Abstract
Purpose: Treatment for head and neck cancer (HNC) results in long-term toxicities and increased physical and psychosocial survivor burden. There are a limited number of treatments for these late effects. Yoga postures, breath work, relaxation, and meditation, may improve these late effects. The purpose of this study was to examine the feasibility of a tailored yoga program in HNC survivors and obtain preliminary efficacy data. Methods: This was a randomized wait-list control study of yoga-naive HNC survivors who were >3 months post–cancer treatment. Baseline data were collected. Participants were randomized to either an 8-week hatha yoga intervention group or a wait-list group. Feasibility and efficacy data were collected. At 4 and 8 weeks, patients underwent a repeat assessment of health. Wait-list control group participants were offered the yoga program after data collection. Descriptive statistics evaluated feasibility. Mixed effects general linear models were used to generate estimates of the efficacy outcomes. Results: Seventy-three individuals were screened and 40 were eligible. All eligible individuals consented and enrolled. Five of the intervention group discontinued early and none in the wait-list control group. Feasibility was affirmed as participants were recruited and retained in the study, there were no adverse events, fidelity to protocol was demonstrated, and satisfaction rates were high. Efficacy measures indicated potential benefit for shoulder range of motion (d = 0.57-0.86, P < .05), pain (d = 0.67-0.90, P ≤ .005), and anxiety (d = 0.59, P = .015). Conclusion: A tailored hatha yoga program is feasible and potentially efficacious for HNC survivors. Preliminary data supports further investigation of yoga in this population is needed.
Keywords: hatha yoga, head and neck cancer, tailored yoga, feasibility, efficacy, function
Introduction
Combined modality therapy is used in the treatment of the majority of patients with locally advanced head and neck cancer (HNC).1,2 Unfortunately, these treatment regimens result in substantial acute and late effects.3,4 While many acute toxicities subside on completion of cancer therapy, HNC survivors frequently experience late effects that result in symptom burden, functional deficits, and decreased quality of life (QOL).1,2,5 Musculoskeletal impairment (MSI) is a critical yet under reported and understudied complication of HNC therapy. It is most commonly manifested as soft tissue damage and dysfunction involving the jaw, neck, shoulders, and chest. Although soft tissue damage due to cancer and surgery have long been known to cause MSI, the critical role of lymphedema and fibrosis are just now being appreciated. Regardless of cause, MSI results in a myriad of problems, including pain, postural abnormalities,6 and loss of critical functions such as jaw or neck range of motion (ROM).7 Furthermore, MSI may also contribute to psychological distress (eg, anxiety, body image disturbance),8,9 increased health care expenses,10 and loss of work.11 MSI itself may be exacerbated by muscular tension and fear of movement resulting from states of high anxiety and distress. The clinically observed consequence is a self-perpetuating spiral that results in worsening functional loss and increasing symptom burden over time.12,13
Yoga has been used to treat diseases in contemporary Western society with increasing frequency since the late 1970s.14 Yoga is often used as a reasonably safe complementary modality for health maintenance and restoration, as well as an adjunct tool in the chronically ill.15-23 In cancer patients, the vast majority of yoga studies have been conducted in survivors of breast cancer.24-26 Studies suggest that yoga is an effective intervention for managing cancer-related symptoms, ameliorating psychosocial distress, and enhancing overall QOL.15-27 Key components of a yoga practice include: postures, breathing practice, relaxation techniques, and meditation.28
Specifically, a growing body of literature supports the positive impact of yoga in reducing stress and improving systemic symptoms such as pain,29 fatigue,30 anxiety,31 depression,32 and insomnia.33 In addition, through the practice of asanas, yoga may improve physical functionion.34
The conceptual framework that guided the study was based on the assumption that yoga has the potential to improve MSI by addressing physical limitations, through the postures that improve ROM and lymph flow. It may decrease anxiety, fear, and muscle tension through meditation, awareness practice, and breathe work. Yoga may address the key factors that contribute to worsening MSI, potentially breaking the cycle that leads to deteriorating function over time. Studies exploring the feasibility, safety, and efficacy of hatha yoga HNC patients are lacking. We conducted a pilot study in HNC survivors to determine the following: (1) manifested physical impairments, (2) need for modified yoga postures, (3) feasibility of using a tailored hatha yoga program in the population, and (4) potential efficacy. In this article, we report on feasibility and preliminary efficacy outcomes.
Patients and Methods
This study used a wait-list control design. Consented patients were randomly assigned either to an 8-week yoga intervention group or to a wait-list group that completed study visits and was then offered the same yoga practice that the intervention group received. Approval for this study was obtained from the Vanderbilt-Ingram Cancer Center Scientific Review Committee, Nashville, Tennessee, USA under the Vanderbilt Institutional Review Board approval number 121357. The study was conducted at the Vanderbilt University School of Nursing.
Recruitment took place from October 2013 until July 2015. Inclusion criteria were (1) ≥18 years old, (2) >3 months post HNC treatment, (3) no active cancer, (4) English speaking, (5) willing/able to conduct home practice and drive to the study site, (6) passing screening tool questions that included the Short Portable Mental Status Questionnaire, (7) completed medically indicated physical therapy, and (8) medical clearance. Exclusion criteria included (1) a prior cancer diagnosis ≤past 3 years, other than HNC; (2) previous radiation therapy or chemotherapy for other type of cancer; (3) medical conditions prohibiting safe implementation of yoga (eg, vertigo, suicidal ideations); or (4) active yoga practice of any frequency ≤past 6 months. Participants received compensation of $110.
Data Collection
Feasibility Measures
Feasibility data were collected as follows:
Recruitment Log: Number screened, eligible, interested, not interested, and enrolled.
Participant Practice Log: Patients completed a self-reported home yoga practice log.
Guided Yoga Sessions Log: The yoga instructor recorded guided practice attendance and reasons for missed sessions.
Fidelity: The consultants reviewed 10% of the videotaped sessions.35
Common Terminology Criteria for Adverse Events (CTCAE Criteria version 4.0): Adverse events were evaluated using the CTCAE grading (severity) scale.36
Columbia Suicide Severity Rating Scale(C-SSRS): Trained staff administered this instrument.37 A positive score required patient assessment.
Satisfaction Questionnaire Form: Patients completed a 9-question self-report rating scale with anchors of 0 totally unsatisfied to 10 highly satisfied with the yoga program, practice log, and yoga booklet.
Efficacy Measures
Physical and emotional outcomes were assessed by trained staff at baseline, 4 weeks, and 8 weeks as follows:
Maximal interincisal opening (MIO), cervical range of motion (CROM), shoulder range of motion (SROM): Standard devices were used to obtain these measurements.38-41
Posture assessment: Spinal deviation from proper alignment by use of photography against a grid and computer analysis of the body.42
The Vanderbilt Head and Neck Symptom Survey (VHNSS–Version 2): This self-report validated measure assessed symptom burden.43,44
Brief Pain Inventory–Short Form (BPI-SF): This self-reported survey captured participant’s pain experience.45
Hospital Anxiety and Depression Scale (HADS): This self-report survey measured anxiety and depression.46,47 The medical oncologist was notified if a participant scored 8 or higher on either scale.
Body Image Quality Life Instrument (BIQLI): Participants completed a self-report scale ranging from very negative (−3) to very positive (+3) effects of body image on 19 life domains.48,49
Procedures
Baseline study measures were obtained. Participants were then randomized via a computer generated, permuted block program, to either an initial yoga evaluation and intervention group, or to a wait-list control group.
Intervention
The protocol addressed all traditional hatha yoga practice components: postures, breathing, relaxation techniques, and meditation. Sixteen core poses were chosen to comprise the head and neck yoga toolbox; these poses were selected because they involved structures critical for optimizing mobility or strength in the neck and shoulders. Participants were given a booklet and the Toolbox, which described the postures, awareness practice, breathing practice, relaxation practice and meditation.
Intervention Group
Prior to initiating the intervention, a comprehensive evaluation was undertaken by the yoga instructor. Evaluation included an assessment of the physical, mental, and psychological limitations with emphasis on jaw, neck, and shoulder dysfunction. The core 16 poses were modified as deemed necessary by the instructor based on this assessment so that they were safe and feasible for the patient to perform and medical approval was obtained for this practice plan. Limitations were discussed with the participant. The practice plan was revised at weeks 3 or 4, or when modifications were indicated. Participants underwent tailored yoga sessions 3 times a week for 4 weeks then 2 times a week for 4 weeks with home practice on all other days. Guided sessions were videotaped. The initial session began with education regarding yoga principles, practice guidelines, and safety. Participants were then guided to observe their physical, psychological, and energy states, in order to enhance self-awareness.28 Participants with lower extremity or pelvic weaknesses initially used a chair for support. As strength improved, they graduated to a standing or a floor pose. Throughout the practice, emphasis was placed on proper alignment and recognition of physical limitations in order to avoid injury. For participants with severe trunk weakness, props (eg, a slightly reclining hard backed chair, pillows, and supports) were used to ensure spinal alignment.
Participants were taught breath awareness, diaphragmatic, thoracic, clavicular breathing, and the complete yoga breath.28 Participants were instructed on tension/systematic relaxation and yoga nidra techniques, including guided imagery.28 Sessions ended with meditation with either chanting a mantra silently or inner silence.
At the start of all subsequent guided sessions, participants were queried about any concerns or yoga related adverse events. Sessions lasted 30 minutes for participants with significant limitations with gradual increase to no more than 90 minutes as physical and mental endurance improved. Physical and psychological limitations and barriers were documented. Personal daily home practice was encouraged.
Wait-List Control Group
Participants in the wait-list control group completed the baseline, 4-week, and 8-week follow-up assessments. They were then offered the tailored yoga program.
Statistical Analysis
Frequency distributions were used to summarize the nominal and ordinal categorical data. Normally distributed continuous data were summarized using mean and standard deviation. However, most of the continuous distributions were skewed. Those were summarized using median and interquartile range (IQR). Comparisons of the demographic and clinical characteristics of the study groups were conducted using chi-square tests of independence (nominal, ordinal data) and Mann-Whitney tests (continuous data). Mixed-level generalized linear modeling with the log-link function was used to assess differences in the patterns of change in the study outcome variables between the two groups. The key effect of interest in these analyses was the interaction effect of group and time of assessment. This effect would indicate that the pattern of change in the intervention group was different from that observed in the control group over the same time period. Cohen’s d effect index was generated for all findings. While this index was the most important statistic used in evaluating the effects of yoga in this preliminary study, a maximum alpha of .05 (P < .05) was used for conclusions of statistical significance.
Results
Participant Characteristics
The study consisted primarily of older, white males living in rural/suburban areas. Summaries of the demographic and disease/treatment, characteristics of the 35 patients who participated in the study (intervention group n = 15, control group n = 20) are shown in Table 1. Fifty-seven percent (24 of 35) had been diagnosed with oropharynx cancer and approximately 74% had stage IV disease, 20% (7 of 35) had a tracheotomy and 17.1% (6 of 35) had a feeding tube during the study.
Table 1.
Demographic and Clinical Characteristics by Study Group (N = 35).
| Characteristic | Overall (N = 35) | Intervention (n = 15) | Control (n = 20) | P |
|---|---|---|---|---|
| Age, y, mean (SD) | 63.2 (8.5) | 65.0 (7.4) | 61.8 (9.2) | .268 |
| Years of education, mean (SD) | 14.4 (2.8) | 15.1 (3.0) | 13.8 (2.5) | .163 |
| Gender, n (%) | .267 | |||
| Female | 13 (37.1) | 4 (26.7) | 9 (45.0) | |
| Male | 22 (62.9) | 11 (73.3) | 11 (55.0) | |
| Race, n (%) | .265 | |||
| Black or African American or Asian | 5 (14.3) | 1 (6.7) | 4 (20.0) | |
| White | 30 (85.7) | 14 (93.3) | 16 (80.0) | |
| Marital status, n (%) | .486 | |||
| Single/Widowed/Other | 14 (40.0) | 7 (46.7) | 7 (35.0) | |
| Married/Partnered | 21 (60.0) | 8 (53.3) | 13 (65.0) | |
| Employment, n (%) | .503 | |||
| Employed | 15 (42.9) | 5 (33.3) | 10 (50.0) | |
| Not employed | 17 (48.6) | 9 (60.0) | 8 (40.0) | |
| Other | 3 (8.6) | 1 (6.7) | 2 (10.0) | |
| Income, $, n (%) | .534 | |||
| ≤30 000 | 11 (31.4) | 5 (33.3) | 6 (30.0) | |
| >30 000 | 19 (54.3) | 9 (60.0) | 10 (50.0) | |
| Don’t care to respond | 5 (14.3) | 1 (6.7) | 4 (20.0) | |
| Residence, n (%) | .486 | |||
| Urban | 14 (40.0) | 7 (46.7) | 7 (35.0) | |
| Rural/Suburban | 21 (60.0) | 8 (53.3) | 13 (65.0) | |
| Time since diagnosis, mo, median [IQR] | 35.7 [9-63] | 45.7 [9-63] | 28.4 [10-67] | .764 |
| Time since last treatment, mo, median [IQR] | 19.8 [4-52] | 43.3 [5-59] | 17.0 [3-43] | .286 |
| Location of primary lesion, n (%) | .564 | |||
| Oral cavity | 7 (20.0) | 2 (13.3) | 5 (25.0) | |
| Oropharynx | 20 (57.1) | 10 (66.7) | 10 (50.0) | |
| Larynx | 4 (11.4) | 1 (6.7) | 3 (15.0) | |
| Salivary gland | 3 (8.6) | 2 (13.3) | 1 (5.0) | |
| Other | 1 (2.9) | 0 (0.0) | 1 (5.0) | |
| Stage,a n (%) | .235 | |||
| I-II | 2 (5.9) | 0 (0.0) | 2 (10.5) | |
| III | 7 (20.6) | 2 (13.3) | 5 (26.3) | |
| IV | 25 (73.5) | 13 (86.7) | 12 (63.2) | |
| Tumor-expressed virus, n (%) | .994 | |||
| None | 7 (20.0) | 3 (20.0) | 4 (20.0) | |
| Human papilloma virus | 12 (34.3) | 5 (33.3) | 7 (35.0) | |
| Not tested | 16 (45.7) | 7 (46.7) | 9 (45.0) | |
| Total treatment received, n (%) | .744 | |||
| Surgery only | 2 (5.7) | 0 (0.0) | 2 (10.0) | |
| Surgery + radiotherapy | 1 (2.9) | 0 (0.0) | 1 (5.0) | |
| Surgery + CCR | 7 (20.0) | 3 (20.0) | 4 (20.0) | |
| Induction + CCR | 6 (17.1) | 3 (20.0) | 3 (15.0) | |
| Surgery + induction + CCR | 12 (34.3) | 6 (40.0) | 6 (30.0) | |
| CCR only | 6 (17.1) | 3 (20.0) | 3 (15.0) | |
| Other | 1 (2.9) | 0 (0.0) | 1 (5.0) |
Abbreviations: CCR, concurrent chemo radiation; IQR, interquartile range.
N = 34, Control = 19.
Feasibility
Recruitment
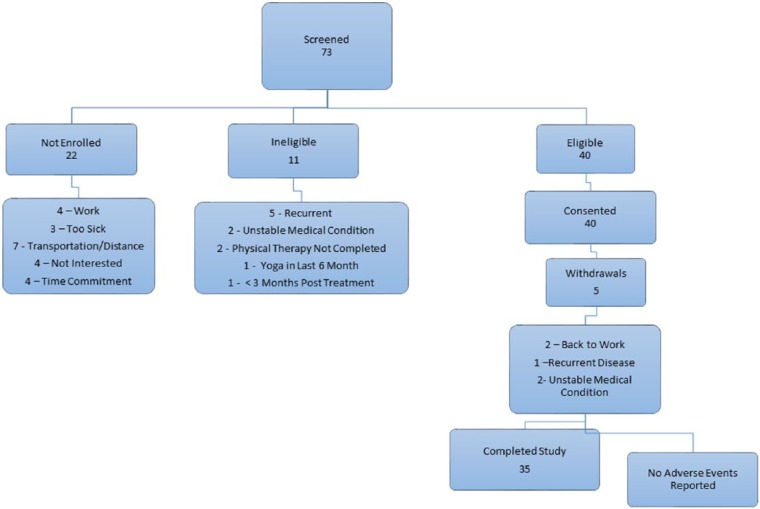
Seventy-three individuals were screened, of whom 40 were eligible. Eleven were ineligible due to recurrence of disease (n = 5), unstable medical condition (n = 2), had not completed physical therapy (n = 2), practiced yoga within the past 6 months (n = 1), and <3 months posttreatment (n = 1). Twenty-two were not interested because of work obligations (n = 4), illness (n = 3), distance to site (n = 7), time commitment (n = 4), and no stated reason (n = 4) (Figure 1).
Figure 1.
Screening, enrollment, and withdrawal.
Forty HNC survivors signed consent, enrolled and were randomized to the study. Five intervention patients withdrew early: returned to work (n = 2), disease recurrence (n = 1), and developed nonstudy-related unstable medical conditions (n = 2) (Figure 1). There were no statistically significant differences in demographic and disease/treatment characteristics between withdrawals and the remainder of the sample (P > .05). Three enrolled patients required financial assistance with transportation. All wait-listed participants began yoga at the conclusion of their wait-list period.
Patient Adherence (Intervention Group Only)
Adherence to the on-site, supervised and home sessions practice was excellent as noted in Table 2. It appeared that the perceived benefits of the practice were not gender specific. One of the male patients summarized his perceptions as, “I used to laugh when someone even mentioned yoga. That happens no longer . . . Yoga has given me much needed positive reinforcement both mentally and physically.”
Table 2.
Patient Adherence to Supervised and Home Sessions Protocol (Intervention Group Only).
| n | Median | Interquartile Range | Minimim | Maximum | |
|---|---|---|---|---|---|
| Supervised sessionsa | |||||
| Number expected | 19 | 20.0 | 20-20 | 3 | 20 |
| Number documented | 19 | 19.0 | 15-20 | 3 | 20 |
| Percent compliance per participant | 19 | 100.0 | 85-100 | 44.4 | 100.0 |
| Home sessionsb | |||||
| Number expected | 16 | 36.0 | 36-36 | 11 | 36 |
| Number documented | 16 | 35.0 | 31-36 | 5 | 41 |
| Percent compliance per participant | 16 | 97.2 | 87-100 | 45.5 | 100.0 |
Nineteen participants received some yoga; 1 participant discontinued prior to starting yoga.
Sixteen intervention participants returned their home practice diaries.
Fidelity
The principal investigator met with the external consultants and then separately with the yoga instructor to discuss findings and review videos. The external yoga consultants summarized fidelity as: Awareness practice was consistent with protocol and excellent breathing techniques were always taught. Posture techniques and practice order were tailored based on patient status. The yoga instructor used touch assistance when required. Relaxation and meditation appeared effective. Nostril breathing was seldom taught due to the short duration of the study; however, it could potentially be incorporated into posture, or alternatively, be removed from the protocol. The yoga instructor was encouraged to be more permissive, more open-ended, and less directive, but patients rated the instructor high.
Safety
No adverse events or suicidal ideations were identified. Eleven patients were referred to the oncologist based on HADS scores. At baseline, 4 of the 15 participants in the intervention group (27%) and 3 of the 20 in the control group (15%) had a score of 8 or higher on either the anxiety or the depression component of the HADS. Those respective numbers at week 4 were 2 of 15 in the intervention (13%) and 5 of 20 in the control (25%); at week 8, were 1 of 15 in the intervention (7%) and 6 of 20 in the control (30%). Overall, 4 patients in the intervention group accounted for all referrals in that group, and only 1 had a score of 8 or higher at week 8. Seven patients in the wait-list group accounted for all referrals, and 6 continued to have high scores at week 8.
Participant satisfaction
The median value satisfaction with all aspects of the program was 10.0 (the highest possible value). See summaries in Table 3.
Table 3.
Intervention Group Participant Satisfaction (N = 15).
| Program Evaluation | Median | Interquartile Range | Minimum | Maximum |
|---|---|---|---|---|
| General satisfaction | 10.0 | 10-10 | 9 | 10 |
| Instruction satisfaction | 10.0 | 10-10 | 10 | 10 |
| Physical progress satisfaction | 10.0 | 8-10 | 7 | 10 |
| Emotional progress satisfaction | 10.0 | 9-10 | 7 | 10 |
| Mental progress satisfaction | 10.0 | 9-10 | 7 | 10 |
| Asanas satisfaction | 10.0 | 8-10 | 6 | 10 |
| Breath work satisfaction | 10.0 | 9-10 | 8 | 10 |
| Meditation/Relaxation satisfaction | 10.0 | 9-10 | 7 | 10 |
| Likelihood of continuing yoga | 10.0 | 10-10 | 8 | 10 |
| Booklet Evaluation | Strongly Disagree, n (%) | Not Sure, n (%) | Agree, n (%) | Strongly Agree, n (%) |
| Wealth of information | 1 (6.7) | 3 (20.0) | 7 (46.7) | 4 (26.7) |
| Helpful tips | 1 (6.7) | 5 (33.3) | 3 (20.0) | 6 (40.0) |
| Motivation | 1 (6.7) | 2 (13.3) | 3 (20.0) | 9 (60.0) |
| Good pictures | 1 (6.7) | 4 (26.7) | 5 (33.3) | 5 (33.3) |
| Encouraged to examine status | 1 (6.7) | 4 (26.7) | 4 (26.7) | 6 (40.0) |
| Engaging, appealing | 1 (6.7) | 4 (26.7) | 6 (40.0) | 4 (26.7) |
| Easy to read, understand | 1 (6.7) | 3 (20.0) | 7 (46.7) | 4 (26.7) |
| Median | Interquartile Range | Minimum | Maximum | |
| Booklet time in minutes | 30.0 | 15-130 | 3 | 420 |
| Practice Log Evaluation | Disagree, n (%) | Not Sure, n (%) | Agree, n (%) | Strongly Agree, n (%) |
| Self-explanatory | 4 (26.7) | 2 (13.3) | 6 (40.0) | 3 (20.0) |
| Easy to complete | 0 (0.0) | 1 (6.7) | 8 (53.3) | 6 (40.0) |
| Didn’t take too much time | 0 (0.0) | 0 (0.0) | 7 (46.7) | 8 (53.3) |
| Helped focus | 0 (0.0) | 4 (26.7) | 4 (26.7) | 7 (46.7) |
| Helped see progress | 0 (0.0) | 4 (26.7) | 7 (46.7) | 4 (26.7) |
| Worth my time | 0 (0.0) | 3 (20.0) | 4 (26.7) | 8 (53.3) |
| Comment section helpful | 1 (6.7) | 3 (20.0) | 5 (33.3) | 6 (40.0) |
| Log reflects practice | 0 (0.0) | 4 (26.7) | 5 (33.3) | 6 (40.0) |
| Median | Interquartile Range | Minimum | Maximum | |
| Time to complete log each time in minutes | 10.0 | 5-20 | 5 | 30 |
Limitations and Modifications
The majority of patients demonstrated MSI that limited movement to the extent that modifications in the core poses were needed. These will be detailed in a separate manuscript.
Efficacy
Jaw, Shoulder Cervical Range of Motion, and Posture
The most consistent effects were observed in the shoulder region for abduction and external rotation on the right side (Table 4). The statistically significant effects (effect sizes Cohen’s d = 0.57-0.86) were those of the intervention group demonstrating generally improving ROM while the control group remained stable or decreased in ROM. No statistically significant effects on jaw (Cohen’s d = 0.20-0.45) or cervical (Cohen’s d = 0.39) ROM were observed (P > .05). However, baseline dysfunction was low. No significant changes in posture were detected.
Table 4.
Selected Shoulder Range of Motion Summaries (N = 35; Intervention n = 15, Control n = 20).a
| Baseline | 4 Weeks | 8 Weeks | |
|---|---|---|---|
| Passive abduction, rightb | (Arm to side, palm up, moving arm away from body toward head as far as possible) | ||
| Intervention | 141.0 [127-155] | 145.0 [138-152] | 154.5 [143-160] |
| Control | 144.5 [138-161] | 140.0 [132-163] | 135.0 [124-158] |
| Passive abduction, left | (Arm to side, palm up, moving arm away from body toward head as far as possible) | ||
| Intervention | 136.5 [131-155] | 149.0 [139-163] | 146.5 [140-162] |
| Control | 130.5 [122-156] | 135.0 [114-150] | 133.5 [116-150] |
| Passive external rotation, rightb | (Arm straight out, bent at elbow with hand up, moving hand back toward head) | ||
| Intervention | 68.5 [63-79] | 74.0 [68-84] | 79.0 [70-82] |
| Control | 80.5 [69-87] | 78.8 [71-87] | 76.3 [70-86] |
| Passive external rotation, left | (Arm straight out, bent at elbow with hand up, moving hand back toward head) | ||
| Intervention | 69.5 [60-77] | 71.0 [68-78] | 72.0 [67-80] |
| Control | 71.3 [54-84] | 68.3 [56-75] | 71.3 [60-77] |
| Active abduction, rightb | (Arm to side, palm up, moving arm away from body toward head as far as possible) | ||
| Intervention | 140.0 [127-148] | 149.0 [137-160] | 152.5 [141-162] |
| Control | 146.0 [139-162] | 140.5 [131-157] | 139.0 [124-158] |
| Active abduction, leftb | (Arm to side, palm up, moving arm away from body toward head as far as possible) | ||
| Intervention | 139.0 [126-151] | 143.5 [133-157] | 147.5 [142-162] |
| Control | 140.0 [118-152] | 139.5 [118-148] | 132.0 [123-148] |
| Active external rotation, rightb | (Arm straight out, bent at elbow with hand up, moving hand back toward head) | ||
| Intervention | 72.5 [64-78] | 76.0 [69-87] | 80.0 [72-84] |
| Control | 79.0 [74-89] | 77.5 [73-86] | 77.0 [70-85] |
| Active external rotation, Left | (Arm straight out, bent at elbow with hand up, moving hand back toward head) | ||
| Intervention | 71.0 [60-74] | 71.0 [62-78] | 72.0 [63-78] |
| Control | 71.5 [50-84] | 66.5 [56-77] | 69.8 [63-76] |
Values are given as median [interquartile range].
Statistically significant (P < .05) differences in the patterns of change in the intervention group compared with that in the control group.
Symptoms
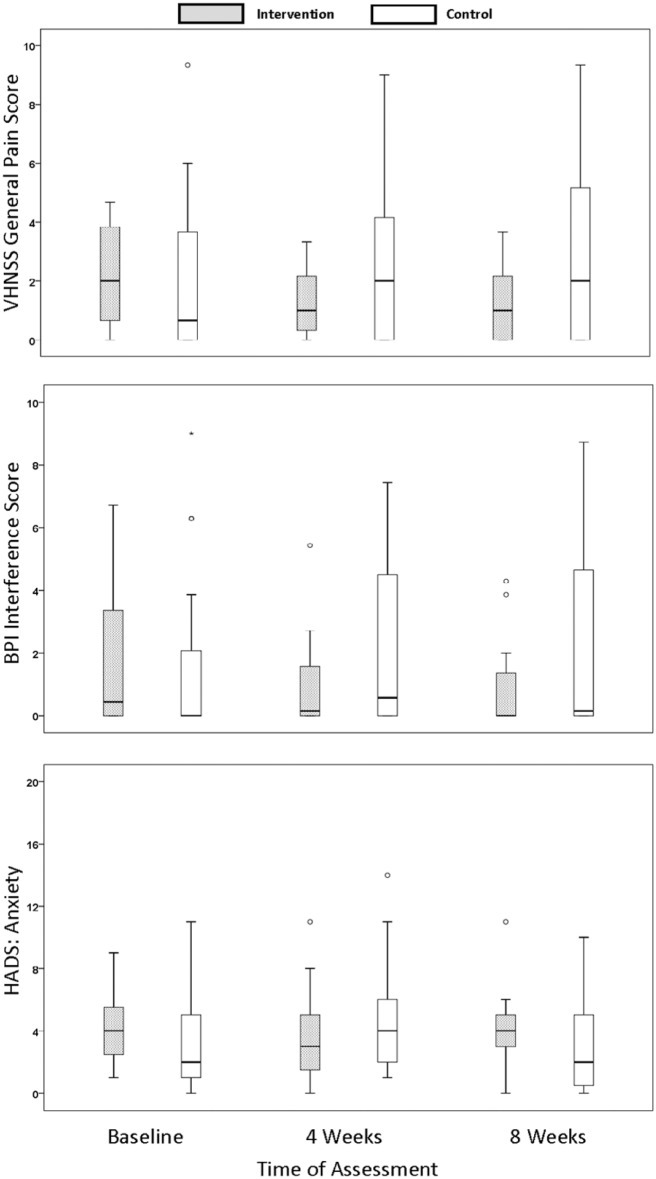
There were statistically significant differences between the study groups in patterns of change in pain (VHNSS General Pain, P < 0.001, effect size Cohen’s d = 0.90) and pain interference with activities of daily living (BPI Interference, P = .005, effect size Cohen’s d = 0.67). The VHNSS General Pain levels decreased for the intervention group, but increased over the same time period for the controls (Figure 2). Median BPI Interference scores were variable and low for both groups. Intervention group variability decreased. Those participants reporting higher levels of interference at baseline tended to demonstrate a decrease in those levels over the course of the study. The opposite pattern was observed for the participants in the control group.
Figure 2.
Summaries of self-reports of pain (VHNSS General Pain), interference from pain (BPI-SF), and anxiety over the study period (HADS) (N = 35; Intervention n = 15, Control n = 20). BPI-SF, Brief Pain Inventory–Short Form; HADS, Hospital Anxiety and Depression Scale; VHNSS, Vanderbilt Head and Neck Symptom Survey.
All demonstrated statistically significant (P < .05) differences in the patterns of change in the intervention group compared with that in the control group.
A statistically significant interaction effect of the yoga intervention was observed for the HADS anxiety scores (P = .015, effect size Cohen’s d = 0.59) but not the depression scores (P = .290, effect size Cohen’s d = 0.31). The intervention participants reported higher anxiety scores at baseline, decreased at 4 weeks and, increased again at 8 weeks. Participants in the control group tended to follow an opposite pattern in that their scores were lower than the intervention group at baseline, increased at 4 weeks, and reduced to baseline levels at 8 weeks (Figure 2).
Body Image
No statistically significant effects on body image were observed in this study (P = .647, effect size Cohen’s d = 0.18).
Discussion
Tailored yoga appears to be safe and feasible in the posttreatment HNC population. No adverse events occurred. Recruitment rates were high as was adherence with guided sessions and home practice. Participants expressed high levels of satisfaction with the yoga instruction and study related tools. Preliminary data indicated that yoga may improve mood, symptom burden, and shoulder function.
Recruitment of HNC survivors to a yoga study was a concern. This population is predominantly male, generally older, and often plagued by late effects that might prove to be barriers to study participation (eg, tracheostomy tubes, feeding tubes). When this study commenced, the majority of yoga studies in the oncology population had been conducted in female survivors of breast cancer.24,25 No known studies had been reported in survivors of HNC. Our study demonstrated that men were willing to participate and were adherent to an intensive yoga program that required both frequent one-on-one guided sessions and daily home practice. However, patients indicated that the duration of the supervised and daily home practice was burdensome. Shorter supervised and tailored home practice should be considered in future studies.
Tracheostomies and feeding tubes presented safety concerns. As such, we incorporated a number of safety measures. The yoga instructor had 500 hours of advanced training in therapeutic aspects of yoga in cancer and chronic illness and spent a year-long preceptorship in the HNC clinic prior to study initiation in order to gain insight into the issues facing this population. Participant’s physical and emotional limitations were thoroughly assessed. One-on-one instruction was given to ensure proper alignment and timely modifications. A registered nurse was on-site during yoga sessions. Although the sample was small, the findings suggested that with modifications, patients with implanted devices can safely participate in a tailored yoga program. Furthermore, issues such as xerostomia did not impair practice. Few community instructors have the skill sets or training to deal with this complex patient population. Ensuring adequate training of yoga instructors in order to maintain safety and efficacy is likely to be a challenge, but possible.
Consistent with studies conducted in other cancer populations,26,30,50-52 we observed improvement in the region of function we anticipated to be most impacted by yoga practice, shoulder range of motion. Lack of measured change in posture may indicate the need for an alternative measurement method. Pain also demonstrated increased improvement in the practice group compared with the control group. Reduced anxiety in the practice group was noted during the 4 weeks when participants underwent 3 supervised sessions a week. It returned to near baseline levels when supervised sessions were reduced. Thus, 3 supervised yoga sessions a week appear to be more effective in decreasing anxiety than 2 sessions per week.
This was a feasibility study; findings should be considered in light of its limitations. The convenience sample may have resulted in selection bias, as participants who agreed to participate may not have been representative of the entire HNC population. A small sample size, only 8 weeks of yoga, and no follow-up after yoga completion should also be considered. Findings from this pilot study may not generalize to other populations. A larger dose of yoga may have yielded better outcomes; however, patients did not desire a more extensive program. The lack of longer term follow-up leaves unanswered questions regarding the uptake of yoga into the daily lives of the participants and fails to capture long-term outcomes. Many of these limitations can be addressed in a larger study that follows participants for an extended period of time. These limitations are also somewhat offset by strengths of the study that include participation by both genders, strict fidelity oversight, and high patient satisfaction.
Conclusion
A tailored yoga program administered by a trained instructor is safe and feasible for HNC survivors. Patient demographics did not limit recruitment. Men and women were eager to participate due to bothersome late effects from their cancer treatment. Some of the late effects such as; mood, symptom burden, and shoulder function improved through the use of yoga. Significant limitations in movement requiring modifications in poses were found. Preliminary efficacy data support further investigation of yoga in this and other chronically ill populations.
Acknowledgments
We wish to acknowledge Amber Green at Vanderbilt University School of Nursing for her assistance with this manuscript. We would like to thank all of the head and neck cancer survivors who spent considerable time and effort supporting this research.
Footnotes
Authors’ Note: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by #R21 CA173202 and the Martha Rivers Ingram Endowed Chair.
ORCID iD: Melissa Adair  https://orcid.org/0000-0002-4973-9231
https://orcid.org/0000-0002-4973-9231
References
- 1. Murphy BA, Gilbert J, Ridner SH. Systemic and global toxicities of head and neck treatment. Expert Rev Anticancer Ther. 2007;7:1043-1053. [DOI] [PubMed] [Google Scholar]
- 2. Nibu K, Ebihara Y, Ebihara M, et al. Quality of life after neck dissection: a multicenter longitudinal study by the Japanese Clinical Study Group on Standardization of Treatment for Lymph Node Metastasis of Head and Neck Cancer. Int J Clin Oncol. 2010;15:33-38. [DOI] [PubMed] [Google Scholar]
- 3. Teymoortash A, Hoch S, Eivazi B, Werner JA. Postoperative morbidity after different types of selective neck dissection. Laryngoscope.2010;120:924-929. [DOI] [PubMed] [Google Scholar]
- 4. Bensadoun RJ, Riesenbeck D, Lockhart PB, et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010;18:1033-1038. [DOI] [PubMed] [Google Scholar]
- 5. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38:E1-E10. [DOI] [PubMed] [Google Scholar]
- 6. Stubblefield M, O’Dell M. Cancer Rehabilitation: Principles and Practice. New York, NY: Demos Medical; 2009. [Google Scholar]
- 7. Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004;40:879-889. doi: 10.1016/j.oraloncology.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 8. Bornbaum CC, Fung K, Franklin JH, Nichols A, Yoo J, Doyle PC. A descriptive analysis of the relationship between quality of life and distress in individuals with head and neck cancer. Support Care Cancer. 2012;20:2157-2165. [DOI] [PubMed] [Google Scholar]
- 9. Rhoten BA, Deng J, Dietrich MS, Murphy B, Ridner SH. Body image and depressive symptoms in patients with head and neck cancer: an important relationship. Support Care Cancer. 2014;22:3053-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harrison JM, Stella PJ, LaVasseur B, et al. Toxicity-related factors associated with use of services among community oncology patients. J Oncol Pract. 2016;12:e818-e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dewa CS, Trojanowski L, Tamminga SJ, Ringash J, McQuestion M, Hoch JS. Advice about work-related issues to peers and employers from head and neck cancer survivors. PLoS One. 2016;11:e0152944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganzer H, Touger-Decker R, Parrott JS, Murphy BA, Epstein JB, Huhmann MB. Symptom burden in head and neck cancer: impact upon oral energy and protein intake. Support Care Cancer. 2013;21:495-503. [DOI] [PubMed] [Google Scholar]
- 13. Deng J, Murphy BA, Dietrich MS, Sinard RJ, Mannion K, Ridner SH. Differences of symptoms in head and neck cancer patients with and without lymphedema. Support Care Cancer. 2016;24:1305-1316. [DOI] [PubMed] [Google Scholar]
- 14. Tandon MK. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax. 1978;33:514-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Ann N Y Acad Sci. 2009;1172:54-62. [DOI] [PubMed] [Google Scholar]
- 16. Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18:465-475. [DOI] [PubMed] [Google Scholar]
- 17. Carlson LE, Bultz BD. Mind-body interventions in oncology. Curr Treat Options Oncol. 2008;9:127-134. [DOI] [PubMed] [Google Scholar]
- 18. Field T. Yoga clinical research review. Complement Ther Clin Pract. 2011;17:1-8. [DOI] [PubMed] [Google Scholar]
- 19. Chen KM, Fan JT, Wang HH, Wu SJ, Li CH, Lin HS. Silver yoga exercises improved physical fitness of transitional frail elders. Nurs Res. 2010;59:364-370. [DOI] [PubMed] [Google Scholar]
- 20. Chen KM, Chen MH, Hong SM, Chao HC, Lin HS, Li CH. Physical fitness of older adults in senior activity centres after 24-week silver yoga exercises. J Clin Nurs. 2008;17:2634-2646. [DOI] [PubMed] [Google Scholar]
- 21. Ranjita R, Hankey A, Nagendra HR, Mohanty S. Yoga-based pulmonary rehabilitation for the management of dyspnea in coal miners with chronic obstructive pulmonary disease: a randomized controlled trial. J Ayurveda Integr Med. 2016;7:158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasanpour-Dehkordi A, Jivad N, Solati K. Effects of yoga on physiological indices, anxiety and social functioning in multiple sclerosis patients: a randomized trial. J Clin Diagn Res. 2016;10:VC01-VC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desveaux L, Lee A, Goldstein R, Brooks D. Yoga in the management of chronic disease: a systematic review and meta-analysis. Med Care. 2015;53:653-661. doi: 10.1097/mlr.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 24. Littman AJ, Bertram LC, Ceballos R, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer. 2012;20:267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desai K, Bowman MA, Galantino ML, et al. Predictors of yoga use among patients with breast cancer. Explore (NY). 2010;6:359-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kvillemo P, Bränström R. Experiences of a mindfulness-based stress-reduction intervention among patients with cancer. Cancer Nurs. 2011;34:24-31. [DOI] [PubMed] [Google Scholar]
- 27. Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253-2260. [DOI] [PubMed] [Google Scholar]
- 28. Stiles M. Structural Yoga Therapy: Adapting to the Individual. Newburyport, MA: Red Wheel/Weiser Books; 2000. [Google Scholar]
- 29. Michalsen A, Traitteur H, Lüdtke R, et al. Yoga for chronic neck pain: a pilot randomized controlled clinical trial. J Pain. 2012;13:1122-1130. [DOI] [PubMed] [Google Scholar]
- 30. Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23:135-142. [DOI] [PubMed] [Google Scholar]
- 31. Ranjita R, Badhai S, Hankey A, Nagendra HR. A randomized controlled study on assessment of health status, depression, and anxiety in coal miners with chronic obstructive pulmonary disease following yoga training. Int J Yoga. 2016;9:137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma A, Barrett MS, Cucchiara AJ, Gooneratne NS, Thase ME. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 2017;78:e59-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mustian KM. Yoga as treatment for insomnia among cancer patients and survivors: a systematic review. Eur Med J Oncol. 2013;1:106-115. [PMC free article] [PubMed] [Google Scholar]
- 34. Noradechanunt C, Worsley A, Groeller H. Thai Yoga improves physical function and well-being in older adults: a randomised controlled trial. J Sci Med Sport. 2017;20:494-501. [DOI] [PubMed] [Google Scholar]
- 35. Borrelli B, Sepinwall D, Ernst D, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol. 2005;73:852-860. [DOI] [PubMed] [Google Scholar]
- 36. US Department of Health and Human Services, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed December 15, 2011.
- 37. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. CranioRehab.com. TMJ and jaw motion rehab. http://www.craniorehab.com/jaw-motion-rehab-systems-orastretch-therabite.html. Accessed December 27, 2017.
- 39. Reynolds J, Marsh D, Koller H, Zenenr J, Bannister G. Cervical range of movement in relation to neck dimension. Eur Spine J. 2009;18:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams MA, McCarthy CJ, Chorti A, Cooke MW, Gates S. A systematic review of reliability and validity studies of methods for measuring active and passive cervical range of motion. J Manipulative Physiol Ther. 2010;33:138-155. [DOI] [PubMed] [Google Scholar]
- 41. Rehaboutlet.com. Goniometers. http://www.rehaboutlet.com/goniometers.htm. Accessed October 31, 2012.
- 42. Posture zone grids. http://www.posturezone.com/categories/Posture-Grids/. Accessed December 31, 2011.
- 43. Murphy BA, Dietrich MS, Wells N, et al. Reliability and validity of the Vanderbilt Head and Neck Symptom Survey: a tool to assess symptom burden in patients treated with chemoradiation. Head Neck. 2010;32:26-37. [DOI] [PubMed] [Google Scholar]
- 44. Cooperstein E, Gilbert J, Epstein JB, et al. Vanderbilt Head and Neck Symptom Survey version 2.0: report of the development and initial testing of a subscale for assessment of oral health. Head Neck. 2012;34:797-804. [DOI] [PubMed] [Google Scholar]
- 45. Atkinson TM, Rosenfeld BD, Sit L, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage. 2011;41:558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69-77. [DOI] [PubMed] [Google Scholar]
- 47. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [DOI] [PubMed] [Google Scholar]
- 48. Cash TF, Jakatdar TA, Williams EF. The Body Image Quality of Life Inventory: further validation with college men and women. Body Image. 2004;1:279-287. [DOI] [PubMed] [Google Scholar]
- 49. Rusticus SA, Hubley AM, Zumbo BD. Measurement invariance of the appearance schemas inventory-revised and the body image quality of life inventory across age and gender. Assessment. 2008;15:60-71. [DOI] [PubMed] [Google Scholar]
- 50. Van Puymbroeck M, Schmid A, Shinew KJ, Hsieh PC. Influence of hatha yoga on physical activity constraints, physical fitness, and body image of breast cancer survivors: a pilot study. Int J Yoga Ther. 2011;21:49-60. [PubMed] [Google Scholar]
- 51. Ulger O, Yağlı NV. Effects of yoga on balance and gait properties in women with musculoskeletal problems: a pilot study. Complement Ther Clin Pract. 2011;17:13-15. [DOI] [PubMed] [Google Scholar]
- 52. Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18:360-368. [DOI] [PMC free article] [PubMed] [Google Scholar]