperative gastric cancer. Med Recapitulate. 2013;19:2087-2088. [Google Scholar]
- 24. He W. Clinical study on combined therapy of traditional Chinese and Western medicine in the treatment of advanced gastric cancer. J New Chin Med. 2014;46:161-165. [Google Scholar]
- 25. Meng Y, Cao F, Jun C, Chen P. Fifty cases of postoperative gastric cancer treated with combined therapy of traditional Chinese and conventional chemotherapy. Global Tradit Chin Med. 2015;8:995-997. [Google Scholar]
- 26. Fu YZ. Fifty cases of postoperative gastric cancer treated with combined therapy of traditional Chinese and Western medicine. Henan Tradit Chin Med. 2016;36:682-683. [Google Scholar]
- 27. Sterne JAC, Gavaghan D, Egger M. Publication and related bias in meta-analysis. J Clin Epidemiol. 2000;53:1119-1129. [DOI] [PubMed] [Google Scholar]