Abstract
Objective: This study sought to describe changes in the health-related quality of life (HRQOL) of women who do and do not seek naturopathic oncology (NO) complementary and alternative medicine (CAM) care during and immediately after breast cancer treatment, and to explore the predictive role of NO CAM care, demographic characteristics, and involvement in decision-making on HRQOL in breast cancer survivors. Methods: Matched cohorts of breast cancer survivors who did and did not choose to supplement their breast cancer treatment with NO care within 2 years of diagnosis participated. NO users were identified through naturopathic doctors’ clinics and usual care (UC) controls with similar prognosis were identified through a cancer registry. The registry provided information about all participants’ age, race, ethnicity, marital status, stage of cancer at time of diagnosis, date of diagnosis, and use of conventional medical treatments (surgery, chemotherapy, radiation, and endocrine therapy). Data of participants’ self-reported involvement in decision-making and HRQOL were collected at study enrollment and at 6-month follow-up. Results: At 6-month follow-up, the NO patients reported significantly more involvement in decision-making about care and better general health than did UC patients (P < .05). Self-reported involvement in decision-making about cancer treatment was associated with better role-physical, role-emotional, and social-functional well-being (P < .05). Race, age, marital status, and congruence of preferred and achieved levels of involvement also predicted aspects of HRQOL in breast cancer survivors (P < .05). Conclusions: Both NO CAM care and involvement in decision-making about cancer treatment may be associated with better HRQOL in breast cancer survivors.
Keywords: breast cancer, survivorship, CAM, integrative oncology, quality of life
Breast cancer treatment often involves making important decisions about surgery, chemotherapy, radiation, endocrine therapy, whether or not to use complementary and alternative medicine (CAM), and whether or not to seek a CAM provider. CAM care can include multiple supplements and recommendations for mind-body treatments including exercise and meditation. Some of these treatments are intended to reduce the side effects associated with conventional care and some are provided with the intent of complementing that care and further reducing risk of cancer recurrence.1 Some biologically based supplements should be evaluated in light of possible interactions with conventional treatments.2,3 It is estimated that between 50% and 80% of cancer patients supplement their care with CAM treatments, while only a small percentage use naturopathic oncology (NO) and receive care from naturopathic doctors (NDs) with special training in oncology. While there is scientific evidence for many of the CAM treatments patients use, and NO providers offer their patients, a review of the literature found no studies describing the health and health-related quality of life (HRQOL) effects of NO care as commonly practiced in communities. This study sought to fill that gap in knowledge.
However, in evaluating CAM care it is important to realize patients seeking NO care might differ from those who do not seek NO care in several ways including their desire to be involved in decision-making about their cancer treatment.4-8 Patient involvement in decisions about medical treatment has been found to predict a variety of short-term positive outcomes including reduced levels of decision-regret and better patient satisfaction with care.9,10 Involvement in decision-making about breast cancer treatment specifically and about breast cancer surgery has been found to be associated with lower levels of anxiety11 and increased short-term well-being.10,12 Some studies have even found self-reported involvement in decision-making predicts long-term outcomes including cancer survivor HRQOL10,13 even years after diagnosis.14,15
Although most studies of involvement in decision-making about cancer treatment have focused on perceived involvement in surgery choice among women with breast cancer,16 some have focused on decision-making about CAM, physician behavior, objective assessments of involvement,10 and congruence between patients’ preferred and perceived actual participation in medical decision-making.14-17 Effects on outcomes have so far been found to be most strongly associated with self-reported involvement and congruence.10
This article describes the results of a matched longitudinal study that recruited women seeking NO care for breast cancer and comparison cases recruited via a cancer registry. The purpose of the study is to describe the content and cost of NO care as provided in community clinics and to explore the effectiveness of NO in improving breast cancer patient HRQOL during early survivorship and any potential influence of involvement in decision-making on this effect.
Methods
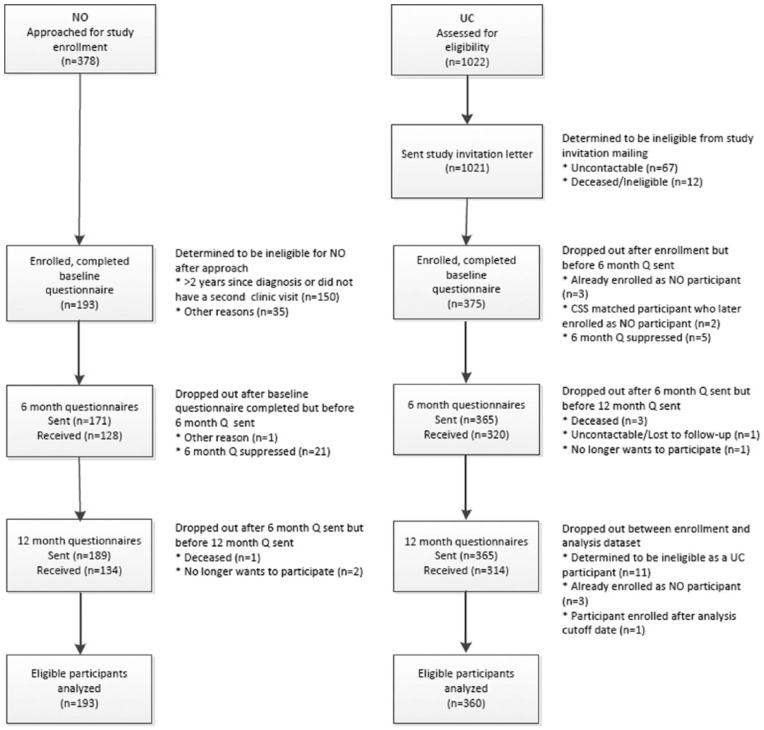
This study is registered with clinical trials as (NCT01366248). Study methods and questionnaires were approved by the institutional review boards of the Fred Hutchinson Cancer Research Center, Seattle, WA, and Bastyr University, Kenmore, WA. The women involved are part of a longitudinal assessment of 2 matched cohorts of women with breast cancer. Breast cancer patients in the NO cohort were eligible for the study if they spoke English fluently enough to complete surveys, were over age 21, and were diagnosed with breast cancer less than 2 years prior to their visit to a participating ND’s clinic. Usual care (UC) cohort members were selected from the registry based on their similarity to an enrolled NO clinic patient. Potential participants received a packet containing the informed consent form, a medical records release, and the enrollment questionnaire. Participants included a group of patients receiving treatment from local naturopathic physicians with specialty training in NO, and a larger group of women recruited from the local cancer registry that were matched on their similarity to the NO patients in demographic characteristics and stage of cancer at time of diagnosis. Analyses describing the similarities and differences between the 2 cohorts are described elsewhere.2,18 Women seeking NO care either consented for the study and completed the forms in the clinic or consented during a telephone call with the documents returned by mail. UC women identified through the cancer registry completed consent and recruitment documents by mail. Figure 1 describes recruitment and retention of all study participants.
Figure 1.
Participant flow through the protocol.
Reasons for ineligibility included in the “other” categories included a few women from both cohorts were found to be ineligible to participate in the study and these analyses after completing the enrollment paperwork because they were identified in the registry for a second, not their first, cancer diagnosis. UC women could also be disqualified for continued participation in the UC cohort if they visited an NO clinic. After completion of the baseline questionnaire 5 women originally identified as UC participants enrolled in the study at an ND clinic. Because these participants’ use of NO care disqualified them from the UC cohort, and made them eligible to participate in the NO cohort, they were dropped from the UC group and enrolled as members of the NO cohort. New UC comparison women were recruited from the registry to replace these women as comparisons for their original NO matches, and as matches for them as new NO cohort members. In total 553 women (193 in the NO cohort and 360 from the UC cohort) are included in these analyses.
Measures
In addition to the standard assessments of demographics, the enrollment questionnaire for the study also included items assessing use of a variety of CAM supplements and CAM activities, a 4-item measure of involvement in decision-making about cancer treatment used in several prior studies,14,15 and the Short Form-36 (SF-36), a commonly used measure of overall HRQOL.19 Participants also signed medical records releases allowing for collection and retention of information about participants’ diagnosis with and treatment for breast cancer from the cancer registry and from their personal medical records, which were abstracted by trained study personnel. Additional information about study procedures available in prior reports describing the cost and content of NO care provided to the NO cohort and reports of describing at-enrollment characteristics of our cohorts.3
Involvement in Decision-Making About Treatment
The involvement in decision-making scale used in this study has been previously used in several studies assessing the effects of involvement on HRQOL in breast cancer survivorship.14,15 After assuring patients that
every condition is different as is every patient. Some people prefer to make decision about their medical treatment themselves: other prefer to have their physician or someone else make decisions for them. We want to know how involved you feel you are or were in making some of the decisions about your care.
The measure included items assessing involvement in decision-making about surgery, chemotherapy and radiation, CAM, or “any other therapeutic activities” received, and about overall involvement in decision-making. For each item respondents were given response options, including 1 = “Not involved: others made decisions for you”; 2 = “A fair bit”; and 3 = “Very involved: I made all the decisions myself,” with an additional option “Not applicable: In my case there were few if any decisions to be made by me or my doctors.” An item asking about preferences regarding decision-making was also included. This item asked, “Would you have preferred to be more or less involved in making decisions about your treatment? Would you say:” For this item response options ranged from “Much less involved” to “Much more involved.”
Consistent with prior uses of these items, and consistent with the scales’ focus on patients’ personal feelings of involvement in decision-making, women indicating that a question (eg, CAM decision-making) was “Not Applicable” in their case as there were “no decisions to be made” were considered to have been “not at all involved” in that decision and given a score of 1 for that item.
Responses to the items were used to create 2 involvement in decision-making scales. Scale scores were calculated by summing the items to be included in each scale. In the creation of both scales study participants who did not respond to one or more questionnaire item did not have scale scores created due to missing data. The first included 4 items: overall involvement, involvement in surgery decision-making, involvement in chemotherapy/radiation decision-making, and involvement in CAM decision-making. Although prior research suggested high levels of correlation between the items suggesting that these items assess a single factor about patients and their desire and ability to be involved in treatment decision-making. A second scale was created that used only 3 of the items, dropping the involvement in CAM decision-making item. This second scale was used to examine the influence of the CAM decision-making item, as it could be particularly important in differences between the study cohorts in this study context.
Scores ranged from 4 to 12 (for the 4-item version) and from 3 to 9 (for the version excluding the CAM question). Cronbach’s αs for the 2 scales were acceptable at .72 for the full 4-item scale and at .81 for the 3-item scale that did not include decision-making about CAM. Suggesting that either scale assesses involvement as a characteristic and/or ability of the participant and that the situation-specific elements associated with each of the decisions assessed has at most modest influence on scale scores. Scale scores were also examined for correlation over time and this revealed the scale scores to be strongly correlated (r = .61) for the overall scale from enrollment to 6-month follow-up, while the version of the scale excluding the CAM item was also highly correlated (r = .59), suggesting good test-retest reliability over a period of 6 months for either scale.
Health-Related Quality of Life
The SF-36 is a widely used measure of functional status and overall HRQOL that is frequently used in intervention and longitudinal studies.19,20 The SF-36 measures quality of life across a broad range of general function levels and is sensitive to changes in life function common in both healthy and ill populations. The SF-36 is scored by calculating 8 subscales: functional status, role-physical function, role-emotional function, pain, general health, mental health, vitality, and social functioning.
Analysis Plan
After examining the demographics of the cohorts, their cancer treatment, use of CAM supplements, and the reliability of the involvement scales using standard methods including χ2 tests and t tests were used to compare the cohorts. Power analyses reveal that using χ2 test has, in general, the ability to detect at power of 95%, a difference of about 12 percentage points between the groups with a significance level of .05, and with continuous variables (eg, the SF-36 scales) a difference of approximately 4 points. Multivariate regression analyses examining the predictors of involvement in decision-making and of HRQOL at enrollment and follow-up were planned. In preparation for multivariate analyses minor simplifications of race, marital status, stage, surgery, chemotherapy, radiation, and endocrine therapy variables were made. Race was reduced to White/non-White, marital status to married or partnered/single or divorced, and stage to 0-2/3-4. Surgery was simplified to none/lumpectomy including breast conserving procedures, re-excision, excision, lumpectomy, and segmental mastectomy, and mastectomy and bilateral mastectomy. Individuals coded as “unknown” for chemotherapy, radiation, and adjuvant endocrine therapy (AET) were recoded as “No.” These analyses were all conducted using R (v 3.1.1), and differences were considered statistically significant at the P < .05 level.
Results
Description of the Cohorts and the Effectiveness of Matching
Overall the study participants were typical of breast cancer patients in the greater metropolitan area, in that they were predominantly non-Hispanic White and averaged 54 years of age. See Table 1 for additional information. Matching does appear to have worked in that no statistically significant differences between the cohorts were found for any of the variables used for recruitment and matching of the UC cohort. Additional information on the similarity of the patients in both cohorts available Table 1.
Table 1.
Characteristics of the Study Cohorts.
| NO Clinic Cohort | UC Cohort | |
|---|---|---|
| Age (years) | 53.3 (SD = 11.19) | 54.8 (SD = 10.30) |
| Race | ||
| Percentage White | 183 (94.8%) | 343 (95.3%) |
| Ethnicity: Hispanic | 2 (1.0%) | 6 (1.7%) |
| Marital status | ||
| Married | 121 (64.7%) | 268 (75.7%) |
| Domestic partner | 14 (7.5%) | 24 (6.8%) |
| Divorced | 30 (16.0%) | 30 (8.5%) |
| Single | 19 (10.2%) | 27 (7.6%) |
| Stage | ||
| 0 | 20 (10.4%) | 30 (8.3%) |
| I | 62 (32.1%) | 142 (39.4%) |
| II | 77 (39.9%) | 128 (35.6%) |
| III | 26 (13.5%) | 48 (13.3%) |
| IV | 7 (3.6%) | 7 (1.9%) |
| Unknown | 1 (0.5%) | 5 (1.4%) |
| Time since diagnosis at study enrollment in years | Median 0.31 years | Median 0.81 years |
| Treatment | ||
| Surgery | ||
| None | 6 (3.1%) | 7 (1.9%) |
| Re-excision | 57 (29.5%) | 88 (24.4%) |
| Lumpectomy | 53 (27.5%) | 96 (26.7%) |
| Mastectomy | 56 (29.0%) | 119 (33.1%) |
| Bilateral mastectomy | 21 (10.9%) | 50 (13.9%) |
| Chemotherapy | 106 (54.9%) | 175 (48.6%) |
| Radiation | 118 (61.1%) | 203 (56.4%) |
| Endocrine therapy | 108 (56.0%) | 239 (66.4%) |
Abbreviations: NO, naturopathic oncology; UC, usual care.
The 2 cohorts did however differ, as was expected based on the recruitment strategy in time since diagnosis at study enrollment. Women seeking ND clinic care often sought care shortly after diagnosis and were, at median, enrolled 0.31 years (about 4 months) postdiagnosis. In contrast, the cancer registry (CSS) identification and mail-based recruitment resulted in a UC cohort that was at median 0.81 years, (9.7 months) postdiagnosis at time of study enrollment. The averages for the cohorts are presented in Table 1 and were found to be statistically significant at the P < .05 level, and thus time since diagnosis was used as an adjustment variable in all multivariate analyses.
After review of the study matching criteria, we examined the 2 cohorts seeking to identify any differences between them prognostic of HRQOL in survivorship. These analyses including review of CSS collected breast cancer treatment data found the 2 cohorts to be similar overall in their use of breast cancer surgeries, chemotherapy, and radiation. A trend toward a difference between the cohorts was found however in use of AET (χ2 = 5.925; P < .052). Women in the NO cohort appear less likely to use AET (56.0% vs 66.4%) than those in the UC cohort. When women with unknown data were assumed to be nonusers, the direction of the relationship was unchanged and was found to be statistically significant (χ2 = 5.8477; P < .02).
Use of CAM and Involvement in Decision-Making and Use of CAM Supplements
As study enrollment for the NO cohort occurred at the initial ND clinic visit, prior to the establishment of an ND patient-physician partnership, self-reports of CAM supplement and activity use and decision-making about CAM at study baseline would generally describe decisions patients made about CAM independent of any specialist CAM provider. These would be decisions made in consultation with friends, family, chiropractors, and conventional physicians including oncologists or primary care providers. Many conventional providers do recommend some CAM supplements and CAM activities for breast cancer patients including use of vitamin D or may have suggested common CAM treatments for nausea and lack of appetite during treatment including ginger and/or other herbal supplements. Review of patient self-reports of these activities showed substantial use consistent with other reports, but few statistically significant differences in the frequency of use of CAM supplements between our cohorts at study enrollment (data not shown).
Participants generally reported being very involved in making decisions about their care overall, about surgery, chemotherapy, and radiation (see Table 2). A majority of women responded that they were “very involved” in decision-making on each of these items. Most women also reported being very involved in making decisions about any CAM treatments they had used or were using, although more women reported decision-making about CAM to be “not applicable” in their particular case than did so for questions about conventional therapies. Approximately 85% of our participants reported that their level of participation in decision-making about cancer treatment had been “about right for them.”
Table 2.
Self-Reported Involvement in Decision-Making About Cancer Treatment.
| Item | Not Involved | Some Involvement | Very Involved | N/A | |
|---|---|---|---|---|---|
| Overall decisions | 11 (2.6%) | 209 (25.3%) | 304 (70.7%) | 6 (1.4%) | |
| Surgery decisions | 30 (7.1%) | 99 (23.3%) | 296 (69.6%) | 0 (0.0%) | |
| Chemotherapy/radiation decisions | 39 (9.4%) | 107 (25.7%) | 226 (54.2%) | 45 (10.8%) | |
| CAM decisions | 0 (0%) | 39 (10.8%) | 162 (45.0%) | 159 (44.2%) | |
| Much Less | Less | About Right | Some More | Much More | |
| Congruence of preference for involvement with level of involvement achieved | 1 (0.2%) | 6 (1.4%) | 361 (85.3%) | 43 (10.2%) | 12 (2.8%) |
Abbreviation: CAM, complementary and alternative medicine.
Involvement in decision-making does appear to be different between the study cohorts. At study enrollment prior to NO care, women about to be enrolled in the NO cohort reported higher levels of involvement in cancer treatment decision-making than those in the UC cohort on the scale assessing involvement in decision-making about treatment that included decision-making about CAM (10.5 vs 9.1; P < .01). Differences, similar but smaller and not statistically significant, were found using the second version of the scale that excluded the CAM question (means 7.8 vs 7.5). Differences between the cohorts were statistically significant on both versions of the scale at the 6-month follow-up assessment with the members of the NO cohort again reporting greater involvement in decision-making about their cancer treatment (P < .05 for each).
Replication of the t tests exploring differences between cohorts using the simplified versions of predictors selected for use in multivariate analyses again revealed no statistically significant differences by cohort for any of these patient characteristics other than marital status and AET. When self-reported marital status was reduced to either “single” or “partnered.” With the “single” category including those who reporting either being “single” divorced, and the “partnered” category including both those who were married and those reporting a domestic partnership, women in the NO cohort were more likely to report being “single” than were those in the UC cohort. When women for whom CSS recorded “unknown” regarding their AET use were considered nonusers, the apparent difference between the cohorts was statistically significant (data not shown).
Predictors of Involvement in Decision-Making
Multivariate regression analyses examining demographic and disease-related predictors of self-reported involvement in decision-making about cancer treatment at study enrollment included cohort, time since diagnosis, race, ethnicity, marital status, age, and stage. These analyses revealed cohort and race to be strong statistically significant predictors of involvement using either scale. Stage also predicted the 3-item involvement measure that excluded the involvement in CAM decision-making question (data not shown).
Predictors of Quality of Life
Analyses examining the HRQOL of patients in the cohorts at time of enrollment revealed that women in the NO cohort reported reduced levels of HRQOL at study enrollment in spite of their similarity in stage, demographics, and the similarity of their recorded treatment. In unadjusted Welch 2-sample t test comparisons, women in the NO cohort reported poorer HRQOL on 4 of the 8 SF-36 scales, including Role Physical function, Social functioning, Role emotional functioning, and Mental health (P < .05 in all cases). This might well be associated with the differences between the cohorts in their time since diagnosis as a greater proportion of the NO women were still undergoing primary chemotherapeutic treatment.
When multivariate analyses were run to examine the effects of controlling for study matching variables (age, race, ethnicity, marital status, and stage), time since diagnosis at study enrollment, and treatment (type of surgery received, use of chemotherapy, use of radiation), these models revealed age, stage, marital status, time since diagnosis, and use of chemotherapy to be predictors of several of the SF-36 HRQOL scales at time of enrollment. The inclusion of these additional predictors reduced the estimated β coefficient for the cohort effect to nonsignificance in all models where it had been significant in unadjusted tests. In the adjusted models cohort was a statistically significant predictor of physical functioning after adjustment. See Table 3.
Table 3.
Predictors of SF-36 Scale Scores at Study Enrollment.
| Step 1 | Physical Functioning, β | Role-Physical, β | Bodily Pain, β | General Health, β | Vitality, β | Social Functioning, β | Role-Emotional, β | Mental Health, β |
|---|---|---|---|---|---|---|---|---|
| Cohort: NO | Ref | |||||||
| Cohort UC | −4.39* | |||||||
| Age | 0.25* | 0.19* | 0.30** | 0.47*** | 0.64*** | 0.47*** | ||
| Time since DX | 3.65* | 17.21*** | 3.52* | 3.22* | 6.82*** | 6.10* | 6.82*** | |
| Race: non-White | Ref | |||||||
| Race | ||||||||
| Ethnicity: non-Hispanic | Ref | |||||||
| Ethnicity: Hispanic | ||||||||
| Stage: early | Ref | |||||||
| Stage: late | −6.31* | |||||||
| Stage: unknown | 35.89* | |||||||
| Marital status: partnered | Ref | |||||||
| Marital status: single | −10.62** | −11.64*** | −11.33* | −7.41** | ||||
| Chemotherapy | −9.87* | −4.66* | ||||||
| Radiation | ||||||||
| Surgery: BCS | Ref | |||||||
| Mastectomy | ||||||||
| None | ||||||||
| Model R2 | 0.08 | 0.09 | 0.06 | 0.05 | 0.05 | 0.10 | 0.08 | 0.08 |
| Model F | 3.92*** | 4.52*** | 2.56** | 2.33*** | 2.35*** | 5.04*** | 3.15*** | 5.04*** |
Abbreviations: SF-36, Short-Form-36; NO, naturopathic oncology; UC, usual care; DX, diagnosis; BCS, breast cancer surgery.
P < .05. **P < .01. ***P < .001.
At 6-month follow-up women’s self-reported HRQOL was improved on almost all scales in both cohorts as would be expected in early posttreatment survivorship although changes were small in some cases for the UC cohort. Differences between the cohorts were not now statistically significantly different in unadjusted tests, with the exception of the General Health scale where the difference between the scores was slightly more than 4 points (NO mean 76.5 vs UC mean 72.1) and was statistically significant (t[258 df] = 2.30; P < .05). See Table 4.
Table 4.
Differences in SF-36 Scale Scores Based on Cohort at Study Enrollment.
| NO Cohort | UC Cohort | Welsh 2-Sample t Test | |
|---|---|---|---|
| Physical function | 79.2 | 78.1 | NS |
| Role physical | 43.1 | 53.5 | t(388 df) = −2.64; P < .05 |
| Pain | 69.6 | 69.7 | NS |
| General health | 72.8 | 71.8 | NS |
| Vitality | 49.5 | 49.8 | NS |
| Social function | 63.9 | 71.9 | t(424 df) = −3.61; P < .05 |
| Role emotional | 59.8 | 71.7 | t(348 df) = −310; P < .05 |
| Mental health | 69.8 | 73.9 | t(359 df) = −2.72; P < .05 |
Abbreviations: SF-36, Short-Form-36; NO, naturopathic oncology; UC, usual care; NS, not significant; df, degree of freedom.
Multiple regression analyses examined change in SF-36 scale scores to 6-month follow-up (Table 5) and included baseline scores as a predictor. Additional predictors examined included the following: study enrollment matching variables, time since diagnosis at enrollment, treatment received (surgery type, use of chemotherapy, and use of radiation), and involvement in decision-making (with each of the 2 involvement scales, and congruence of involvement with preferences run as a separate models). These analyses revealed cohort to be a statistically significant predictor of change in the General Health subscale of the SF-36 such that women in the NO cohort reported larger improvements over the 6-month interval immediately following the initiation of NO treatment (P < .05; in all versions of analyses). This difference appears to be in the range of 4 additional points on the general health scale.
Table 5.
Predictors of 6-Month SF-36 Scale Scores.
| Step 1 | Physical Functioning, β | Role-Physical, β | Bodily Pain, β | General Health, β | Vitality, β | Social Functioning, β | Role-Emotional, β | Mental Health, β |
|---|---|---|---|---|---|---|---|---|
| Level of measure at enrollment | 0.56*** | 0.38*** | 0.49*** | 0.69*** | 0.64*** | 0.57*** | 0.35*** | 0.69*** |
| Cohort: NO | Ref | |||||||
| Cohort UC | −3.53* | |||||||
| Age | −0.19* | |||||||
| Time since DX | −3.75* | |||||||
| Race: non-White | Ref | |||||||
| Race | 10.79* | 19.88* | ||||||
| Ethnicity: non-Hispanic | Ref | |||||||
| Ethnicity: Hispanic | ||||||||
| Stage: early | Ref | |||||||
| Stage: late | ||||||||
| Stage: unknown | ||||||||
| Marital status: partnered | Ref | |||||||
| Marital status: single | −6.10** | |||||||
| Chemotherapy | ||||||||
| Radiation | ||||||||
| Surgery: BCS | Ref | |||||||
| Mastectomy | ||||||||
| None | ||||||||
| Involvement in decision-making | 1.92* | 1.02* | 2.32** | |||||
| Model R2 | 0.41 | 0.21 | 0.29 | 0.54 | 0.42 | 0.38 | 0.21 | 0.46 |
| Model F | 18.51*** | 6.90*** | 10.72*** | 31.89*** | 19.43*** | 16.39*** | 7.15*** | 22.36*** |
Abbreviations: SF-36, Short-Form-36; NO, naturopathic oncology; UC, usual care; DX, diagnosis; BCS, breast cancer surgery.
P < .05. **P < .01. ***P < .001.
Involvement in decision-making, as assessed using the 4-item scale, was also associated with improved HRQOL on the role-physical, social function, and role-emotional function subscales (P < .05). The 3-item version of the involvement scale, excluding the CAM question, also predicted social function and role-emotional function (P < .05 in cases) but could only be described as a “trend” for the role-physical function scale (P = .07). Congruence of level of involvement with preferences predicted physical function, general health, mental health, social function, and pain (P < .05). Other predictors of SF-36 HRQOL scales at 6-month follow-up included age, race, marital status, stage, and time since diagnosis at enrollment.
Discussion
This study, which may be the first to report on the effects of specialist CAM oncology care by NDs at community clinics, found evidence to suggest that patients who seek NO CAM care experience improved HRQOL at 6-month follow-up associated with that care. The difference found was modest, but at 4 points on the SF-36 general health scale could be considered clinically significant.21 Although this was not a randomized controlled trial as our NO seeking breast cancer patient cohort, and the UC cohort patients we enrolled as a matched comparison group, were found to have similar demographic and diagnostic characteristics and received comparable primary course of conventional care, it appears the improvement in self-assessed general health reported by NO women may be attributable to the NO CAM care they received from their ND clinic providers. How exactly specialist naturopathic oncology care improves self-assessed general health status could not be assessed in this study given its whole systems approach consistent with some recent calls to use health services research methods to better understand CAM care in real-world settings.22 Future studies exploring both the effects of NO care as a package of services in this or other settings23 would help confirm our findings as would explorations of the specific supplements, recommendations, and/or CAM activities that might contribute to the overall effect of NO care.2
We also found that women who are involved in making decisions about their cancer care also appear to report improved HRQOL including better role-physical, role-emotional, and social functioning 6 months later. This is consistent with prior studies that have found similar effects 5 to 30 years later.2,14,15 We also found women in NO care reported higher levels of involvement in decision-making about their care both at study enrollment and 6-months later, suggesting that NO patient may have benefited from higher levels of involvement. However, the HRQOL subscales on which the improvement associated with involvement was found were not the general health scale, suggesting that patient involvement in decision-making and desire to be involved in treatment decision-making were not confounding predictors of the improvements in HRQOL found associated with NO clinic treatment. For self-reported congruence of involvement with preferences, women who indicated their level of involvement was “right for them” at study enrollment also predicted better HRQOL 6 months later, and while this included the general health subscale the inclusion of congruence did not reduce the significance of the NO cohort effect on HRQOL.
Limitations
Time since diagnosis served in this study as, perhaps, a proxy for current treatment and may present a design explanation for the substantial improvements in role-physical, social, and emotional functioning and in mental health NO care patients report in the 6 months following the initiation of NO care that for some women was during active primary treatment. This would suggest that much of the return to usual functioning that we see here in the NO group might have occurred in the absence of specific NO assistance. That said, the self-reported improved general health status found at 6-month follow-up occurred when few patients were receiving treatments other than AET. Differences in the general health scale at enrollment were also particularly modest, limiting the degree to which these results are amenable to any regression to the mean explanation. They appear to reflect a genuine, if modest, advantage associated with receiving NO care. We did not control for AET use in our multivariate analyses as it would not be clear if all participating women for whom we have evidence of AET use would in fact have been using AET long enough to experience arthralgia and mood-related side effects at the time of our 6-month follow-up. In addition, AET use is a late element of primary treatment that might be influenced by ND providers’ recommendations for or against use. It was, thus, unclear if adjusting for this variable would, in principle, adjust out a potential means by which NO care influences HRQOL among those who use it.
Differences in perceived involvement in decision-making likely reflect women’s circumstances, personal internal control preferences, and biases in perception that influenced both the degree to which they actively sought to be involved and the degree to which they were inclined to indicate that they were “Very involved: I made all the decisions myself” as contrasted with “a fair bit.” Again, this is consistent with the small body of research on involvement in treatment decision-making that has assessed health outcomes, all of which have found perceived involvement in decision-making, not objectively assessed involvement, or physician perceptions of involvement to be the significant predictor of outcome.10 Still the differences of a few points on this measure would be associated with clinically significant differences on multiple self-assessed HRQOL scales suggests the potentially profound importance of perceived involvement, whether this is a preexisting characteristic of women or something physicians can encourage and promote. Analyses examining congruence between perceived and preferred levels of involvement in care were also significant. Thus, congruence in involvement letting patients be involved to the degree they desire may improve HRQOL. In reflecting on the congruence findings it is important to note that very few women reported being more involved than they wished to be and inability to be as involved as they would have preferred was far more common, suggesting again that higher levels of involvement are protective of long-term HRQOL.10
Finally, women in this study were predominantly white, well-educated, and married. Replication of this work including women in other parts of the country and those receiving other forms of CAM specialist care would be valuable in describing the generalizability of these findings.
Conclusions
NO care may help women with breast cancer improve their HRQOL in early survivorship. More research is needed to understand how ND’s care improves patient well-being, both through the provision of specific treatments and through provision of care and support more generally. Involvement in decision-making about cancer treatment also improves HRQOL in early survivorship, and the effects of involvement appear to be independent of use of NO care. This finding adds to the modest body of research documenting positive health and psychosocial outcomes for patients who are involved in decision-making about their cancer treatment, and for those who are able to participate consistent with their preferences. NDs providing CAM services may assist their patients through their ability to provide additional CAM care options where women have greater leeway to be involved in treatment decision-making then may be the case in conventional care offices where there are often a standard or guideline-based treatment that is strongly encouraged to assure the best chance for important clinical outcomes. NDs caring for breast cancer patients clearly have many possible means by which they may improve patient well-being during treatment by supporting patients’ efforts to be informed decision-makers in their own care, but this alone does not explain their value. The degree to which individual CAM activities or supplements contribute to the overall benefits found here associated with NO care should be a topic of additional research.
Acknowledgments
The authors would also like to thank Dr Chad Aschtgen, Dr Laura James, Dr Janile Martin, Dr Eleanora Naydis, and Dr Paul Reilly for their efforts recruiting study participants.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Center for Complementary and Integrative Health (RO1 AT 5873, T32 AT815).
References
- 1. Deng G, Cassileth B. Integrative oncology: an overview. Am Soc Clin Oncol Educ Book. 2014:233-242. [DOI] [PubMed] [Google Scholar]
- 2. Andersen MR, Afden K, Hager S, Gaul M, Sweet E, Standish LJ. (2015) The “cause’ of my cancer, beliefs about cause among breast cancer patients and survivors who do and do not seek IO care. Psycho-Oncology, Do women with breast cancer who choose adjunctive naturopathic oncology care receive different standard oncology treatment? doi: 10.1002/pon.4028. [DOI] [PubMed] [Google Scholar]
- 3. Sweet E, Dowd F, Zhou M, Standish LJ, Andersen MR. The use of complementary and alternative medicine supplements of potential concern during breast cancer chemotherapy. Evid Based Complement Alternat Med. 2016;2016:4382687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boon H, Brown JB, Gavin A, Kennard MA, Stewart M. Breast cancer survivors’ perceptions of complementary/alternative medicine (CAM): making the decision to use or not to use. Qual Health Res. 1999;9:639-653. [DOI] [PubMed] [Google Scholar]
- 5. Vincent C, Furnham A. Why do patients turn to complementary medicine? An empirical study. Br J Clin Psychol. 1996;35(pt 1):37-48. [DOI] [PubMed] [Google Scholar]
- 6. Montbriand MJ, Laing GP. Alternative health care as a control strategy. J Adv Nurs. 1991;16:325-332. [DOI] [PubMed] [Google Scholar]
- 7. Boon H, Brown JB, Gavin A, Westlake K. Men with prostate cancer: making decisions about complementary/alternative medicine. Med Decis Making. 2003;23:471-479. [DOI] [PubMed] [Google Scholar]
- 8. Boon H, Westlake K, Deber R, Moineddin R. Problem-solving and decision-making preferences: no difference between complementary and alternative medicine users and non-users. Complement Ther Med. 2005;13:213-216. [DOI] [PubMed] [Google Scholar]
- 9. Zdenkowski N, Butow P, Tesson S, Boyle F. A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast. 2016;26:31-45. [DOI] [PubMed] [Google Scholar]
- 10. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35:114-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris J, Royle GT. Offering patients a choice of surgery for early breast cancer: a reduction in anxiety and depression in patients and their husbands. Soc Sci Med. 1988;26:583-585. [DOI] [PubMed] [Google Scholar]
- 12. Ashcroft JJ, Leinster SJ, Slade PD. Breast cancer—patient choice of treatment: preliminary communication. J R Soc Med. 1985;78:43-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15:9-19. [DOI] [PubMed] [Google Scholar]
- 14. Andersen MR, Bowen DJ, Morea J, Stein KD, Baker F. Involvement in decision-making and breast cancer survivor quality of life. Health Psychol. 2009;28:29-37. [DOI] [PubMed] [Google Scholar]
- 15. Andersen MR, Sweet E, Lowe KA, Standish LJ, Drescher CW, Goff BA. Involvement in decision-making about treatment and ovarian cancer survivor quality of life. Gynecol Oncol. 2012;124:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keating NL, Guadagnoli E, Landrum MB, Borbas C, Weeks JC. Treatment decision making in early-stage breast cancer: should surgeons match patients’ desired level of involvement? J Clin Oncol. 2002;20:1473-1479. [DOI] [PubMed] [Google Scholar]
- 17. Brom L, Hopmans W, Pasman HR, Timmermans DR, Widdershoven GA, Onwuteaka-Philipsen BD. Congruence between patients’ preferred and perceived participation in medical decision-making: a review of the literature. BMC Med Inform Decis Mak. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Standish, et al. Do women with breast cancer who choose adjunctive naturopathic oncology care receive different standard oncology treatment? In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ware JE, Jr, Phillips J, Yody BB, Adamczyk J. Assessment tools: functional health status and patient satisfaction. Am J Med Qual. 1996;11:S50-S53. [PubMed] [Google Scholar]
- 20. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217-227. [DOI] [PubMed] [Google Scholar]
- 21. Ward MM, Guthrie LC, Alba MI. Clinically important changes in Short Form-36 scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res (Hoboken). 2014;66:1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wardle J, Oberg EB. The intersecting paradigms of naturopathic medicine and public health: opportunities for naturopathic medicine. J Altern Complement Med. 2011;17:1079-1084. [DOI] [PubMed] [Google Scholar]
- 23. Ritenbaugh C, Verhoef M, Fleishman S, Boon H, Leis A. Whole systems research: a discipline for studying complementary and alternative medicine. Altern Ther Health Med. 2003;9:32-36. [PubMed] [Google Scholar]