Abstract
Introduction: Women with breast cancer are often prescribed aromatase inhibitors, which can cause rapid loss of bone mass leading to significant potential for morbidity. Vibration training has been shown to be helpful in reducing bone turnover in postmenopausal women without cancer. Aim: To examine the effect of vibration stimulus on markers of bone turnover in breast cancer patients receiving aromatase inhibitors. Methods: Thirty-one breast cancer survivors undergoing treatment with aromatase inhibitors were randomized to vibration stimulus (n = 14) or usual care control (n = 17). Low-frequency and low-magnitude vibration stimulus (27-32 Hz, 0.3g) was delivered in supervised sessions via standing on a vibration platform for 20 minutes, 3 times per week for 12 weeks. The primary outcome was blood markers of bone resorption (serum N-telopeptide X/creatine) and formation (serum type 1 procollagen N-terminal propeptide; P1NP). Other study outcomes body composition as well as measures of physical functioning. Outcomes were compared between groups using analysis of covariance adjusted for baseline values as well as time on aromatase inhibitors. Outcomes: On average, participants were 61.5 years old and overweight (ie, body mass index = 28.5 kg/m2). Following vibration training, there was no significant difference between groups for bone resorption (adjusted group difference 0.5, P = .929) or formation (adjusted group difference 5.3, P = .286). There were also no changes in any measure of physical functioning body composition. Conclusions: Short-term low-magnitude vibration stimulus does not appear to be useful for reducing markers of bone turnover secondary to aromatase inhibitors in breast cancer patients; nor is it useful in improving physical function or symptoms. However, further investigations with larger samples and higher doses of vibration are warranted. Trial Registration: Australian and New Zealand Clinical Trials Registry (ACTRN12611001094965).
Keywords: aromatase inhibitors, hormone therapy, bone metabolism, vibration
Introduction
Breast cancer is the most common cancer in women.1 About 40% of women diagnosed will be postmenopausal and have tumors that are sensitive to estrogen or progesterone.2 For these women, aromatase inhibitors are standard pharmacological treatment, which has been shown to improve disease-free survival.3,4 However, aromatase inhibitors act by blocking the production of estrogen, profoundly depleting oestrogen levels within days of administration. Consequently, this results in a severe loss of bone mass where the inhibition of estrogen causes a marked increase in bone resorption (ie, breakdown),4 resulting in net bone loss at twice the rate of normal physiological postmenopausal bone loss.5,6 Low bone mineral density is a significant health problem as it increases the risk of fracture; the most serious complication of osteoporosis, which is associated with significantly increased morbidity, mortality, and health care expenditure.7 The loss of bone density and increased incidence of fragility fractures associated with aromatase inhibitor use is higher when compared to other treatments such as tamoxifen.8
The current treatment to reverse increased bone resorption is administration of oral bisphosphonates. However, these are costly pharmaceutical interventions with little benefit observed in bisphosphonate use beyond the first 5 to 10 years.9 While generally well tolerated, they have low compliance levels (20%-57% over 1 year) due to the rigid dosing requirements5 and are associated with negative side effects, including suppression of bone formation, gastrointestinal irritations, and its long term use is associated with secondary cancers.10 Exercise interventions that involve an increased loading on the bone such as resistance training, jumping activity, and other weightbearing activities, have also been shown to improve bone density in pre- and postmenopausal women when performed regularly.11 However, research to date regarding the role of exercise for bone health in breast cancer survivors reports poor intervention adherence,12-15 as well as a lack of evidence for improvements in bone mineral density.12-17 Investigation of other supportive care interventions aimed at decreasing bone resorption in breast cancer patients on aromatase inhibitors is required.
Whole-body vibration training (WBV) is a relatively new form of therapy that has been shown in certain populations to increase bone density, develop muscle function, and improve balance.18,19 This time efficient form of treatment involves having the patient stand on a platform that produces vertical accelerations at a specific frequency and amplitude in order to stimulate physiological responses in the bone and muscle tissue. Low-magnitude vibration training has previously been shown to reduce the rate of bone resorption in postmenopausal women.20 Vibration exposure 3 times per week reduced a marker of bone resorption in postmenopausal women without cancer by 34.6% over 8 weeks.20 By comparison, reductions of 25% in markers of bone resorption have been reported in postmenopausal women with osteoporosis/osteopenia following walking programs of 12 months duration.21 Vibration may be more accessible to those with greater symptoms and lower mobility when compared with exercise training, given the low physical demand of standing.
In this study, we examined the effect of vibration stimulus on markers of bone turnover in postmenopausal breast cancer patients receiving aromatase inhibitor therapy. We hypothesized that the application of low-magnitude vibration stimulus for 12 weeks would decrease the bone resorption marker, N-telopeptide X/creatinine (Ntx/Cr), compared with no vibration exposure. In addition, we explored the effects of vibration stimulus on body composition and physical functioning.
Patients and Methods
Participants
Women undergoing aromatase inhibitor (AI) therapy were recruited between August 2012 and November 2014 from Perth, Australia through referral from oncologists. Participants were eligible if they were willing to continue taking any bone altering medications or supplements they were previously taking for the duration of the study, including calcium, vitamin D, or hormone replacement therapy (HRT); able to stand unassisted for sustained periods of time (ie, 20 minutes); received medical clearance from their general practitioner to participate. Exclusion criteria included taking bisphosphonates, cognitive impairment; contraindications to vibration platform training (including pacemaker and fracture within the past six months); diagnosis of bone metastases, and the diagnosis of diseases other than osteoporosis affecting bone. The study protocol was approved by the Edith Cowan University Human Research Committee and was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12611001094965). All participants gave informed written consent prior to enrolment.
Design
The study was a single blinded randomized controlled trial, with an intention-to-treat analytic strategy. The intervention consisted of a 12-week whole body vibration exposure program. Serum and urine markers of bone turnover, body composition and physical function, muscular strength, bone density, quality of life, and pain/stiffness of joints were measured at baseline and postintervention. All testing was conducted at the Edith Cowan University, Exercise Medicine Research Institute, and training was conducted either there or at Sir Charles Gairdner Hospital Department of Physiotherapy, Perth, Australia.
Randomization
Participants were randomized into a vibration-training group, or a control group following completion of baseline assessments. Randomization was performed using computer-generated, randomly permuted blocks by a researcher who was not involved in testing and training of participants. Participants were stratified by bone mineral density T-score obtained via DXA (dual-energy X-ray absorptiometry) scan (ie, ≤1 vs >1), and current physical activity participation obtained via self-report from Godin Leisure Time Exercise Questionnaire,22 (ie, moderate and vigorous physical activity <150 vs ≥150). Participants were informed of their group allocation by means of sealed opaque envelopes given to them after completion of all baseline testing.
Vibration Exposure
Low-frequency, low-magnitude vibration was applied (30 Hz, 0.1 mm, 0.3g) using a vibration platform (Juvent Medical, Somerset, NJ, USA). This is consistent with current research that suggests that low magnitude is anabolic to bone23 as it does not cause damage to physiology possibly associated with high-magnitude vibration.24 Past literature has reported that low frequency vibration has greatest transmission up the axial skeleton, with this transmissibility decreasing as higher vibration frequencies are used.25 Recently, a meta-analysis exploring the effect of whole-body vibration on bone loss in postmenopausal women showed that low-magnitude vibration (defined as <1g) was effective in reducing bone loss, whereas high-magnitude was not.26 All participants were instructed to stand on the vibration platform with their feet shoulder width apart, knees locked, and hands by their side to receive maximum vibration exposure. The protocol was performed with participants’ shoes removed to prevent any attenuation of vibration that may result from footwear. Sessions included 20 minutes of vibration exposure 3 times a week for 12 weeks.
Control
The control group received usual care from their physician throughout the 12-week period. Those assigned to the control group were given no additional treatment or intervention but were placed on a waiting list giving them access to the vibration training facilities at the completion of the study.
Outcomes
Outcome measures were conducted at baseline (prior to randomization) and postintervention.
Primary Outcomes
Markers of bone formation (serum type 1 procollagen N-terminal propeptide [P1NP]) and resorption (N-telopeptide X/creatinine [NTx/Cr]) were assessed via blood and urine tests performed without batching by an independent NATA-accredited laboratory blinded to group allocation. Blood samples were collected between 72 and 120 hours following the last vibration exposure session to standardize previously reported acute bout effects on markers of bone metabolism.27
Secondary Outcomes
Demographic and health history information was collected via questionnaire at baseline assessment. Baseline 25-OH vitamin D status was assessed by blood test, and analysis performed without batching by an independent NATA (National Association of Testing Authorities, Australia)–accredited laboratory blinded to group allocation.
Anthropometric Measures, Body Composition, and Bone Mineral Density
Height and weight were assessed by stadiometer (Seca GmbH & Co KG, Hamburg, Germany). Waist and hip circumference were assessed as a horizontal measure taken at the narrowest part of the torso or between the iliac crest and 12th rib, and the maximal circumference of the hip.28 Whole body dual-energy X-ray absorptiometry scan (DXA; Hologic Discovery A, Waltham, MA, USA) was used to assess whole body and regional lean and fat mass.29 Additionally, to characterize the study population, DXA scans were used to assess bone mineral density (BMD, g/cm2) of total hip (ie, femoral neck), spine (ie, lumbar spine L2-L4), and whole body.
Physical Functioning
Physical functioning was assessed by a series of tests. Cardiovascular fitness was assessed by the 400-m walk test.30 Functional ability was assessed via repeated chair rise and stair climb.31 To assess ambulatory ability, electronic timing gates were used to assess usual, fast-paced and backward 6-m walk.32 Functional tests were performed in triplicate with standardized rest-times given between trials.31 Static balance was assessed in six different positions (feet apart in parallel stance, feet together in parallel stance, half tandem stance, tandem stance, one-legged stance, one-legged stance eyes closed), without the use of assistive device for a maximum of 15 seconds in each position. Total static balance is calculated by summing the time recorded for each of the six stances.33 Maximal strength was by a 1-repetition maximum test for lower body (leg press) and upper body (chest press).34
Patient-Reported Outcomes
Patient-rated outcomes were assessed using well-validated self-report questionnaire. The Western Ontario and McMaster Universities Arthritis Index (WOMAC) was applied to assess joint pain and stiffness and difficulty with daily activities.35 Fatigue was assessed by the Functional Assessment of Cancer Therapy–Fatigue subscale.36 Exercise behavior was assessed by a validated modified version of the Godin Leisure Time Exercise Questionnaire.22,37
Sample Size Calculations
Sample size estimates were driven by hypothesized differences between the vibration and control subjects in NTx over the study. Changes were derived from a previous study using vibration training in postmenopausal women.20 A priori, 2-tailed power calculations at an alpha of .05 and beta of .20 gave an actual power of 0.82 for a total sample size of 40 using G-Power software (University of Trier, Germany).
Statistical Analysis
Data were analyzed using the SPSS statistical software package (version 22.0, IBM Corp, Armonk, NY, USA). Data distributions were inspected for normality. Normally distributed data were described using mean ± SD and non-normally distributed data using median and interquartile range (IQR). Non-normally distributed continuous variables were log-transformed prior to use with parametric statistics if possible, otherwise nonparametric statistics were used. Analyses included standard descriptive statistics, unpaired t tests, chi-square, and Mann-Whitney U tests. Analysis of covariance (ANCOVA) models were constructed to compare groups, using the change scores as the dependent variable, with adjustment for baseline score and time on aromatase inhibitors. Although this difference between groups for time on AI therapy at baseline was not statistically significant (P = .06), we felt the difference between groups was clinically meaningful and therefore included it as a covariate in the analysis. Association between variables was determined by Pearson correlation. An intention-to-treat analysis was performed with missing outcome scores imputed from participants’ baseline scores (last observation carried forward). An alpha level of .05 was set as the criterion for statistical significance. Clinical relevance was assessed relative to the meaningfulness of bone and musculoskeletal outcomes compared with existing literature.
Results
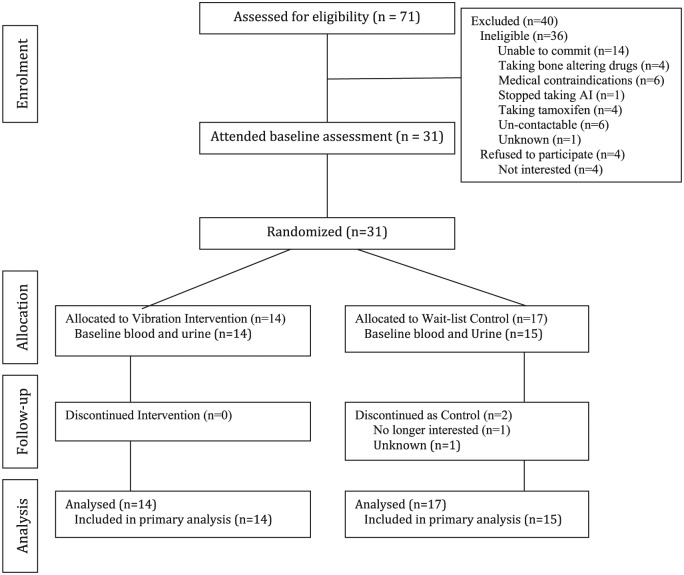
Thirty-one participants were enrolled into the study. Recruitment ended at the completion of the funding period. Participant flow is presented in Figure 1. On average, participants attended 91.5% (range 78%-100%) of WBV training sessions.
Figure 1.
Flow of subject progress through each stage of the trial.
Participant Characteristics
Baseline participant characteristics are presented in Table 1. The cohort had a mean age of 61.6 ± 8.3 years. The participants were on average overweight (body mass index = 28.8 ± 4.7 kg/m2), and the majority were nonsmokers. Baseline characteristics of the participants did not differ between groups, except for duration of AI therapy with participants in the vibration group having been on treatment for longer than the control (median 19 months, IQR 26.5 vs median 3 months IQR 13.5; P = .06). Approximately half (53.3%) of the participants were taking anastrozole, with the remainder taking letrozole. Osteoporosis was present in only 4 participants, with no group differences (P = .35). Baseline levels of 25-OH vitamin D, P1NP, and NTx/Cr were not different between groups (P > .53).
Table 1.
Demographics and Health Status.a
| Variable | Whole Cohort (n = 31) | Vibration (n = 14) | Control (n = 17) | P |
|---|---|---|---|---|
| Age, years | 61.6 ± 8.3 | 61.6 ± 9.2 | 61.6 ± 7.8 | .98 |
| Vitamin D, nmol/L | 86.8 ± 25.9 | 85.6 ± 25.8 | 87.8 ± 26.8 | .83 |
| Body mass index,b kg/m2 | 28.8 ± 4.7 | 28.4 ± 3.9 | 29.1 ± 5.3 | .72 |
| Education, % | .06 | |||
| High school | 83.8 | 85.7 | 81.2 | |
| Undergraduate | 9.7 | 0.0 | 18.8 | |
| Postgraduate | 6.5 | 14.3 | 0.0 | |
| Smoking status, % | .15 | |||
| Past smoker | 35.5 | 35.7 | 35.2 | |
| Current smoker | 9.7 | 0.0 | 5.7 | |
| Alcohol drinks per week | 3.8 ± 4.8 | 3.3 ± 2.8 | 4.3 ± 6.1 | .59 |
| Caffeine drinks per week | 20.3 ± 10.1 | 21.4 ± 7.3 | 19.3 ± 12.3 | .59 |
| Months of AI therapy at baseline, median (IQR) | 10 (20) | 3 (13.5) | 19 (26.5) | .06c |
| Bone mineral density, g/cm3 | ||||
| Hip | 0.874 ± 0.105 | 0.871 ± 0.093 | 0.877 ± 0.116 | .88 |
| Spine | 1.011 ± 0.177 | 0.987 ± 0.159 | 1.032 ± 0.193 | .49 |
| Whole body | 1.053 ± 0.106 | 1.03 ± 0.061 | 1.073 ± 0.131 | .24 |
Abbreviations: AI, aromatase inhibitor; IQR, interquartile range.
All data presented as mean ± SD unless otherwise specified.
Body mass index: an indicator of body fat calculated by weight (kg)/height2 (m). Normal values range from 18.5 to 24.9kg/m2. Values >25 kg/m2 are considered overweight, and >30 kg/m2 are considered obese.
Mann-Whitney test.
Markers of Bone Resorption and Formation
Results are presented in Table 2. Following 12 weeks of vibration training, NTx/Cr levels did not change differentially between groups (adjusted group difference −0.5, P = .929). Change in NTx/Cr was significantly negatively associated with duration of aromatase inhibitors (r = −0.46, P = .009), as well as baseline vitamin D (r = −0.41, P = .026). The change in NTx/Cr was not associated with changes in body composition (Ps > .657) or changes in physical function (Ps > .147), with the exception of chair rise, which was significantly negatively correlated (r = −0.38, P = .039).
Table 2.
Changes in Markers of Bone Resorption and Formation Following the Intervention.
| Vibration Group (Mean ± SD) | Control Group (Mean ± SD) | Adjusted Group Difference in Mean Changea | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Mean Change (95% CI) | P | |
| NTx/Cr (BCE/mmol Cr) | 43.3 ± 20.0 | 45.9 ± 25.6 | 39.0 ± 16.4 | 38.7 ± 12.9 | 0.5 (–10.6 to 11.5) | .929 |
| P1NP (µg/L) | 62.3 ± 27.0 | 64.0 ± 25.6 | 62.2 ± 25.3 | 59.5 ± 26.2 | 5.3 (–4.7 to 15.2) | .286 |
Abbreviations: BCE, bone collagen equivalent; Cr, creatinine; NTx, N-telopeptide X; P1NP, type 1 procollagen N-terminal propeptide.
Adjusted for baseline value and time on aromatase inhibitors at baseline.
P1NP levels did not change differentially between groups over the 12-week intervention (adjusted group difference +5.3, P = .286). Changes in P1NP were not associated with aromatase inhibitor duration (r = 0.80, P = .670), or baseline vitamin D levels (r = 0.089, P = .645). Changes in P1NP were not associated with changes in body composition (Ps > .135) or physical function (Ps > .089), with the exception of chair rise, which was significantly negatively correlated (r = −0.36, P = .049).
Secondary Outcomes
All results of secondary outcomes are reported in Tables 3 and 4. No measures of physical functioning changed differentially between groups (Ps > .140). Changes in maximal strength and functional ability are illustrated in Figures 2 and 3. Body composition measures of lean and fat mass remained unchanged after 12 weeks (Ps > .548). There was no difference between groups for changes in joint pain, stiffness and difficulty with daily activities as measured by the WOMAC scale (P > 0.051). The intervention had no significant effect on fatigue (P = .079).
Table 3.
Changes in Physical Functioning and Patient-Rated Outcomes Following the Intervention.
| Vibration Group (Mean ±
SD) |
Control Group (Mean ±
SD) |
Adjusted Group Difference in Mean Changea |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Mean (95% CI) | P | |
| Physical function | ||||||
| Leg press 1RM, kg, median (IQR) | 65.0 (48.8) | 71.3 (46.2) | 63.0 (50.0) | 65.2 (47.9) | 3.5 (0.0, 10.0) | .140b |
| Chest press 1RM, kg,c median (IQR) | 22.5 (12.5) | 23.8 (11.3) | 17.5 (12.5) | 25.0 (11.9) | 0.0 (0.0, 2.5) | .402b |
| Seated row press 1RM, kg,c median (IQR) | 45.0 (7.9) | 46.2 (5.0) | 42.8 (11.2) | 45.0 (15.7) | 0.0 (0.0, 2.9) | .347b |
| 400-m walk, s | 264.1 ± 31.0 | 256.3 ± 33.8 | 273.5 ± 41.2 | 269.0 ± 36.3 | −5.0 (–20.5, 10.5) | .514 |
| Chair rise, reps | 11.2 ± 2.3 | 11.0 ± 1.6 | 11.4 ± 2.6 | 10.5 ± 2.5 | 0.65 (–0.59, 1.88) | .292 |
| Stair climb, s | 5.2 ± 1.46 | 5.30 ± 1.70 | 5.88 ± 2.20 | 5.09 ± 1.69 | 1.04 (–0.42, 2.49) | .154 |
| 6-m walk usual pace, s | 4.60 ± 0.49 | 4.39 ±0.0.41 | 4.56 ± 0.71 | 4.47 ± 0.54 | 0.65 (–0.59, 1.88) | .292 |
| 6-m walk fast pace, s | 3.35 ± 0.32 | 3.36 ± 0.36 | 3.33 ± 0.58 | 3.44 ± 0.46 | −1.46 (–0.35, 0.60) | .158 |
| 6-m backward walk, s | 18.56 ±4.69 | 16.9 ± 4.34 | 17.3 ± 6.55 | 16.1 ± 6.2 | −0.94 (–3.80, 1.91) | .503 |
| Balance | 80.0 ± 6.9 | 77.8 ± 6.7 | 75.8 ± 6.8 | 77.3 ± 6.9 | 3.04 (–7.56, 1.53) | .185 |
| Patient-rated outcomes | ||||||
| WOMAC Pain, median (IQR) | 0.0 (1.0) | 0.0 (3.3) | 1.0 (6.8) | 2.0 (6.8) | 0.0 (0.0, 1.0) | .334b |
| WOMAC Stiffness, median (IQR) | 0.0 (1.3) | 1.0 (2.0) | 2.0 (3.0) | 2.5 (3.8) | 0.0 (0.0, 1.0) | .224b |
| WOMAC Difficulty, median (IQR) | 0.5 (5.5) | 2.5 (8.5) | 7.0 (20.0) | 6.0 (19.8) | 0.0 (0.0, 3.0) | .063b |
| WOMAC Total, median (IQR) | 1.0 (7.3) | 4.5 (13.8) | 9.0 (27.0) | 9.5 (30.0) | 0.0 (–1.0, 4.3) | .051b |
| Fatigued | 13.1 ± 6.4 | 11.6 ± 4.4 | 13.4 ± 8.8 | 14.2 ± 7.9 | −2.9 (–6.2, 0.4) | .079 |
Abbreviations: 1RM, one repetition maximum; IQR, interquartile range; WOMAC, Western Ontario and McMaster Universities Arthritis Index (higher scores indicate worse symptoms).
Adjusted for baseline value and time on aromatase inhibitor at baseline.
Mann-Whitney test.
n = 28 due to inability to complete upper body strength measures.
Fatigue was assessed by the Functional Assessment of Cancer Therapy–Fatigue subscale (higher scores indicate greater fatigue).
Table 4.
Changes in Body Composition and Bone Mineral Density Outcomes Following the Intervention.
| Vibration Group (Mean ±
SD) |
Control Group (Mean ±
SD) |
Adjusted Group Difference in Mean Changea |
||||
|---|---|---|---|---|---|---|
| Body Composition | Pre | Post | Pre | Post | Mean (95% CI) | P |
| Waist to hip ratio | 0.83 ± 0.07 | 0.82 ± 0.06 | 0.83 ± 0.67 | 0.83 ± 0.70 | 0.012 (–0.30, 0.55) | .548 |
| Whole body mass, kg | 75.1 ± 13.3 | 74.4 ± 12.3 | 75.6 ± 14.3 | 75.6 ± 14.2 | −0.1 (–2.7, 2.4) | .912 |
| Whole body lean mass, kg | 41.4 ± 5.3 | 41.3 ± 5.4 | 43.3 ± 5.7 | 43.0 ± 5.7 | 0.3 (–0.9, 1.6) | .573 |
| Appendicular lean mass, kg | 16.5 ± 2.4 | 16.2 ± 2.4 | 17.4 ± 2.5 | 17.2 ± 2.4 | 0.1 (–0.9, 1.1) | .820 |
| Whole body fat mass, kg | 30.7 ± 8.3 | 31.0 ± 7.5 | 30.9 ± 9.6 | 31.1 ± 9.3 | 0.1 (–1.0, 1.2) | .803 |
| Whole body fat percentage, % | 40.9 ± 4.6 | 41.3 ± 4.1 | 40.2 ± 5.7 | 40.6 ± 5.3 | 0.1 (–1.2, 1.4) | .890 |
| Trunk fat mass, kg | 14.6 ± 4.6 | 14.9 ± 4.1 | 14.6 ± 5.4 | 14.7 ± 5.3 | −0.2 (–1.1, 0.7) | .607 |
Adjusted for baseline value and time on aromatase inhibitor at baseline.
Figure 2.
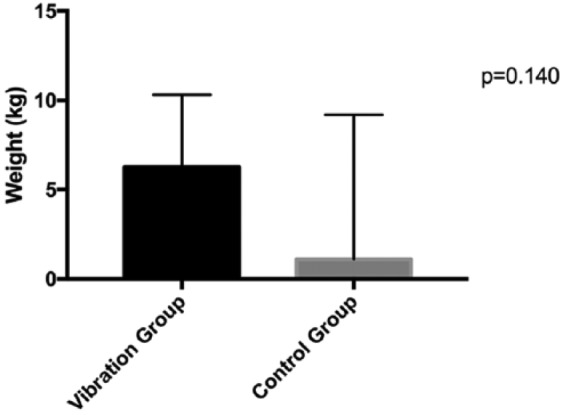
Median change in lower body strength (leg press) at 12 weeks.
Figure 3.
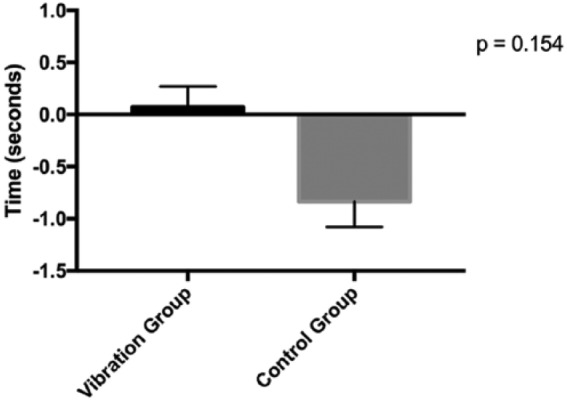
Mean change in stair climb time at 12 weeks.
Adverse Events
Three adverse events occurred during the trial. One participant reported syncope during a training session. Another experienced increased swelling in her arm after exercise testing. A third experienced extreme distress on being allocated to the control group. All participants were able to continue with the intervention and the intervention was otherwise well tolerated.
Discussion
Aromatase inhibitor use secondary to breast cancer results in significant bone loss that puts women at risk for morbidity. This study examined the effect of short-term, low amplitude and low g-force vibration stimulus on markers of bone turnover in postmenopausal breast cancer patients receiving aromatase inhibitor therapy. Following 12 weeks of vibration training, there were no differences between intervention and control groups for markers of bone turnover, physical functioning, body composition, arthralgia symptoms, or fatigue.
Vibration training was well-tolerated by participants. Twenty minutes of vibration exposure 3 times a week for 12 weeks was not effective in changing markers of bone resorption or bone formation in the current study. Previous studies have shown beneficial effects on bone metabolism after vibration training in postmenopausal women,20 and after combined aerobic and resistance training in female cancer survivors.38 However changes observed in bone metabolism may not consistently translate into clinically or statistically significant improvements in bone density or strength. A recent meta-analysis suggests that vibration training in postmenopausal women has little effect on bone outcomes, as measured by DXA, at 6-month follow-up.39 Comparison with other studies, however, is problematic because of large variations in study durations and dose of vibration.40
A range of pharmaceutical interventions are available to improve bone mass. Bisphosphonates such as alendronate increase bone mineral density by approximately 5% to 15%, with better results observed in the lumbar spine and more modest results at the femoral neck.41,42 Different forms of exercise have been shown to be effective in improving bone density. Combined impact protocols, impact exercise training combined with resistance training, is the most effective option for maintaining or preserving bone mineral density in postmenopausal women.43 A meta-analysis of the effect of exercise on bone mineral density on postmenopausal women showed a small effect at both the lumbar spine and femoral neck of a magnitude that is similar to vitamin D and calcium supplementation, but smaller than observed with bisphosphonates.42,44 Recent research suggests that 12 months of aerobic and resistance training does not improve bone mineral density in postmenopausal breast cancer patients taking aromatase inhibitors16 or in those who have completed treatment.13 The authors suggest that the amount of exercise required to sufficiently provide osteogenic stimulus for healthy postmenopausal women could be inadequate for those on aromatase inhibitor therapy.16 The same could be true for the level of WBV stimulus tested in this study.
The mechanism by which vibration stimulus affects bone is not fully understood. It has been hypothesized that the anabolic effect of vibration on bone may be a result of stress exerted on bone through repeated loading and unloading, resulting in increased fluid flow.45 Greater activation of muscle through enhanced sensitivity of mechanoreceptors, and increased osteogenesis in osteoblasts have also been observed, suggesting that vibration is anabolic at a cellular level.46,47 Human studies have reported potential benefits in bone,23,24,48-55 muscle function,52,54,56,57 balance and prevention of falls,58 reduction of muscle spasticity in those with cerebral palsy,59 and postural control in those with Parkinson’s disease.60 However, the optimal time course, dose, and frequency of vibration to elicit optimal changes in bone are currently not established, with a wide variety of vibration exposure protocols leading to variation in changes in bone outcomes. Inconsistency in study nomenclature, design, and measurement of bone outcomes, reporting of adverse effects and noncompliance, highlight the lack of uniformity and small pool of information available on the effects of vibration on bone.61 While improvements in BMD have been demonstrated in postmenopausal women following 6 months of WBV training,54 the lack of effect observed in the current study may be partially explained by the short study duration, the dose of exposure, or the lack of intermittent vibration exposure.62 However, the challenge of overcoming bone loss during AI therapy is great. While treatment with bisphosphonates has shown to be effective in improving BMD, in the adjuvant setting among women undergoing AI therapy such treatment does not prevent fractures.63
No improvements in physical function or body composition were observed in the current study. Previous research indicates that WBV can improve muscular strength as well as physical functioning in older women,64 as well as those with fibromyalgia.65 Even low doses of vibration have previously improved physical function, with increases in lower limb muscle strength in older adults of 14% observed after 13 weeks.66
Given the lack of change in physical function or muscle strength, the lack of change in body composition is unsurprising. Whole body vibration has previously been shown to improve body composition in other clinical populations, such as people with rheumatoid arthritis67 and obese women.68 However these interventions used a much higher dose of vibration of approximately 5g to 6g compared to our 0.3g—a dose chosen for its osteogenic effects.23 It has been suggested that for muscular adaptation, higher frequencies and amplitudes are optimal.69
Arthralgia is a commonly reported side effect of AI therapy with the incidence reported in clinical trials from 16.8% to 35.9%.70 While aerobic and resistance exercise have been shown to improve symptoms of AI-induced arthralgia,71 we did not observe such an effect with WBV. Similarly, while numerous studies have demonstrated an improvement in cancer–related fatigue with exercise,72 no such effect was observed with WBV. As WBV has only a modest effect on energy expenditure and heart rate,73,74 the muscle contractions and perturbation of the cardiovascular system in short 20-minute bouts may not be sufficient to elicit such adaptations.
This study has limitations that should be considered in interpreting the results. We did not reach our target sample size, as such we were underpowered to detect a significant difference between groups, although this was unlikely to have affected the primary outcomes given no trend was observed. Further research is required in order to determine if higher doses of vibration, intermittent vibration combined with exercise on the platform, or longer duration of training (eg, 24 weeks) can elicit beneficial effects on bone during AI therapy. Recent evidence from meta-analyses suggest that side-alternating vibration platforms produce superior outcomes in postmenopausal women39,75—future research should focus on this mode of vibration delivery. Further research into the potential benefits of other forms of prescribed exercise on bone health during AI therapy should be considered.
To our knowledge this is the first application of WBV therapy in breast cancer patients undergoing AI therapy. Well-validated outcomes measured assessed markers of bone turnover, body composition, as well as a comprehensive assessment of physical functioning. We also report an excellent adherence rate to WBV training.
Conclusion
Short-term, low-amplitude and low g-force vibration stimulus does not appear to be effective for altering markers of bone turnover secondary to aromatase inhibitors in breast cancer patients. There also appears to be no benefit in physical functioning, body composition, arthralgia symptoms, or fatigue.
Footnotes
Authors’ Note: The funding source played no role in the design, collection, analysis, or interpretation of the data.
Nigel spry is also affiliated to Genesis Cancer Care, Joondalup, Western Australia, Australia.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by The Cancer Council of Western Australia.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 2. Nordman IC, Spillane AJ, Hamilton AL. The aromatase inhibitors in early breast cancer: who, when, and why? Med J Aust. 2005;183:24-27. [DOI] [PubMed] [Google Scholar]
- 3. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619-629. [DOI] [PubMed] [Google Scholar]
- 4. Geisler J. Differences between the non-steroidal aromatase inhibitors anastrozole and letrozole-of clinical importance? Br J Cancer. 2011;104:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011;22:2546-2555. [DOI] [PubMed] [Google Scholar]
- 6. Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73-82. [DOI] [PubMed] [Google Scholar]
- 7. Hodgson SF, Watts NB, Bilezikian JP, et al. ; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract. 2003;9:544-564. [DOI] [PubMed] [Google Scholar]
- 8. Rizzoli R, Body JJ, DeCensi A, Reginster JY, Piscitelli P, Brandi ML; European Society for Clinical and Economical aspects of Osteoporosis and Osteoarthritis (ESCEO). Guidance for the prevention of bone loss and fractures in postmenopausal women treated with aromatase inhibitors for breast cancer: an ESCEO position paper. Osteoporos Int. 2012;23:2567-2576. [DOI] [PubMed] [Google Scholar]
- 9. Black DM, Schwartz AV, Ensrud KE, et al. ; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. [DOI] [PubMed] [Google Scholar]
- 10. Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int. 2000;67:10-18. [DOI] [PubMed] [Google Scholar]
- 12. Winters-Stone K, Dobek J, Nail L, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011;127:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saarto T, Sievanen H, Kellokumpu-Lehtinen P, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2012;23:1601-1612. [DOI] [PubMed] [Google Scholar]
- 14. Winters-Stone KM, Leo MC, Schwartz A. Exercise effects on hip bone mineral density in older, post-menopausal breast cancer survivors are age dependent. Arch Osteoporos. 2012;7:301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim SH, Cho YU, Kim SJ, Hong S, Han MS, Choi E. The effect on bone outcomes of adding exercise to supplements for osteopenic breast cancer survivors: a pilot randomized controlled trial. Cancer Nurs. 2016;39:144-152. [DOI] [PubMed] [Google Scholar]
- 16. Knobf MT, Jeon S, Smith B, et al. Effect of a randomized controlled exercise trial on bone outcomes: influence of adjuvant endocrine therapy. Breast Cancer Res Treat. 2016;155:491-500. [DOI] [PubMed] [Google Scholar]
- 17. Simonavice E, Liu PY, Ilich JZ, Kim JS, Arjmandi B, Panton LB. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl Physiol Nutr Metab. 2014;39:730-739. [DOI] [PubMed] [Google Scholar]
- 18. Mikhael M, Orr R, Fiatarone Singh MA. The effect of whole body vibration exposure on muscle or bone morphology and function in older adults: a systematic review of the literature. Maturitas. 2010;66:150-157. [DOI] [PubMed] [Google Scholar]
- 19. Rehn B, Lidström J, Skoglund J, Lindström B. Effects on leg muscular performance from whole-body vibration exercise: a systematic review. Scand J Med Sci Sports. 2007;17:2-11. [DOI] [PubMed] [Google Scholar]
- 20. Turner S, Torode M, Climstein M, et al. A randomized controlled trial of whole body vibration exposure on markers of bone turnover in postmenopausal women. J Osteoporos. 2011;2011:710387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y. Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Metab. 2004;22:500-508. [DOI] [PubMed] [Google Scholar]
- 22. Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77:359-361. [PubMed] [Google Scholar]
- 23. Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343-351. [DOI] [PubMed] [Google Scholar]
- 25. Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine (Phila Pa 1976). 2003;28:2621-2627. [DOI] [PubMed] [Google Scholar]
- 26. Ma C, Liu A, Sun M, Zhu H, Wu H. Effect of whole-body vibration on reduction of bone loss and fall prevention in postmenopausal women: a meta-analysis and systematic review. J Orthop Surg Res. 2016;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maimoun L, Simar D, Malatesta D, et al. Response of bone metabolism related hormones to a single session of strenuous exercise in active elderly subjects. Br J Sports Med. 2005;39:497-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 29. Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44-47. [DOI] [PubMed] [Google Scholar]
- 30. Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127-132. [DOI] [PubMed] [Google Scholar]
- 31. Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340-347. [DOI] [PubMed] [Google Scholar]
- 32. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029-3034. [PubMed] [Google Scholar]
- 33. Rossiter-Fornoff JE, Wolf SL, Wolfson LI, Buchner DM. A cross-sectional validation study of the FICSIT common data base static balance measures. Frailty and injuries: cooperative studies of intervention techniques. J Gerontol A Biol Sci Med Sci. 1995;50:M291-M297. [DOI] [PubMed] [Google Scholar]
- 34. Whaley MH, Brubaker PH, Otto RM, Armstrong LE. ACSM’s Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 35. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-1840. [PubMed] [Google Scholar]
- 36. Cella D, Eton DT, Lai JS, Peterman AH, Merkel D. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547-561. [DOI] [PubMed] [Google Scholar]
- 37. Godin G, Shepard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141-146. [PubMed] [Google Scholar]
- 38. Almstedt H, Grote S, Korte JR, et al. Combined aerobic and resistance training improves bone health of female cancer survivors. Bone Rep. 2016;5:274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oliveira LC, Oliveira RG, Pires-Oliveira DA. Effects of whole body vibration on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Osteoporos Int. 2016;27:2913-2933. [DOI] [PubMed] [Google Scholar]
- 40. Thompson WR, Yen SS, Rubin J. Vibration therapy: clinical applications in bone. Curr Opin Endocrinol Diabetes Obes. 2014;21:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bone HG, Hosking D, Devogelaer JP, et al. ; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. [DOI] [PubMed] [Google Scholar]
- 42. Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C; Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23:570-578. [DOI] [PubMed] [Google Scholar]
- 43. Xu J, Lombardi G, Jiao W, Banfi G. Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sports Med. 2016;46:1165-1182. [DOI] [PubMed] [Google Scholar]
- 44. Kelley GA, Kelley KS, Kohrt WM. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2012;13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445-452. [DOI] [PubMed] [Google Scholar]
- 46. Tanaka SM, Alam IM, Turner CH. Stochastic resonance in osteogenic response to mechanical loading. FASEB J. 2003;17:313-314. [DOI] [PubMed] [Google Scholar]
- 47. Tanaka SM, Li J, Duncan RL, Yokota H, Burr DB, Turner CH. Effects of broad frequency vibration on cultured osteoblasts. J Biomech. 2003;36:73-80. [DOI] [PubMed] [Google Scholar]
- 48. Beck BR, Marcus R. The effect of low strain, high frequency mechanical loading on pre-menopausal women with low bone mass: pilot data. Med Science Sports Exerc. 1999;31(5 suppl):S82. [Google Scholar]
- 49. Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464-1474. [DOI] [PubMed] [Google Scholar]
- 50. Iwamoto J, Takeda T, Sato Y, Uzawa M. Effect of whole-body vibration exercise on lumbar bone mineral density, bone turnover, and chronic back pain in post-menopausal osteoporotic women treated with alendronate. Aging Clin Exp Res. 2005;17:157-163. [DOI] [PubMed] [Google Scholar]
- 51. Pitukcheewanont PMD, Safani DBA. Extremely low-level, short-term mechanical stimulation increases cancellous and cortical bone density and muscle mass of children with low bone density: a pilot study. Endocrinologist. 2006;16:128-132. [Google Scholar]
- 52. Russo CR, Lauretani F, Bandinelli S, et al. High-frequency vibration training increases muscle power in postmenopausal women. Arch Phys Med Rehabil. 2003;84:1854-1857. [DOI] [PubMed] [Google Scholar]
- 53. Torvinen S, Kannus P, Sievänen H, et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res. 2003;18:876-884. [DOI] [PubMed] [Google Scholar]
- 54. Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352-359. [DOI] [PubMed] [Google Scholar]
- 55. Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360-369. [DOI] [PubMed] [Google Scholar]
- 56. Torvinen S, Kannu P, Sievänen H, et al. Effect of a vibration exposure on muscular performance and body balance. Randomized cross-over study. Clin Physiol Funct Imaging. 2002;22:145-152. [DOI] [PubMed] [Google Scholar]
- 57. Delecluse C, Roelants M, Verschueren S, Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Science Sports Exerc. 2003;35:1033-1041. [DOI] [PubMed] [Google Scholar]
- 58. Bautmans I, Van Hees E, Lemper JC, Mets T. The feasibility of whole body vibration in institutionalised elderly persons and its influence on muscle performance, balance and mobility: a randomised controlled trial [ISRCTN62535013]. BMC Geriatr. 2005;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med. 2006;38:302-308. [DOI] [PubMed] [Google Scholar]
- 60. Turbanski S, Haas CT, Schmidtbleicher D, Friedrich A, Duisberg P. Effects of random whole-body vibration on postural control in Parkinson’s disease. Res Sports Med. 2005;13:243-256. [DOI] [PubMed] [Google Scholar]
- 61. Lorenzen C, Maschette W, Koh M, Wilson C. Inconsistent use of terminology in whole body vibration exercise research. J Sci Med Sport. 2009;12:676-678. [DOI] [PubMed] [Google Scholar]
- 62. Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports Exerc. 2002;34:196-202. [DOI] [PubMed] [Google Scholar]
- 63. Valachis A, Polyzos NP, Georgoulias V, Mavroudis D, Mauri D. Lack of evidence for fracture prevention in early breast cancer bisphosphonate trials: a meta-analysis. Gynecol Oncol. 2010;117:139-145. [DOI] [PubMed] [Google Scholar]
- 64. Machado A, Garcia-Lopez D, Gonzalez-Gallego J, Garatachea N. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand J Med Sci Sports. 2010;20:200-207. [DOI] [PubMed] [Google Scholar]
- 65. Tapp LR, Signorile JF. Efficacy of WBV as a modality for inducing changes in body composition, aerobic fitness, and muscular strength: a pilot study. Clin Interv Aging. 2014;9:63-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mikhael M, Orr R, Amsen F, Greene D, Singh MA. Effect of standing posture during whole body vibration training on muscle morphology and function in older adults: a randomised controlled trial. BMC Geriatr. 2010;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Prioreschi A, Makda MA, Tikly M, McVeigh JA. In patients with established RA, positive effects of a randomised three month WBV therapy intervention on functional ability, bone mineral density and fatigue are sustained for up to six months. PLoS One. 2016;11:e0153470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Milanese C, Piscitelli F, Zenti MG, Moghetti P, Sandri M, Zancanaro C. Ten-week whole-body vibration training improves body composition and muscle strength in obese women. Int J Med Sci. 2013;10:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Da Silva-Grigoletto ME, De Hoyo M, Sañudo B, Carrasco L, Garcia-Manso JM. Determining the optimal whole-body vibration dose-response relationship for muscle performance. J Strength Cond Res. 2011;25:3326-3333. [DOI] [PubMed] [Google Scholar]
- 70. Younus J, Kligman L. Management of aromatase inhibitor-induced arthralgia. Curr Oncol. 2010;17:87-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liao LR, Ng GY, Jones AY, Pang MY. Cardiovascular stress induced by whole-body vibration exercise in individuals with chronic stroke. Phys Ther. 2015;95:966-977. [DOI] [PubMed] [Google Scholar]
- 74. Maikala RV, Bhambhani YN. Cardiovascular responses in healthy young women during exposure to whole-body vibration. Int J Ind Ergon. 2008;38:775-782. [Google Scholar]
- 75. Fratini A, Bonci T, Bull AM. Whole body vibration treatments in postmenopausal women can improve bone mineral density: results of a stimulus focussed meta-analysis. PLoS One. 2016;11:e0166774. [DOI] [PMC free article] [PubMed] [Google Scholar]