Abstract
Plants from the Bursera genus are widely distributed in the tropical dry forests of Mexico. In traditional medicine, extracts from different species of Bursera have been used for a wide range of biological activities, including the treatment of cancer-related symptoms. Compounds present in the Bursera genus include lignans, flavonoids, steroids, short-chain aliphatic alkanes, acetates, alcohols, ketones, and terpenoids. In some instances, secondary metabolites of these classes of compounds may induce cytotoxicity, and therefore we sought to investigate the effects of B. copallifera leaf extracts in breast cancer cell lines to evaluate their potential therapeutic value for the treatment of breast cancer, one of the most prevalent types of cancer in women worldwide. Two B. copallifera leaf extracts exerted cytotoxic effects on both the MCF7 and MDA-MB-231 breast cancer cell line models. The cytotoxic effect was more evident in the MDA-MB-231 triple negative cell line inhibiting also the migration of these cells. We identified hydroxycinnamic acid and flavonol derivatives as major phenolic components of the extracts. Our results strongly suggest a potential use of the Bursera leaf extracts rich in phenolic compounds, their individual phenolic compounds, or their combinations for the treatment of breast cancer.
Keywords: Bursera copallifera, breast cancer, cytotoxicity, migration
Introduction
The plant family Burseraceae consists of approximately 20 genera and 600 species that are widely distributed at low elevations in subtropical regions.1 The most representative genus is Bursera, with over a hundred species present from the south of the United States to Peru.2 In Mexico, Bursera is one of the most important physiognomic components of the tropical dry forests and is locally known as cuajiote or copal. In many places, the genus becomes the dominant or codominant woody taxon, surpassing the legumes in diversity and abundance3,4 and is notable for its terpenoid secretions and exudates,5-7 which are known to provide chemical defense against specialized herbivores.8,9 The genus Bursera is the past and current source of copal and incense of Aztec and Maya Indians.10 The word copal comes from the Nahuatl word copalli, name given to the different aromatic resins used as incense, independent of the plant from which they are extracted.11,12 Since pre-Hispanic times, the use of these resins has had a great social, economic, religious-ceremonial relevance, as well as in daily life. In this regard, there are current references by the populace on the consumption (dissolved or swallowed) of the fruits of copal (B. copallifera) “to get rid of abscesses or lumps in the breast.”13-15
Bursera extracts and compounds have been investigated for a wide range of biological activities, for example, antioxidant, cytotoxic, antihelmintic, bactericidal, antiparasitic, acaricidal, anti-inflammatory, and antiviral activities.16-26 Phytochemical studies of the genus Bursera have reported the presence of lignans, bilignans, flavonoids, flavonoid glycosides, steroids, short-chain aliphatic alkanes, acetates, alcohols, ketones, and terpenoids, the latter mostly monoterpenes while diterpenes and triterpenes occur at lesser extent.5,6,18,27-33 Secondary metabolites belonging to these groups of compounds have been reported to have cytotoxic properties,34-36 further suggesting a potential use of Bursera extracts in the treatment of cancer. Accordingly, extracts of B. ariensi, B. bicolor, B. linanoe, B. galeottiana, B. fagaroides, B. graveolens, B. microphylla, and B. schlechtendalli have been found to induce growth inhibitory effects in different cancer cell lines, and extracts from B. fagaroides, B. permollis, B. morelensis, B. microphylla, B. klugii, and B. schlechtendalii have also been shown to exert antitumor activity in mice.17,18,37,38 Recently, the cytotoxic activity of B. copallifera extracts from stem and leaves on different types of cancer cell lines has been also demonstrated.39
Since there are references by the populace on the consumption of B. copallifera to get rid of abscesses or lumps in the breast,13-15 we evaluated the effects of this particular species on breast cancer cell lines. Also, presence of resins and essential oils is characteristic of the genus and many studies have focused on the chemical characterization of bark or volatiles; however, little is known about the compounds present in leaves and their biological activity.18,19,40,41
Materials and Methods
Sample Extraction
Samples of B. copallifera collected in Iguala, Guerrero, Mexico (CFA) and Taxco de Alarcón Guerrero (TA) in October–November were identified and preserved at the IMSS (Instituto Mexicano del Seguro Social) herbarium in Mexico City for future reference, with the voucher number 24578. B. copallifera powdered dried leaves (10 g) were extracted with methanol using a soxtherm apparatus (Soxtherm automatic, Gerhardt, Germany) for 1.30 hours at 60°C, with reduction intervals of 2 minutes 30 seconds and reduction pulse of 2 seconds. The extract was filtered and concentrated under reduced pressure to dryness and the residue was dissolved in DMSO and protected from light until use on cell cultures.
Identification of Phenolic Compounds by HPLC-DAD-ESI/MSn by RP-HPLC-DAD
For the chemical analysis, each sample was mixed with 1.5 mL of methanol:water:formic acid (25:24:1, v:v:v), then it was vortexed and sonicated in an ultrasonic bath for 60 minutes. The samples were kept at 4°C overnight and sonicated again for 60 minutes. A centrifugation was performed for 10 minutes at 10 000 rpm to separate the supernatant from the solid residue. The supernatant was filtered through a 22 μm PVDF (polyvinylidene fluoride) filter before analysis. The chromatographic analyses for identification were carried out on a Luna C18 column (250 × 4.6 mm, 5 µm particle size; Phenomenex, Macclesfield, UK). Water:formic acid (99:1, v/v) and acetonitrile were used as the mobile phases A and B, respectively, with a flow rate of 1 mL/min. The linear gradient started with 8% solvent B, reaching 15% solvent B at 25 minutes, 22% at 55 minutes, and 40% at 60 minutes, which was maintained to 70 minutes. The injection volume was 20 µL. The HPLC-DAD-ESI/MSn analyses were carried out using an Agilent HPLC 1100 series model equipped with a photodiode array detector and a mass detector in series (Agilent Technologies, Waldbronn, Germany). The equipment consisted of a binary pump (model G1312A), an autosampler (model G1313A), a degasser (model G1322A), and a photodiode array detector (model G1315B). The HPLC system was controlled by ChemStation software (Agilent, version 08.03). The mass detector was an ion trap spectrometer (model G2445A) equipped with an electrospray ionization interface, and was controlled by LCMSD software (Agilent, version 4.1). The ionization conditions were 350°C and 4 kV, for capillary temperature and voltage, respectively. The nebulizer pressure and nitrogen flow rate were 65.0 psi and 11 L/min, respectively. The full-scan mass covered the range of m/z from 100 to 1200. Collision-induced fragmentation experiments were performed in the ion trap using helium as the collision gas, with voltage ramping cycles from 0.3 to 2 V. The mass spectrometry data were acquired in the positive ionization mode for anthocyanins and in the negative ionization mode for other flavonoids. The MSn was carried out in the automatic mode on the more-abundant fragment ion in MS(n − 1).
For the quantification, all samples were also centrifuged for 5 minutes at 9500 × g. Each supernatant was filtered through a 0.22 μm PVDF filter (Millex HV13, Millipore, Bedford, MA, USA) before injection into the HPLC-DAD system. Chromatograms were recorded at 280, 320, and 360 nm. Flavonols were determined as quercetin 3-O-glucosides at 360 nm, and phenolic acids and derivatives as 5-O-caffeoylquinic acid at 320 nm.
Cell Culture
The MCF7 and the MDA-MB-231 cell lines were cultured in RPMI and DMEM high glucose medium, respectively, both containing 10% fetal calf serum (FCS, full medium). Cells were kept in a humidified incubator containing 5% CO2. For each experiment, cells were trypsinized, plated at the indicated densities, and treated after 24 hours. For B. copallifera extract treatment, the methanolic extract with a concentration of 172 mg/mL was diluted as a 1:1000 work solution in serum free media. Before treatment, full medium was replaced with serum-free media and the extract was used at concentrations between 2.5 to 200 µg/mL for 24 hours as described in each experiment.
Hematoxylin-Eosin and Giemsa Staining
MCF7 and MDA-MB-231 cells were plated on coverslips in 6-well plates at a density of 5 × 105 cells/ well. The cells were treated with the indicated concentrations of the B. copallifera extract and after 24 hours, they were fixed in methanol for 3 minutes and washed with sterile phosphate buffered saline (PBS) 3 times. Cells were incubated with Harris hematoxylin (Omnichem, Lima, Peru) for 5 minutes and washed with distilled water. Cells were stained with eosin for 1 minute, washed twice with PBS and mounted with sterile water. For Giemsa staining, the cells were fixed in methanol for 5 minutes, washed with PBS, and stained for 5 minutes with a working solution of Giemsa stain prepared from a commercially available stock solution (Golden Bell, Jalisco, Mexico) according to the manufacturer’s recommendations. The coverslips were then washed with distilled water to remove the excess dye and lastly, air dried.
Viability Tests
For MTT experiments, cells were plated in 96-well plates, at a density of 5000 cells/well. They were treated with different concentrations of the B. copallifera extract and after 24 hours, they were washed with PBS and incubated with MTT working solution containing 0.5 mg/mL MTT (thiazolyl blue tetrazolium bromide, Sigma, St Louis, MO, USA), 7% FCS and penicillin/streptomycin in PBS for 4 hours. After incubation, the dye was solubilized with DMSO and the plate was read in a LP400 microplate reader (Anthos Labtec Instruments, Wals, Austria) at 550 nm with a 620 nm reference. Calcein AM (Molecular Probes, Eugene, OR, USA) and propidium iodide (PI) staining were used to determine cytotoxicity. Cells were plated on coverslips at a density of 75 000 cells per dish (35 mm), treated with different concentrations of the B. copallifera extract and after 24 hours, they were incubated with 0.5 nM calcein AM in serum-free medium for 15 minutes and then incubated with 1 µM PI for 5 minutes, both at 37°C. Cells were mounted with medium and observed in a Zeiss Observer.Z1 microscope equipped with an Axiocam MRm camera with green filter set 44 (1114-459, excitation BP 475/40 and emission BP 530/50) or red filter set 45 (1114-462, excitation BP 460/40 and emission BP 630/75). Clonogenic assays were used to determine long-term survival of the cells after treatment with the extract. Briefly, cells were plated on 24-well plates at a density of 3000 cells for MCF7 and 1500 for cells/well for MDA-MB-231 and treated for 24 hours with different concentrations of the B. copallifera extract. After treatment, cells were washed with PBS and medium was replaced with full media. The cells were left in culture for 5 more days, they were fixed (10% acetic acid, 10% methanol, 80% water) and stained with crystal violet solution (0.4% crystal violet, 20% ethanol in water). Stain was solubilized with 30% acetic acid and absorbance was measured at 540 nm in a Synergy 4 (BioTek, Winooski, VT, USA) microplate reader. Controls were treated with the amount of methanol present in the highest concentration of the extract (0.2 µL/mL) since we previously found no differences in cell viability in cells treated with different amounts of vehicle (data not shown).
Migration Assay
Cells were plated in a 24-well plate at a density of 50 000 cells/well. After 24 hours in culture, 3 wounds per well were made with the laser beam of a PALM Microbeam Zeiss Observer.Z1 Microscope. The wound was 328 µm wide and 750 µm long. After the wound was made, the medium was changed to serum free medium and cells were treated with the B. copallifera extract. After 24 hours, cells were stained with calcein AM as previously described and imaged in a Zeiss Observer.Z1 fluorescence microscope with green filter set 44. Green fluorescence intensity was calculated as intensity mean value using Zen 2 software (Carl Zeiss Microscopy, GmbH, 2011).
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical differences were determined using GraphPad Prism 6.04 software. P values ≤.05 were considered as statistically significant. IC50 (inhibitory concentration 50) values were determined using GraphPad Prism 6.04 software with a logarithmic versus response nonlinear fit adjustment.
Results
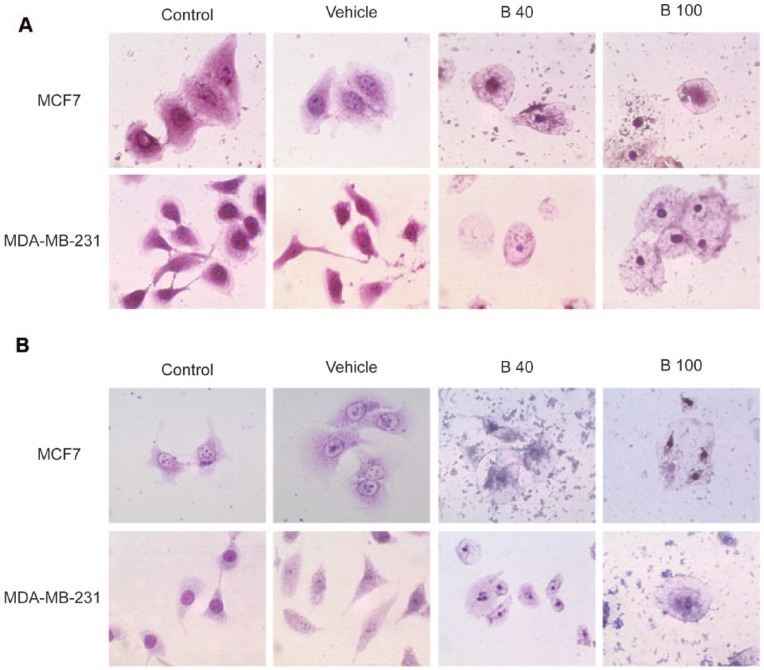
We investigated the effects of 2 B. copallifera extracts on 2 breast cancer cell lines that belong to different subtypes of breast cancer. The MCF7 cell line is an ER (estrogen receptor)– and PR (progesterone receptor)–positive cell line and the MDA-MB-231 is a triple negative breast cancer cell line (ER, PR, and human epidermal growth factor receptor 2 [HER2] negative).43 Hematoxylin-eosin and Giemsa staining of cells treated with B. copallifera showed dramatic morphological changes in both cell lines characterized by cell swelling, cytoplasmic vacuolization and nuclear condensation when evaluated in hematoxylin-eosin (Figure 1A) or Giemsa-stained slides (Figure 1B). Interestingly, the TA extract–induced morphological changes at lower concentrations than the CFA extract (data not shown).
Figure 1.
Bursera copallifera extract–induced morphological changes in breast cancer cell lines. Hematoxylin-eosin (A) or Giemsa (B) staining of MCF7 and MDA-MB-231 cells treated with a B. copallifera extract at 40 and 100 μg/mL (B40 and B100). Cells were treated for 24 hours with the extract and then stained as indicated in the Materials and Methods section.
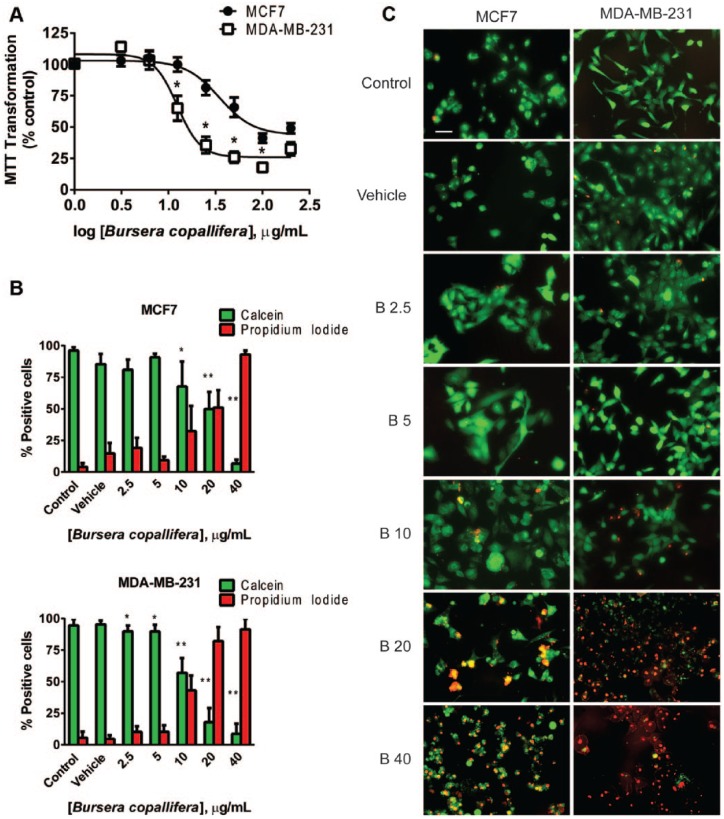
Tetrazolium salt reduction by NADH-dependent dehydrogenases in the cell has been extensively used as an indicator of cell viability.44 Thus, we tested a tetrazolium salt, MTT, transformation efficiency in both cell lines using both B. copallifera extracts. Both the TA and CFA extracts decreased MTT transformation in a dose-dependent manner and the TA extract had a more pronounced effect than the CFA extract in both cell lines (Figure 2). In the MCF7 cells, TA extract was more effective at reducing MTT transformation at 25 and 50 µg/mL when compared to the CFA extract. In MDA-MB-231 cells, the same difference was observed at 12.5 and 50 µg/mL. The MDA-MB-231 cell line showed the highest sensitivity to B. copallifera treatment when compared with MCF7 cells (Figure 3A). According to the National Cancer Institute (NCI), plant extracts with cytotoxic ED50 (effective dose 50) values ≤30 µg/mL are considered active.37 The calculated IC50 doses were 34.88 and 12.43 µg/mL for MCF7 and MDA-MB-231 cell lines respectively, suggesting that the B. copallifera extract might have a therapeutic potential particularly for breast cancers like the MDA-MB-231 triple negative cell line.
Figure 2.
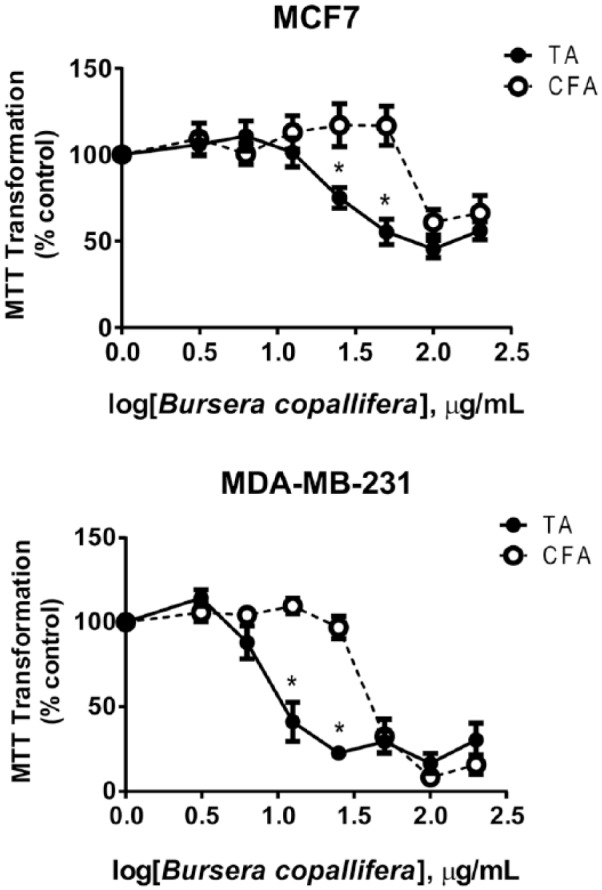
Bursera copallifera extracts induced a decrease in cell number in breast cancer cell lines. MTT transformation was used to determine the effect of both the TA and CFA extracts on cell viability of MCF7 and MDA-MB-231 cell lines. Graphs show the mean + SE of 4 independent experiments performed in triplicate. *Different from the other extract at the same concentration, p < .05.
Figure 3.
The Bursera copallifera extract was cytotoxic to breast cancer cell lines. In (A), we used MTT transformation to compare the sensitivity to the extract between the MCF7 and MDA-MB-231 cell lines. The graph shows mean + SE of 4 independent experiments performed in triplicate. *Different from the other cell line, p < .05. In (B) and (C), Calcein/propidium iodide staining were used to test cytotoxic effects of the extract. In (B), graphs show the mean + SE of 3 independent experiments. *Different from vehicle-treated cells, P < .05. A representative image is shown in (C) for the different concentrations of the extract tested (μg/mL).
The TA and CFA samples were analyzed for the presence of (poly) phenolic compounds and the results displayed mainly hydroxycinnamic acid and flavonol derivatives, which were determined by HPLC-DAD-ESI-MSn analysis. Based on retention times, ultraviolet spectra, mass fragmentation and comparison with available data in the literature, a total of 16 compounds were clearly identified in B. copallifera. Data on retention time, fragmentation pattern, and presence in the samples are presented in Table 1. Most of the 16 identified compounds were present and common to both samples analyzed. Only a few compounds were different according to the sample used, such as Myricetin-3-glc and Myricetin-3-rha present only in TA samples and Q-3-rut (Rutin) which was found to be present only in the CFA extract. In this regard, the presence of the two compounds in the TA but not the CFA extract (myricetin-3-glc and myricetin-3-rha), suggests that the increased efficiency of the TA extract at reducing cell viability could be due to the presence of these glycosilated flavonols, to their interaction with other phenolic compounds or to a higher concentration of the other phenolic compounds with known cytotoxic activities present in the extracts.
Table 1.
Compounds Identified in the Samples of Bursera copallifera by HPLC-DAD-ESI-MSn.
| Phenolic Compounds | Rt (Minutes) | [M]− m/z | MSn m/z | TA | CFA |
|---|---|---|---|---|---|
| 5-CQA (chlorogenic acid) | 18.7 | 353a | 191 | + | + |
| Quercetin-3-rha-X-glcb | 25.9 | 609 | 447, 301 | + | + |
| 5 p-coumaroyl quinic acid | 27.2 | 337 | 191, 163 | + | + |
| Cafferoyl shikimic acid | 28.9 | 335 | 179, 135 | + | + |
| Feruloyl quinic acid | 31.2 | 367 | 191 | + | + |
| Myricetin-3-gal | 35.0 | 479 | 316, 179 | + | + |
| Myricetin-3-glc | 35.9 | 479 | 316, 179 | + | − |
| Quercetin-3-glc-gallic | 40.5 | 615 | 463, 301 | + | + |
| Myricetin-3-rha | 42.3 | 463 | 316, 179 | + | − |
| Q-3-rut (rutin) | 43.1 | 609 | 301 | − | + |
| Q-3-gal | 44.2 | 463 | 301 | + | + |
| Q-3-glc | 45.6 | 463 | 301 | + | + |
| Q-3-xyl | 48.8 | 433 | 301 | + | + |
| Q-3-arabp | 50.5 | 433 | 301 | + | + |
| Q-3-arabf | 52.5 | 433 | 301 | + | + |
| Q-3-rha | 54.0 | 447 | 301 | + | + |
Abbreviations: HPLC-DAD-ESI-MSn, high-performance liquid chromatography–photodiode array detection–electrospray ionization–mass spectrometry; rut, rutinoside; gal, galactoside; glc, glucoside; rha, rhamnoside, xyl, xyloside; arabp, arabinopyranoside; arabf, arabinofuronoside.
Dimeric adduct [M]− = 707 m/z.
Quercetin 3-rhamnoside-X-glucoside or Quercetin 3-glucoside-X-rhamnoside.
A decrease in cell number or in MTT transformation efficiency could be due to decreased proliferation or to increased cell death. To determine if the B. copallifera extract had a cytotoxic effect, we performed death assays and long-term clonogenic assays in both cell lines using the TA extract. Nonfluorescent calcein AM is converted to green fluorescent calcein by intracellular esterase activity. Calcein is well retained within live cells, producing an intense green fluorescence in them. Also, propidium iodide (PI) staining enters the cells with damaged membranes and binds nucleic acids, thereby producing a red nuclear fluorescence in dead cells. In agreement with the MTT results, B. copallifera treatment induced a dose-dependent decrease in cell viability (green staining) and an increase in cell death (red staining) in both cell lines (Figure 3B and C). However, there was a decrease in cell viability in the MDA-MB-231 cell line starting at 2.5 µg/mL after 24 hours of treatment and this decrease was evident in the MCF7 cell line until 10 µg/mL. The different sensitivity of the cell lines to the extract was more evident at 20 µg/mL where MCF7 cells showed a 49.89% in cell viability (50.88% cell death) and the MDA-MB-231 showed a 17.85% of cell viability (82.14% cell death).
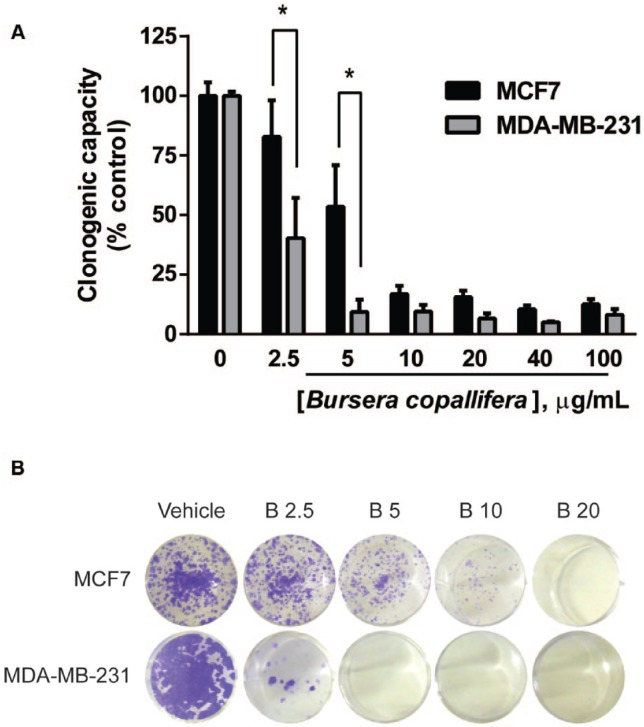
Long-term colony formation experiments have been used to determine cell death after treatment with radiation or chemotherapy.45 Since viability experiments were performed after 24 hours of treatment, our MTT and calcein/PI staining results suggest that the B. copallifera extract is cytotoxic after a short-term treatment. However, these results did not rule out the possibility that the extract was causing cell damage that would eventually result in cell death at lower concentrations as radiation or chemotherapy do. We treated both cell lines with different concentrations of the extract for 24 hours, removed the treatment, and allowed the cells to recover and proliferate in full medium for 5 days (Figure 4). A decrease in cell number was observed even at 2.5 µg/mL particularly in the MDA-MB-231 cell line. Our data show that despite being nontoxic at low concentrations at 24 hours, the B. copallifera extract induced cell damage that ultimately resulted in cell death at later time points. There was a decrease in viability starting at 2.5 µg/mL in both cell lines and the MDA-MB-231 cell line was more sensitive than the MCF7 cells starting at the lowest concentration tested. Concentrations of 5 and 20 µg/mL were the lowest that completely killed the MDA-MB-231 and MCF7 cells, respectively (Figure 4A and B).
Figure 4.
The Bursera copallifera extract was cytotoxic to breast cancer cell lines and decreased long-term survival at low concentrations. We used clonogenic survival experiments to determine the long-term effects of the extract in both breast cancer cell lines. The graph in (A) shows the mean + SE of 3 independent experiments. *Different from the other cell line, p < .05. In (B), a representative image is shown from stained wells containing cells treated with the different concentrations of the extract (μg/mL).
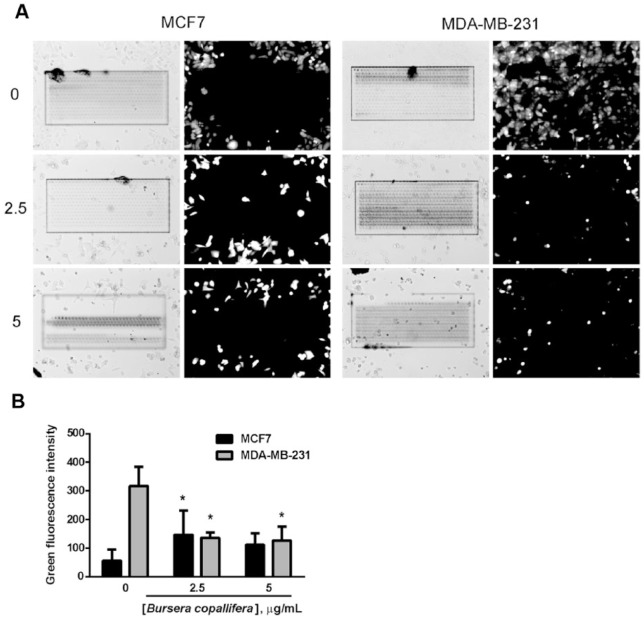
We used concentrations of the extract where cell toxicity induced by the B. copallifera extract was minimal in both cell lines at 24 hours (Figure 3) to assess migration inhibition in a wound assay for both cell lines. In the MCF7 cell line, we did not observe a dramatic increase in green fluorescence as measured by calcein green fluorescence intensity in the wound area in the control cells. There was an increase in fluorescence intensity at the concentration of 2.5 µg/mL and no difference at 5 µg/mL. On the other hand, in the MDA-MB-231, a highly metastatic cell line, we observed a dramatic wound recovery and the wound area was almost completely recovered after 24 hours. Treatment with the extract dramatically reduced cell number in the wound area in this cell line (Figure 5A and B).
Figure 5.
The Bursera copallifera extract decreased migration of the highly metastatic MDA-MB-231 cell line. We used the scratch-wound assay to evaluate changes in migration ability in both cell lines treated with the B. copallifera extract. Cells were treated for 24 hours with nontoxic concentrations of the extract at this time point. In (A), a representative image is shown of both cell lines with vehicle treatment (0) or 2.5 and 5 μg/mL B. copallifera extract. The wound is shown in the brightfield image. Cells were stained with calcein after 24 hours of treatment and the green fluorescent intensity inside the wound area was quantified. In (B), the graph shows the mean + SE of a representative experiment performed in triplicate. *Different from vehicle-treated cells, p < .05.
Discussion
Breast cancer is the leading cause of cancer death in women worldwide. Although breast cancer has a higher incidence in developed countries, an estimated 60% of deaths provoked by this disease occur in the developing world.42 Although some developed countries have achieved a decrease in mortality rate in women with breast cancer,43 other countries still show increasing incidence and mortality rates.42
Breast cancer is a highly heterogeneous disease. In the clinic, it has been classified by the presence of biological markers including the presence or absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2). Hormone receptor–positive breast cancers (ER+ and/or PR+) are associated with the most favorable short-term prognosis since the expression of hormone receptors allows treatment with hormonal therapy. HER2-enriched breast cancers are more aggressive but the use of targeted therapies against HER2 signaling has reversed much of the adverse prognosis of these types of cancers. Finally, triple negative breast cancers do not express any of the receptors. Women diagnosed with this type of breast cancer have the worst short-term prognosis since there are no targeted therapies for these tumors.44 The heterogeneity of breast cancer, the high toxicity and adverse effects of chemotherapy as well as the lack of targeted treatments for the triple negative disease make it imperative to find alternative treatments that could lead to an increase in life expectancy of breast cancer patients.
Plants from the Bursera genus are known to contain cytotoxic/antitumoral lignans35 and scientific evidence supports their use as cytotoxic agents. Extracts from stems and fruits of B. copallifera have been found to have cytotoxic effects on some cancer cell lines, including the MCF7 breast cancer cell line despite the absence of podophyllotoxin and/or related lignans, which are compounds with known cytotoxic activities.35 In agreement with these results, we did not find podophyllotoxin or any related lignan in the HPLC-DAD-ESI-MSn analysis of the B. copallifera extracts that we used. Our results suggest that the cytotoxic activity in the B. copallifera extracts was due to the hydroxycinnamic acid and flavonol derivatives present in the extracts, since some of these compounds are known to possess anticancer activities. For instance, chlorogenic acid, quercetin derivatives, and myricetin, have been proposed to have anticancer activities in breast45-47 and other types of cancer.46-48 Importantly, myricetin-3-glc and myricetin-3-rha, 2 glycosilated flavonoids were present only in the extract with the highest cytotoxic activity. So, the higher cytotoxic activity found in this extract could be due to the presence of these compounds or to their interaction with the other phenolic compounds present in the extract.
The available literature on flavonoids from Bursera spp. is very limited, and only a few communications on the prenylflavonoids of B leptophloeos were available.49 The characterization of flavonols in B. copallifera was not previously documented to the best of our knowledge,50 and for the purpose of this work, the glycosylated flavonoids in the samples are potential candidates for exerting the observed cytotoxic effects, based on their potential for bioactivity.51
Recently, the hexane fraction of a methanolic resin extract of B microphylla was shown to have antiproliferative and proapoptotic effects in different types of cancer cell lines, including the MCF7 breast cancer cell line.38,52 The mechanism by which this extract induced cytotoxicity involved a p53-independent increase in p21 together with a decrease in cyclin E, reduced Rb phosphorylation, and caspase-3 activation. It will be important to test if B. copallifera extracts, any of its components or the combination of constituents in the extract exert cytotoxicity by a similar mechanism.
Metastasis formation is the main cause of cancer-related death and it is the main characteristic of advanced-stage cancers.53 During metastasis, cancer cells migrate through the basement membrane and vasculature to get to target organs. Therefore, a cancer therapy that inhibits cell migration is highly desirable. The scratch wound assay has been used to evaluate cell migration, a critical process in the establishment of metastatic foci.54 Our results show that the B. copallifera extract inhibited cell migration in a wound assay in the highly metastatic MDA-MB-231 cell line but not in the MCF7 cell line (Figure 5), which is poorly metastatic in vivo.55 Decreased migration in the MDA-MB-231 cell line could be explained as decreased metastatic abilities or decreased proliferation ability which would later result in cell death.
Conclusions
In Mexico, more than 90% of the population use medicinal plants in common practice for the empirical treatment of several diseases including cancer.34 Cancer patients perceive medicinal plants as efficient and safe because of their natural origin, even though there are few toxicological and pharmacological studies supporting their use. Importantly, approximately 60% of drugs currently used for cancer treatment have been isolated from natural products,34 underscoring the therapeutic potential of plant extracts in cancer treatment.
Our results show that the B. copallifera leaf extract had a cytotoxic effect on the breast cancer cell lines MCF7 and MDA-MB-231. The MDA-MB-231 triple negative cell line was more sensitive to the extract, showed migration inhibition in a wound assay and had an IC50 of 12.43 µg/mL, suggestive of a therapeutic potential of the extract in this type of breast cancer. Our data suggests that the cytotoxic activity in the B. copallifera was mainly due to the hydroxycinnamic acid and flavonol derivatives present in the extracts, since some of these compounds are known to possess anticancer activities. The results shown here support the therapeutic potential of B. copallifera extracts and the need to generate knowledge on the influence of B. copallifera bioactive compounds (either individually or synergistically) for the treatment of breast cancer, particularly of the triple negative subtype, which currently does not have a targeted therapy.
Acknowledgments
We would like to acknowledge Dr Julio Morán Andrade, M.C. Guadalupe Domínguez Macouzet (Instituto de Fisiología Celular, UNAM) for kindly providing reagents used in this study.
Footnotes
Authors’ Note: Fabiola Domínguez is also affiliated to Centro de Investigación Biomédica de Oriente (CIBIOR). Paola Maycotte is now affiliated to CONACYT-CIBIOR, Instituto Mexicano del Seguro Social, Mexico. Diego A. Moreno and Federico Ferreres are now affiliated to Campus Universitario Espinardo-25, Murcia, Spain. Martín Pérez-Santos is now affiliated to Benemérita Universidad Autónoma de Puebla, Mexico.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported with the following projects from the Instituto Mexicano del Seguro Social: FIS/IMSS/PROT/G11/974 and CTFIS/1ORD/012/2011. Adilene Acosta-Casique had a CONCYTEP (Consejo de Ciencia y Tecnología del Estado de Puebla) scholarship. Part of this work was carried out in international collaboration within the CYTED Programme (Ref. 112RT0460) CORNUCOPIA Thematic Network (URL: redcornucopia.org).
ORCID iD: Paola Maycotte  https://orcid.org/0000-0003-4059-0554
https://orcid.org/0000-0003-4059-0554
References
- 1. Weeks A, Daly DC, Simpson BB. The phylogenetic history and biogeography of the frankincense and myrrh family (Burseraceae) based on nuclear and chloroplast sequence data. Mol Phylogenet Evol. 2005;35:85-101. [DOI] [PubMed] [Google Scholar]
- 2. Daly DC. Notes on Bursera in South America, including a new species. Studies in neotropical Burseraceae VII. Brittonia. 1993;45:240-246. [Google Scholar]
- 3. Rzedowski J, Lemos R, Rzedowski G. Inventario del conocimiento taxonómico, así como de la diversidad y del endemismo regionales de las especies mexicanas de Bursera (Burseraceae). Acta Bot Mex. 2005;70:85-111. [Google Scholar]
- 4. Rzedowski J, Kruse H. Algunas tendencias evolutivas en Bursera (Burseraceae). Taxon. 1979;28:103-116. [Google Scholar]
- 5. Becerra JX. Squirt-gun defense in Bursera and the chrysomelid counterploy. Ecology. 1994;75:1991-1996. [Google Scholar]
- 6. Becerra JX, Venable DL. Rapid-terpene-bath and “squirt-gun” defense in Bursera schlechtendalii and the counterploy of chrysomelid beetles. Biotropica. 1990;22:320-323. [Google Scholar]
- 7. Mooney HA, Emboden WA., Jr. The relationship of terpene composition, morphology, and distribution of populations of Bursera microphylla (Burseraceae). Brittonia. 1968;20:44-51. [Google Scholar]
- 8. Islam MA, Becerra JX. Comparative analyses of chemical composition in the leaves of three Bursera species and their effect on insect pest. J Expt Biosci. 2011;2:29-34. [Google Scholar]
- 9. Vandenabeele P, Grimaldi DM, Edwards HGM, Moens L. Raman spectroscopy of different types of Mexican copal resins. Spectrochim Acta A Mol Biomol Spectrosc. 2003;59:2221-2229. [DOI] [PubMed] [Google Scholar]
- 10. Lucero P. Chemical Analysis of Resinous Materials Employed in Artistic Pre-Hispanic Mexico: Application to Aztec and Maya Archaeological Samples [thesis]. University d’Avignon; 2012. [Google Scholar]
- 11. Stacey R, Cartwright C, McEwan C. Chemical characterization of acient Mesoamerican “copal” resins: preliminary results. Archaeometry. 2006;48:323-340. [Google Scholar]
- 12. Peterson AA, Peterson AT. Aztec exploitation of cloud forests: tributes of liquidambar resin and quetzal feathers. Global Ecology Biogeography Lett. 1992;2:165-173. [Google Scholar]
- 13. Jiménez Ramírez J, Flores K, Durán R. Balsas (Sapindaceae), Género Nuevo de la Cuenca del Río Balsas en el Estado de Guerrero, México. Novon A J Bot Nomencl. 2011;21:196-200. [Google Scholar]
- 14. Reyes-García T, Maradiaga-Ceceña F, Catalán-Heverástico C, Ávila-Sánchez P, Jiménez-Hernández J. Flora leñosa del municipio de Cocula, Guerrero, México. Polibotánica. 2012;34:21-49. [Google Scholar]
- 15. Chazaro-Basañez M, Mostul B, Lara F. Los copales mexicanos (Bursera spp). Bouteloua. 2011;7:57-70. [Google Scholar]
- 16. Alvarez AL, Habtemariam S, Parra F. Inhibitory effects of lupene-derived pentacyclic triterpenoids from Bursera simaruba on HSV-1 and HSV-2 in vitro replication. Nat Prod Res. 2015;29:2322-2327. [DOI] [PubMed] [Google Scholar]
- 17. Acevedo M, Nunez P, Gónzalez-Maya L, CardosoTaketa A, Villarreal L. Cytotoxic and anti-inflammatory activities of Bursera species from Mexico. J Clin Toxicol. 2015;5:232. [Google Scholar]
- 18. Rojas-Sepúlveda AM, Mendieta-Serrano M, Mojica MYA, et al. Cytotoxic podophyllotoxin type-lignans from the steam bark of Bursera fagaroides var. fagaroides. Molecules. 2012;17:9506-9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jutiviboonsuk A, Zhang H, Tan GT, et al. Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry. 2005;66:2745-2751. [DOI] [PubMed] [Google Scholar]
- 20. Puebla-Pérez AM, Huacuja-Ruiz L, Rodríguez-Orozco G. Cytotoxic and antitumour activity from Bursera fagaroides ethanol extract in mice with L5178Y lymphoma. Phyther Res. 1998;12:545-548. [Google Scholar]
- 21. Claudia Alina CM, Rocio RL, Marco Aurelio RM, et al. Chemical Composition and in vivo anti-inflammatory activity of Bursera morelensis Ramírez essential oil. J Essent Oil Bearing Plants. 2014;17:758-768. [Google Scholar]
- 22. Serrano Parrales R, Vázquez Cruz B, Segura Cobos D, Anaya Lang A, Jimenez-Estrada M, Canales Martínez M. Anti-inflammatory, analgesic and antioxidant properties of Bursera morelensis bark from San Rafael, Coxcatlán, Puebla (México): implications for cutaneous wound healing. J Med Plants Res. 2011;6:5609-5615. [Google Scholar]
- 23. Zuniga B, Guevara-Fefer P, Herrera J, et al. Chemical composition and anti-inflammatory activity of the volatile fractions from the bark of eight Mexican Bursera species. Planta Med. 2005;71:825-828. [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Aroche U, Salinas-Sanchez DO, Mendoza de, Gives P, et al. In vitro nematicidal effects of medicinal plants from the Sierra de Huautla, Biosphere Reserve, Morelos, Mexico against Haemonchus contortus infective larvae. J Helminthol. 2008;82:25-31. [DOI] [PubMed] [Google Scholar]
- 25. Rosas-Arreguin P, Arteaga-Nieto P, Reynoso-Orozco R, et al. Bursera fagaroides, effect of an ethanolic extract on ornithine decarboxylase (ODC) activity in vitro and on the growth of Entamoeba histolytica. Exp Parasitol. 2008;119:398-402. [DOI] [PubMed] [Google Scholar]
- 26. Yasunaka K, Abe F, Nagayama A, et al. Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. J Ethnopharmacol. 2005;97:293-299. [DOI] [PubMed] [Google Scholar]
- 27. Clifford MN, Knight S, Kuhnert N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS(n). J Agric Food Chem. 2005;53:3821-3832. [DOI] [PubMed] [Google Scholar]
- 28. Lucero-Gómez P, Mathe C, Vieillescazes C, Bucio L, Belio I, Vega R. Analysis of Mexican reference standards for Bursera spp. resins by gas chromatography–mass spectrometry and application to archaeological objects. J Archaeol Sci. 2014;41:679-690. [Google Scholar]
- 29. Maldini M, Montoro P, Piacente S, Pizza C. Phenolic compounds from Bursera simaruba Sarg. bark: phytochemical investigation and quantitative analysis by tandem mass spectrometry. Phytochemistry. 2009;70:641-649. [DOI] [PubMed] [Google Scholar]
- 30. Dominguez X, Rzedowki J, Gutierres M, Gomez M. A phytochemical survey of 21 species of the genus Bursera (Burseraceae) natives of Mexico. Rev Latinoam Quím. 1973;4:108-111. [Google Scholar]
- 31. Peraza-Sanchez SR, Pena-Rodriguez LM. Isolation of picropolygamain from the resin of Bursera simaruba. J Nat Prod. 1992;55:1768-1771. [DOI] [PubMed] [Google Scholar]
- 32. Becerra JX, Venable DL, Evans PH, Bowers WS. Interactions between chemical and mechanical defenses in the plant genus Bursera and their implications for herbivores. Am Zool. 2001;41:865-876. [Google Scholar]
- 33. Mc Vaugh R, Rzedowski J. Synopsis of the genus Bursera L. in western Mexico, with notes on the material of Bursera collected by Sessé & Mociño. Kew Bull. 1965;18:317-382. [Google Scholar]
- 34. Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol. 2011;133:945-972. [DOI] [PubMed] [Google Scholar]
- 35. Lautié E, Quintero R, Fliniaux MA, Villarreal ML. Selection methodology with scoring system: application to Mexican plants producing podophyllotoxin related lignans. J Ethnopharmacol. 2008;120:402-412. [DOI] [PubMed] [Google Scholar]
- 36. Gioti K, Tenta R. Bioactive natural products against prostate cancer : mechanism of action and autophagic/apoptotic molecular pathways. 2015;81:543-562. [DOI] [PubMed] [Google Scholar]
- 37. Kintzios SE. Terrestrial plant-derived anticancer agents and plant species used in anticancer research. CRC Crit Rev Plant Sci. 2006;25:79-113. [Google Scholar]
- 38. Adorisio S, Fierabracci A, Gigliarelli G, et al. The Hexane Fraction of Bursera microphylla a gray induces p21-mediated antiproliferative and proapoptotic effects in human cancer-derived cell lines. Integr Cancer Ther. 2017;16:426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Columba-Palomares MFMC, Villareal DM, Acevedo Quiroz MCME, Marquina Bahena MCS, Álvarez Berber DL, Rodríguez-López DV. Anti-inflammatory and cytotoxic activities of Bursera copallifera. Pharmacogn Mag. 2015;11(suppl 2):S322-S328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bianchi E, Sheth K, Cole JR. Antitumor agents from Bursera fagaroides (Burseraceae). (Beta-peltatin-A-methylether and 5’-desemethoxy-beta-peltatin-A-methylether). Tetrahedron Lett. 1969;(32):2759-2762. [DOI] [PubMed] [Google Scholar]
- 41. McDoniel PB, Cole JR. Antitumor activity of Bursera schlechtendalii (Burseraceae): isolation and structure determination of two new lignans. J Pharm Sci. 1972;61:1992-1994. [DOI] [PubMed] [Google Scholar]
- 42. Anaya-Ruiz M, Vallejo-Ruiz V, Flores-Mendoza L, Perez-Santos M. Female breast cancer incidence and mortality in Mexico, 2000-2010. Asian Pac J Cancer Prev. 2014;15:1477-1479. [DOI] [PubMed] [Google Scholar]
- 43. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 44. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52-62. [DOI] [PubMed] [Google Scholar]
- 45. Zhang JQ, Yao ZT, Liang GK, et al. Combination of lapatinib with chlorogenic acid inhibits breast cancer metastasis by suppressing macrophage M2 polarization [in Chinese]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44:493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tajik N, Tajik M, Mack I, Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr. 2017;56:2215-2244. [DOI] [PubMed] [Google Scholar]
- 47. Lei CS, Hou YC, Pai MH, Lin MT, Yeh SL. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018;51:105-113. [DOI] [PubMed] [Google Scholar]
- 48. Kim ME, Ha TK, Yoon JH, Lee JS. Myricetin induces cell death of human colon cancer cells. Anticancer Res. 2014;34:701-706. [PubMed] [Google Scholar]
- 49. Souza M, Machado MIL, Braz-Filho R. Six flavonoids from Bursera leptophloeos. Phytochemistry. 1989;28:2467-2470. [Google Scholar]
- 50. Romero-Estrada A, Maldonado-Magana A, Gonzalez-Christen J, et al. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement Altern Med. 2016;16:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xiao J. Dietary flavonoid aglycones and their glycosides: which show better biological significance? Crit Rev Food Sci Nutr. 2017;57:1874-1905. [DOI] [PubMed] [Google Scholar]
- 52. Messina F, Curini M, Di Sano C, et al. Diterpenoids and triterpenoids from the resin of Bursera microphylla and their cytotoxic activity. J Nat Prod. 2015;78:1184-1188. [DOI] [PubMed] [Google Scholar]
- 53. Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12:153-162. [DOI] [PubMed] [Google Scholar]
- 54. Cory G. Scratch-wound assay. In: Wells CM, Parsons M. eds. Cell Migration: Developmental Methods and Protocols. New York, NY: Springer; 2011:25-30. [DOI] [PubMed] [Google Scholar]
- 55. Sliva D, Mason R, Xiao H, English D. Enhancement of the migration of metastatic human breast cancer cells by phosphatidic acid. Biochem Biophys Res Commun. 2000;268:471-479. [DOI] [PubMed] [Google Scholar]