Abstract
Cachexia has been recognized for a long time as an adverse effect of cancer. It is associated with reduced physical function, reduced tolerance to anticancer therapy, and reduced survival. This wasting syndrome is mainly known for an ongoing loss of skeletal muscle leading to progressive functional impairment and is driven by a variable combination of reduced food intake and abnormal metabolism. Cytokines derived from host immune system or the tumor itself is believed to play a role in promoting cancer cachexia. Circulating levels of cytokines, including IL-1α, IL-6, and TNFα have been identified in cancer patients but they probably only represent a small part of a changed and abnormal metabolism. Murine models have shown that browning of white adipose tissue (WAT) takes place early in the progression of cancer cachexia. Thus, browning of white adipose tissue is believed to be a strong contributor to the increased energy expenditure common in cachectic patients. Despite the severe implications of cancer cachexia for the patients and extensive research efforts, a more coherent and mechanistic explanation of the syndrome is lacking, and for many clinicians, cancer cachexia is still a vague concept. From a lung cancer perspective this commentary reviews the current knowledge on cancer cachexia mechanisms and identifies specific ways of clinical management regarding food intake, systemic inflammation, and muscular dysfunction. Much of what we know comes from preclinical studies. More translational research is needed for a future cancer cachexia screening tool to guide clinicians, and here possible variables for a cancer cachexia screening tool are considered.
Keywords: cancer cachexia, lung cancer, molecular, clinical management, mechanisms, weight loss, inflammation, cytokines, browning of adipose tissue, screening tool
Introduction
Involuntary weight loss is abnormal in assumed healthy individuals, and should make everyone alert, as it may be a symptom of cancer. Yet, this involuntary weight loss may reach significant magnitude before action is taken and the proper diagnosis is found. Once a person is diagnosed with cancer, body weight should routinely be monitored throughout the treatment trajectory. The incidence of weight loss at the time of diagnosis and through the disease trajectory varies greatly according to the tumor type.1 A study of Dewys and colleagues from 1980 reported the frequency of weight loss in patients with newly diagnosed non–small cell lung cancer (NSCLC) to be 61%. A more recent study from 2004 reported the same incidence of weight loss for lung cancer patients on diagnosis. Both studies demonstrated convincingly that weight loss was an independent negative prognostic factor for survival of patients with NSCLC, SCLC, and mesothelioma.2
For many clinicians, weight loss is intertwined with cancer cachexia. An observed weight loss or clear loss of muscle mass can be the visible part of cachexia in many patients (Figure 1). However, as this review will discuss, large and as yet poorly understood metabolic alterations may underlie this syndrome and precede a later weight loss. Cachexia has been recognized for a long time as an adverse effect of cancer, and is associated with reduced physical function, reduced tolerance to anticancer therapy,3,4 and reduced survival.5,6 It is described as
a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. Its pathophysiology is characterized by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism.7
Figure 1.

Warning symptoms: When a cancer patient presents with these symptoms consider cancer cachexia.
Patients who have more than 5% loss of stable body weight over the past 6 months, or a body mass index (BMI) less than 20 kg/m2 and ongoing weight loss of more than 2%, or sarcopenia and ongoing weight loss of more than 2%, but have not entered the refractory stage, are classified as having cachexia.
This consensus definition of cancer cachexia has been adapted by the scientific community, but despite this a more coherent and mechanistic explanation is lacking, which for many clinicians means that there is still no good understanding of the syndrome. To this end, there is a need for predictive factors, as well as a validated and accepted screening tool that can guide clinicians in the decision making regarding palliative chemotherapy and supportive care initiatives.
In the following, current knowledge on cancer cachexia mechanisms and clinical management of the syndrome will be reviewed. The underlying mechanisms leading to the clinical syndrome known as cachexia are not yet fully understood, but here 4 areas are highlighted where mechanistic evidence is accumulating. These include food intake and neuronal input, systemic inflammation, muscular effects, and browning of white adipose tissue.
Mechanisms of Cancer Cachexia
Food Intake and Neuronal Input
Generally, body weight and metabolism are controlled in the brain. Here, afferent signals from several organs, including the gastrointestinal tract and the adipose tissues, are integrated and distributed to other parts of the central nervous system. Two specific populations of neurons in the hypothalamus are generally responsible for the integration, the orexigenic pathway (promoting food intake and reducing energy loss) and the anorexigenic pathway (inhibiting food intake and increasing the use of energy).8
Though anorexia is a common symptom in advanced cancer and the clinical implications of disturbed appetite regulation are clear, the underlying mechanisms of appetite regulation in advanced stage cancer are to be revealed. One possible hypothesis is that pro-inflammatory signaling from peripheral tissues, including the tumor, increases the activity of pro-opiomelacortin neurons in the hypothalamus resulting in anorexia and reduced food intake.9 The neuronal input may be responsible for what is considered to be the primary part of cancer cachexia. This primary part is characterized by abnormal metabolism due to intrinsic neuronal and inflammatory inputs.
Cancer cachexia may also have a second part. Here, complicating symptoms arising from the cancer itself or the treatment given may worsen the condition further. These are called secondary nutrition impact symptoms and can be, for example, pain, dysphagia, or nausea. These symptoms can result in reduced food intake and only exacerbates the weight loss in addition to the changed metabolism. It is assumed that the combination of these metabolic and dietary factors results in the syndrome recognized clinically as cancer cachexia.10 Despite the major impact on food intake in the primary and secondary part of the syndrome, treating cachexia through nutritional intervention alone does not reverse cancer cachexia, indicating that a decreased food intake is not the primary cause of the disease.11
Systemic Inflammation
It is widely accepted that systemic inflammation is a unifying hallmark of the cluster of behaviors associated with injury, infection, and cancer; and that chronic systemic inflammation can drive adipose tissue lipolysis and muscle proteolysis.12 In cancer, inflammation has been described as a double-edged sword. The natural role of the immune system is to fight off infection and transformed cells, thereby not only controlling infections but also controlling tumor growth. However, cancer cells may also hijack the immune system to produce specific cytokines promoting tumor growth, survival, and progression.13 Thus, a prompt pro-inflammatory response is essential to clear infections and nascent tumors, yet equally essential is the ability to resolve the inflammatory response through a later anti-inflammatory phase of the response. The understanding of the balance between the inflammatory mediators in cancer cachexia is emerging.14
Tumor necrosis factor α (TNFα), also named cachectin, is probably the most characterized cytokine in cachexia. It has been demonstrated to promote anorexia and skeletal muscle wasting, mainly through the nuclear factor-κB (NF-κB) pathway. The base of evidence is founded on both comprehensive human integrative physiology studies, as well as animal and in vitro studies. Moreover, systemic TNFα levels have been demonstrated to be upregulated in cachexic cancer patients, but direct proof of its mediating role in driving cachexia in cancer patients is still lacking. To this end, using neutralizing antibodies against TNFα showed no benefit in a trial in non–small cell lung cancer patients, suggesting that targeting TNFα alone is not sufficient to prevent cachexia.15,16 This may suggest that tumors or host tissue secrete more than one cachectic factor, and that targeting one of these factors will not be sufficient to prevent the syndrome.
The additional inflammatory cytokines may include interleukin (IL)-1α and IL-6. Like TNFα, IL-1α is also known to promote anorexia, probably through similar modes of action. Yet using an IL-1 receptor antagonist was not sufficient to impair cachexia progression, as demonstrated in a rat model.17 In parallel, numerous studies have investigated the correlation between IL-6 and cachexia development.18,19 In a murine model, where intestinal polyps were induced and muscle mass, lipid tissue, and circulating levels of IL-6 were measured, it was demonstrated that high levels of systemic IL-6 were related to tumor growth and loss of muscle mass, and thereby essential in the development of cachexia.20 In one study of patients with advanced-stage cancer at different sites, circulating levels of these cytokines (IL-1α, IL-6, and TNFα) were found to be increased. Together with the findings in the aforementioned murine studies, this could support the assumption of a robust system of cytokines collectively promoting cachexia.21 In addition, circulating IL-6 levels in prostate cancer patients have been shown to correlate with cachexia development and poor prognosis.22 Altogether these cytokines most likely all play a role, but in promoting cachexia they are probably only branches in a greater network.
To this end, host genotype and immune response may influence the heterogeneity seen in the presentation of cachexia. Even with the same tumor type and burden, one individual may become cachectic, whereas another will not.
Muscular Effects
The major clinical symptom of cachexia is muscle wasting. To maintain muscle homeostasis, a tight balance between protein synthesis and degradation is required. Thus, a decrease in synthesis or an excessive degradation will result in muscle wasting. During tumor progression the network of anabolic and catabolic factors that normally regulate this tight balance is believed to be severely disrupted.23,24
At the cellular level, three main pathways have been described in skeletal muscle to account for protein degradation. These are
Ubiquitin-mediated proteasome degradation (UPR); part of a comprehensive system regulating cellular processes and homeostasis within the muscles being upregulated by skeletal muscles during cachexia.
Autophagy; believed to be a main promotor of skeletal muscle proteolysis and to be upregulated in cancer cachexia, where increased levels of autophagy mediator BCL-interacting protein 3 (BNIP3) messenger RNA were found in a small cohort of lung cancer patients.25
Calcium-activated protease calpains; a family of Ca2+-dependent cysteine proteases, proposed to initiate a degradative process during cachexia, although knowledge on these is still limited.26
That induction of the aforementioned pathways eventually leads to cachexia has been reported using muscle biopsies from newly diagnosed gastric cancer patients.27 The mRNA levels of markers associated with weight loss correlated with tumor stage but not with pre-illness weight loss, indicating that tumor burden directly initiates early phases of muscle degradation, which is present before muscle wasting is detectable.
In a rat model of cachexia, induced by the Yoshida ascites hepatoma, UPR upregulation was evident following tumor growth, as seen by Atrogin-1 mRNA expression and increased protein ubiquitination.16 Despite the large body of evidence supporting UPR as a major driver of muscle atrophy in murine models, limited evidence is present for this mechanism in human cancer cachexia.
The previously discussed cytokines (eg, TNFα and IL-1) play a central role in muscle catabolism, as these cytokines can elicit each of the protein degradation pathways. Downstream of these pathways, another important factor affecting skeletal muscle has been identified, the TNFα receptor adapter protein (TRAF6). TRAF6 is upregulated during atrophy and has been found to be overexpressed in muscles from cachectic gastric cancer patients in a recent study, where high TNM-stage and weight loss of more than 10% were associated with significant elevated TRAF6 levels.28 Moreover, inhibition of TRAF6 has been shown to prevent skeletal muscle wasting induced by cachexia in experimental models in mice.29
At the same time, pathways involved in the anabolic response are also affected. Decrease in the circulating levels of the anabolic factor insulin-like growth factor-1 (IGF-1) and the development of insulin resistance has been reported in rats bearing the AH-130 hepatoma.30 Another study, however, questioned whether muscle wasting in tumor-bearing animals was directly associated with downregulation of the IGF-1 signaling pathway. The study demonstrated that IGF-1 overexpression in healthy mice resulted in increased muscle fibers and muscle size, but muscle mass was not modified in mice with tumor-driven cachexia.31
These interesting findings are still to be confirmed by studies that do more than investigate the molecular mechanisms characterizing the early cancer-associated myopathy, before treatment is initiated. It is important to explore the entire wasting cascade during anticancer treatment directed at the underlying cancer—which is believed to be the driver of the myopathy and cachectic trajectory—and investigate the extent to which the treatment disrupts the works of the inflammatory pathways and the wasting process.
Fat Browning in Cancer Cachexia
An interesting feature of cancer cachexia is a progressive switch of fat tissue type, from white (white adipose tissue, WAT) to brown (WAT browning [BAT]), which derives its name from the darker color associated with the enrichment of mitochondria. This browning of white adipose tissue is believed to be driven by the cancer itself. The mitochondria characterizing the brown adipose tissue express high levels of the uncoupling protein-1 (UCP-1), which directly promotes thermogenesis by uncoupling the electrochemical gradient during ATP generation. WAT and BAT usually perform opposite physiological functions, with WAT being responsible for energy accumulation in intracellular lipid droplets, and BAT being responsible for energy dissipation through heat production.32 Pro-inflammatory factors are either derived from the host immune system or the tumor contributing to this switch within WAT.33 Browning is believed to be a strong contributor to the increased energy expenditure common in cachectic patients.34
Murine models with genetically engineered mice (GEMMs) with, for example, Kras lung cancer display a loss of more than 15% of total body weight compared with control mice.35 These mice show massive loss of gonadal WAT mass and evidence of WAT browning. Similar results have been shown in mice injected with Lewis lung carcinoma. Other murine models have shown that WAT browning takes place early in the progression of cachexia, preceding loss of body weight and muscle atrophy. Therefore, WAT browning is suggested to be active also in the precachectic state.34 A small study of cachectic cancer patients, including lung cancer patients, found increased UCP-1 staining in the adipose tissue. This indicates that adipocyte atrophy may be associated with thermogenic activity in human cancer cachexia (see figure 7 in Petruzzelli et al34). The sparse knowledge in the field of fat browning and cancer stresses the need for more translational studies to investigate the role of fat browning in human cancer cachexia.
Clinical Management of Cancer Cachexia
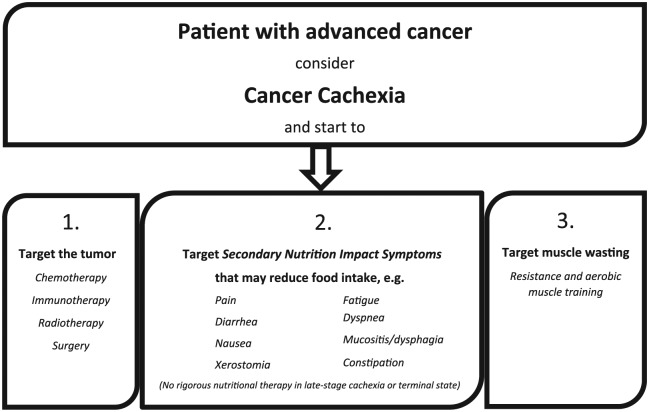
It is clear that cancer cachexia is not a single organ or a single fault disease, but rather a multidimensional syndrome with interdependent components.10 With that in mind search for a single fixer-drug for the syndrome does not make any sense. Although there is no coherent explanation of the syndrome, no effective drugs targeting crucial parts of the pathophysiology, and no good screening tool, cancer cachexia should always be considered in patients with advanced cancer. Even where there is no measurable weight loss an altered metabolism and a still subclinical muscle wasting may already be in progression. In the following, a valuable multimodal approach to the syndrome is illustrated (Figure 2.).
Figure 2.
No single fixer-drug for a multidimensional syndrome. Target the underlying cancer if possible, alleviate secondary nutrition impact symptoms, and stimulate physical training.
Targeting the Tumor
The tumor is considered to be the main driver of the syndrome, thus, the most effective treatment for cancer cachexia is a curative treatment of the cancer itself, for example, radical operation with possible adjuvant systemic treatment. The patient may for a short time suffer from pain and inactivity after surgery, suffer from the side effects of the antineoplastic treatment, possible steroid treatment, nausea, and reduced food intake, and so on, but is expected to recover and regain strength and weight, including lost muscle mass. However, this scenario is rare in lung cancer patients, and often the reality is an incurable disease where life-prolonging and palliative treatment is offered.
Targeting the tumor requires calculating the dose of chemotherapy, and the use of body surface from weight and height has been standard procedure for centuries. However, there is a growing recognition of the condition known as sarcopenic obesity. At the time of cancer diagnosis, overweight and obese patients may have substantial ongoing muscle depletion (sarcopenia being defined as muscle mass of > 2 SD below that of healthy adults).36 Patients with low muscle mass show increased prevalence of toxic adverse effects to treatment, and low muscle mass is an independent risk factor for decreased survival.37,38 When targeting with chemotherapy, toxicity of treatment can be of sufficient severity that it requires dose reductions, treatment delays or termination of treatment, potentially resulting in weight-losing patients not achieving the full potential benefit of their cancer therapy.2,4 Although cachexia is associated with increased toxicity to anticancer treatment and associated with reduced survival, it would be wrong to conclude that cachectic patients only have a poorer outcome because they are treated less intensively with anticancer drugs. Many components of this syndrome probably contribute to the poorer outcome. One of these could be atrophy of respiratory muscles39 also found in patients with chronic obstructive pulmonary disease and only aggravating an already impaired lung function.
Nevertheless, having the increased prevalence of toxic adverse effect in patients with low muscle mass in mind, it could be clinically sound in some cases to consider a smaller dose reduction earlier in fragile patients to avoid treatment delay or termination due to toxicity. This does not necessarily require a special “sarcopenia scan” but clinical judgment of low muscle mass.
Targeting All Secondary Nutritional Impact Symptoms (Secondary Cachexia)
All symptoms that have a negative impact on food intake and well-being should be sought and treated adequately up front. This to ensure optimal conditions for the patient who is about to start systemic treatment with known substantial side effects. Good supportive care must be a cornerstone in all oncological units, because it is a matter of course that good supportive care up front and through the whole treatment trajectory increases the likelihood of high quality of life and reduces the risk of early termination of the life-prolonging treatment.
A crucial part of handling secondary nutritional impact symptoms is securing nutritional support, for example, by relieving nausea or pain. This ensures that intake of needed energy and substrates are normalized. Sufficient intake is sometimes reached by giving high energy and protein supplement in addition to normal food. However, in late-stage cachexia one must always consider whether the patient is in an irreversible catabolic state where rigorous nutritional therapy is futile.40
Addressing the often-accompanying loss of appetite, only corticosteroids and progestins have shown effectiveness. There is no documented positive effect on muscle mass, the effect of corticosteroids on appetite is short-lasting, and both corticosteroids and progestin have known additional unwanted effects. Cannabinoids have shown increased appetite in patients with AIDS, but not in those with cancer,41 but the use of cannabinoids may be an option in selected cachectic patients experiencing chronic nausea, with attention to known cognitive side effects.
In general, securing good supportive care demands a dedicated interdisciplinary teamwork throughout the treatment trajectory for each patient. This is to ensure not only the needed nutritional support but also, for example, good antiemetic treatment and adequate pain relief.
Targeting Systemic Inflammation
As described, inflammatory mediators are believed to play a central role in the development of cancer cachexia. Several anti-inflammatory drugs and nutrients have been tested in clinical trials—as single treatment or in a multimodal approach. A systematic review examining nonsteroidal anti-inflammatory drug (NSAID) treatment in cancer cachexia was conducted in 2012.42 It concluded that NSAIDs might improve weight in cancer patients with cachexia and found some evidence for the effect on physical performance, self-reported quality of life, and inflammatory parameters. But evidence was not considered strong enough to recommend NSAIDs for cachexia outside clinical trials, and the 2016 guidelines from the European Society for Clinical Nutrition and Metabolism (ESPEN) found clinical data insufficiently consistent to recommend NSAID drugs to improve body weight in weight-losing cancer patients.40
The use of n-3 fatty acids with potential anti-inflammatory effect has been investigated. The level of evidence to recommend n-3 fatty acids with the intention of treating cachexia outside clinical trials was considered insufficient in a systematic review from 2012.43 The mentioned 2016 ESPEN guidelines on nutrition in cancer patients came with a weak recommendation for using n-3 fatty acids to improve appetite and body weight in patients with advanced cancer undergoing chemotherapy and at risk of weight loss or malnourished. This weak recommendation was due to the inconsistencies in the reported effects, but with several positive trials have been published during the last few years reporting nutritional benefits, a plausible biological rationale, only mild side effects and no convincingly serious safety issues. One positive study from 2011 demonstrated how patients undergoing chemotherapy for lung cancer maintained weight and muscle mass when given 2.2 g of fish oil per day compared with standard of care/no intervention.44 In conclusion, it seems safe and reasonable to advise patients to eat fat fish or take n-3 fatty acids in capsules.
Targeting Muscle Dysfunction
Currently, there are no approved drugs to target muscle dysfunction. Yet muscle function is highly dependent on its use, and physical training may be the single most efficient intervention in targeting muscle build-up. In cancer patients, there is evidence that physical exercise can reduce fatigue, improve quality of life and relieve adverse side effects during and after treatment.45 Physical exercise is considered well tolerated, feasible and safe during and following cancer treatment.46 This also goes for patients with advanced stage lung cancer undergoing chemotherapy, where both aerobic cardiovascular training and strength training were combined.47 Relative physical inactivity compared to the period before diagnosis is believed to be one of the causes of muscle dysfunction in cancer patients. Physical exercise may be of particular importance for cancer patients with advanced disease in a precachectic or cachectic stage because of its potential positive effects on muscle mass and strength.48 There are even recent studies showing an anticancer effect of exercise in tumor-bearing mice.49 Thus, based on current knowledge, it is considered clinically sound to advise most cancer patients to perform physical exercise. Future clinical studies will likely consider physical training to be an obvious part of standard care.
The ghrelin antagonist anamorelin has shown statistically significant effect on lean body mass (LBM; as an indicator for muscle mass), but the drug is not available. The difference in LBM between active treatment and control group was not convincing, and most important, no positive effect in muscle function measured by hand grip strength was demonstrated and no proven effect on quality of life.50
Levels of C-reactive protein (CRP) and albumin have been considered possible prognostic factors of cachexia development, but so far, nothing supports mandatory measurement. In a study with patients with advanced lung cancer, CRP and albumin levels at the start of chemotherapy did not show a significant association with change in muscle mass.51
Regarding browning of WAT, there is so far no consensus nor substantiated advice to give clinicians or patients. The reason is that knowledge in this field still is very sparse.
Future Perspectives
Late-stage cancer in various tumor types is associated with weight loss being an independent negative prognostic factor. These findings also apply to lung cancer, the largest contributor to cancer deaths worldwide. As a clinician, nutrition, muscle wasting, functional impairment, and dyspnea are just some of many pieces of the puzzle one has to consider when counseling a patient with newly diagnosed lung cancer. There are many important decisions to be made regarding treatment planning and the range of supportive care initiatives for the patient.
For many clinicians, cancer cachexia is a vague concept. The definition of cachexia gives an idea of what cachexia is but does not give any good tools for decision making. Patients are screened for body weight, but the need for a sound and validated screening tool is clear so that clinicians not only take note of the all-too-frequent weight loss, but also get a better idea what is going on behind it. “Negative protein and energy balance” and “abnormal metabolism” are not actionable for the clinician.
Understanding cancer cachexia as a multifactorial syndrome with more contributing factors has led to the acknowledgement of a multimodal approach—nutritional support, supportive care, and physical activity alongside traditional anticancer treatment. There is no specific anticachexia targeting drug available that has shown significant improvements on patient-centered outcomes. Moreover, drugs in the pipeline often only address one out many known factors, for example, increasing appetite or decreasing inflammation, and therefore are not effective in alleviating the spectrum of clinical components that patients with cancer cachexia experience. Possible future specific cachexia drugs are desirable, but a multimodal approach to the patients will always be of great importance.
Seeing cancer cachexia as not only a muscle-wasting syndrome but also a syndrome affecting other organs, not least adipose tissue, has happened in parallel with the development of new imaging technologies that can quantify different tissue compartments (eg, lumbar skeletal-muscle index on computed tomography scans). In future clinical studies on cancer cachexia, quantification of muscle mass on computed tomography scans seems obvious.52 Together with possible prognostic biomarkers, including markers for fat browning, it seems likely that the degree of muscle function and muscle wasting at time of diagnosis will be variables to incorporate in a future cachexia screening tool (Figure 3).
Figure 3.
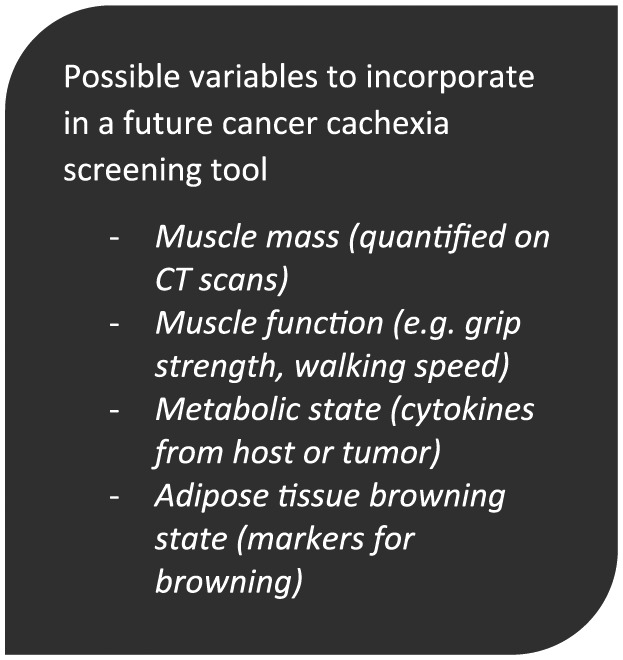
Possible variables to incorporate in a future cancer cachexia screening tool.
Conclusion
In conclusion, effective interventions to prevent muscle loss and physical impairment in patients with cancer cachexia requires early identification of the condition. This cannot be done only by measurement of body weight changes but also, and preferably, with imaging technologies for quantification of muscle mass, measurements of muscle function, and detection of prognostic biomarkers. Much of what we know comes from preclinical studies. The initiation of more translational studies that address these issues—including possible identification of markers for muscle wasting and browning of white fat along the cancer trajectory—are necessary to support this large and growing patient population in the future.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Laviano A, Meguid MM. Nutritional issues in cancer management. Nutrition.1996;12:358-371. [DOI] [PubMed] [Google Scholar]
- 2. Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancer? Br J Cancer. 2004;90:1905-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503-509. [DOI] [PubMed] [Google Scholar]
- 4. Prado CM, Antoun S, Sawyer MB, Baracos VE. Two faces of drug therapy in cancer: drug-related lean tissue loss and its adverse consequences to survival and toxicity. Curr Opin Clin Nutr Metab Care. 2011;14:250-254. [DOI] [PubMed] [Google Scholar]
- 5. Costa G, Donaldson SS. Current concepts in cancer: effects of cancer and cancer treatment on the nutrition of the host. N Engl J Med. 1979;300:1471-1474. [DOI] [PubMed] [Google Scholar]
- 6. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491-497. [DOI] [PubMed] [Google Scholar]
- 7. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661-671. [DOI] [PubMed] [Google Scholar]
- 9. Grossberg AJ, Scarlett JM, Zhu X, et al. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology. 2010;151:606-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90-99. [DOI] [PubMed] [Google Scholar]
- 11. Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:371-385. [DOI] [PubMed] [Google Scholar]
- 12. Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35:802-807. [DOI] [PubMed] [Google Scholar]
- 13. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153-166. [DOI] [PubMed] [Google Scholar]
- 15. Jatoi A, Ritter HL, Dueck A, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non–small cell lung cancer patients (N01C9). Lung Cancer. 2010;68:234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costelli P, Llovera M, Carbó N, García-Martínez C, López-Sorianoq FJ, Argilés JM. Interleukin-1 receptor antagonist (IL-1ra) is unable to reverse cachexia in rats bearing an ascites hepatoma (Yoshida AH-130). Cancer Lett. 1995;95:33-38. [DOI] [PubMed] [Google Scholar]
- 18. Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer. 1996;73:1560-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep. 2009;21:1091-1095. [DOI] [PubMed] [Google Scholar]
- 20. Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393-R401. [DOI] [PubMed] [Google Scholar]
- 21. Mantovani G, Macció A, Mura L, et al. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med (Berl). 2000;78:554-561. [DOI] [PubMed] [Google Scholar]
- 22. Kuroda K, Nakashima J, Kanao K, et al. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007;69:113-117. [DOI] [PubMed] [Google Scholar]
- 23. Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58-74. [DOI] [PubMed] [Google Scholar]
- 25. Op den Kamp CM, Langen RC, Snepvangers FJ, et al. Nuclear transcription factor κB activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr. 2013;98:738-748. [DOI] [PubMed] [Google Scholar]
- 26. Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM. Ca2+-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol. 2005;37:2134-2146. [DOI] [PubMed] [Google Scholar]
- 27. Bossola M, Muscaritoli M, Costelli P, et al. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun YS, Ye ZY, Qian ZY, Xu XD, Hu JF. Expression of TRAF6 and ubiquitin mRNA in skeletal muscle of gastric cancer patients. J Exp Clin Cancer Res. 2012;31:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paul PK, Gupta SK, Bhatnagar S, et al. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol. 2010;191:1395-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Costelli P, Muscaritoli M, Bossola M, et al. IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2006;291:R674-R683. [DOI] [PubMed] [Google Scholar]
- 31. Penna F, Bonetto A, Muscaritoli M, et al. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved? Int J Cancer. 2010;127:1706-1717. [DOI] [PubMed] [Google Scholar]
- 32. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277-359. [DOI] [PubMed] [Google Scholar]
- 33. Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petruzzelli M, Schweiger M, Schreiber R, et al. A switch from white to brown fat increases energy expenditure in cancer associated cachexia. Cell Metab. 2014;20:433-447. [DOI] [PubMed] [Google Scholar]
- 35. Puyol M, Martín A, Dubus P, et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non–small cell lung carcinoma. Cancer Cell. 2010;18:63-73. [DOI] [PubMed] [Google Scholar]
- 36. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755-763. [DOI] [PubMed] [Google Scholar]
- 37. Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973-6979. [DOI] [PubMed] [Google Scholar]
- 38. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635. [DOI] [PubMed] [Google Scholar]
- 39. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10:236-241. [DOI] [PubMed] [Google Scholar]
- 40. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11-48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 41. Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24:3394-3400. [DOI] [PubMed] [Google Scholar]
- 42. Solheim TS, Fearon KC, Blum D, Kaasa S. Non-steroidal anti-inflammatory treatment in cancer cachexia: a systematic literature review. Acta Oncol. 2013;52:6-17. [DOI] [PubMed] [Google Scholar]
- 43. Ries A, Trottenberg P, Elsner F, et al. A systematic review on the role of fish oil for the treatment of cachexia in advanced cancer: an EPCRC cachexia guidelines project. Palliat Med. 2012;26:294-304. [DOI] [PubMed] [Google Scholar]
- 44. Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer. 2011;117:1775-1782. [DOI] [PubMed] [Google Scholar]
- 45. Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87-100. [DOI] [PubMed] [Google Scholar]
- 46. Oldervoll LM, Loge JH, Lydersen S, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16:1649-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quist M, Rørth M, Langer S, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer.2012;75:203-208. [DOI] [PubMed] [Google Scholar]
- 48. Argiles JM, Busquets S, López-Soriano FJ, Costelli P, Penna F. Are there any benefits of exercise training in cancer cachexia? J Cachexia Sarcopenia Muscle.2012;3:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554-562. [DOI] [PubMed] [Google Scholar]
- 50. Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519-531. [DOI] [PubMed] [Google Scholar]
- 51. Stene GB, Helbostad JL, Amundsen T, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol.2015;54:340-348. [DOI] [PubMed] [Google Scholar]
- 52. Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Palliat Care. 2009;3:269-275. [DOI] [PubMed] [Google Scholar]