Abstract
Background: Syndrome (ZHENG in Chinese) in traditional Chinese medicine (TCM) refers to the intrinsic characteristics of a pathological process at a certain stage; these characteristics are influenced by internal and external environments and reveal the nature of a disease. Proper syndrome differentiation is the basic principle that guides clinical treatment. Objective: To have a good understanding of tumor progression and the different mechanisms related to ZHENG that have occurred before and after tumor development and to explore the valid evaluation criteria of different pancreatic cancer syndromes to improve the guiding role of TCM syndrome differentiation in pancreatic cancer treatment. Methods: In this study, we established mouse subcutaneous pancreatic cancer models, namely, Con (control), Pi-Xu (Spleen-Deficiency), Shi-Re (Dampness-Heat), and Xue-Yu (Blood-Stasis). Then, for the first time, we compared the different effects of “ZHENG-first” (referring to a different disease status that occurred before tumor occurrence) and “Tumor-first” (referring to the change in the tumor microenvironment and the resulting changes in the state of the body) conditions on tumor progression and evaluated the associated molecular mechanisms. Results: We found that tumor growth in the “ZHENG-first” and “Tumor-first” conditions was different. In the “Tumor-first” model, the tumor growth in the Pi-Xu group was faster than that in the other groups. However, in the “ZHENG-first” model, the tumor growth trend was most obvious in the Shi-Re group. There was a difference in tumor-associated macrophage infiltration between the 2 models. The expression levels of the inflammatory cytokines IL-6, IL-10, and P-STAT3 were also differentially altered. Conclusion: The emergence of ZHENG conditions before or after tumor occurrence had different impacts on pancreatic cancer development, and these impacts may be related to differences in tumor-associated macrophage infiltration and the involved inflammatory cytokines IL-6, IL-10, and P-STAT3. The study results uncovered the molecular basis of syndrome differentiation in pancreatic cancer progression, which might provide more specific guidance for TCM treatment of pancreatic cancer.
Keywords: pancreatic cancer, ZHENG, syndrome, traditional Chinese medicine, TCM, TAM, inflammatory cytokines
Introduction
Pancreatic cancer remains a leading cause of cancer mortality. The overall 5-year survival rate is still less than 5% to 7%.1 Curative resection is one of the most important factors that determines the outcomes in patients with pancreatic cancer.2 However, 80% of patients with nonspecific symptoms are not diagnosed until the terminal clinical stage.3 Therefore, chemotherapy is still the most effective systemic therapy for advanced pancreatic cancer.4 However, overall survival has not significantly improved due to chemotherapy resistance.5 Thus, it is urgent for us to shift our thinking and to better understand the molecular basis of pancreatic cancer.
Unlike modern medicine, a deductive science with a specific focus but in which the general needs of the individual might be neglected, traditional Chinese medicine (TCM) is a form of complementary medicine that might not be related to highly specific targets but aims to improve the well-being of individuals by maintaining balance in various physiological functions. TCM emphasizes overall coordination of the environment inside and outside the human body. This allows the individual to maintain a biologically balanced status to build up better bodily defenses and fight physiological problems.6,7 An increasing number of studies have indicated that TCM may be a strong therapeutic candidate for tumors, improving clinical symptoms and prolonging survival rate.8,9
ZHENG, or syndrome differentiation, is known as the cornerstone of TCM. It is based on comprehensive analysis of clinical information collected through the main TCM diagnosis procedures: observation, listening, questioning, and pulse analyses. TCM syndrome differentiation is the most essential principle that guides the prescription of Chinese herbal formulae.10 However, in the era of evidence-based medicine, TCM ZHENG has encountered a bottleneck in biomedical science owing to the shortage of evidence-based theoretical interpretations and credible evidence of the efficacy of ZHENG-based treatment.11
At the same time, there is still not a clear understanding of ZHENG-specific molecules and their effects on pancreatic cancer. Our previous study suggested that the existence of TCM ZHENG may influence tumor growth in pancreatic cancer via specific chemokines and their receptors.12 We also previously showed that the activity of cancer-associated fibroblasts (CAFs) and the infiltration of tumor-associated macrophages (TAMs) were altered under different ZHENG conditions. Accordingly, the expression levels of CAF-derived chemokines and TAM-derived cytokines were altered, such as CAF-related SDF-1/CXCR4 and TAM-related CCL5/CCR5.13 These observations demonstrated a relationship between ZHENG and inflammatory cytokines in pancreatic cancer, and this relationship may ultimately promote tumor progression.
In this study, we first established 2 subcutaneous pancreatic cancer models, namely, “ZHENG-first” (referring to different disease status before tumor occurrence) and “Tumor-first” (referring to the change in the tumor microenvironment and the resulting changes in the state of the body). Correspondingly, we compared the influence of ZHENG on tumor growth and analyzed the related inflammatory cytokines underlying the 2 models.
Materials and Methods
Cell Line and Mice
The pancreatic cancer cell line panc02 was purchased from the American Type Culture Collection and cultivated in complete growth medium according to the recommendations supplied by the manufacturer. The cultured cells were maintained in Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin (Thermo Fisher Scientific, Waltham, MA) in a humidified 5% CO2 atmosphere at 37°C.
C57 female mice (6 weeks old, weighing 20-22 g) were obtained from Vital River Laboratories, Beijing Vital River Laboratory Animal Technology Co, Ltd, and housed in laminar flow cabinets with food and water under specific pathogen-free conditions. All the experiments on mice were conducted according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol for the use of live animals in teaching and research was also approved by the Shanghai Medical Experimental Animal Care Committee.
Drugs and Reagents
Mirabilite and rhubarb herb powders were supplied by the Pharmacy of Traditional Medicine, Fudan University Shanghai Cancer Center, Shanghai, China. Adrenaline hydrochloride for injection was obtained from the Central Pharmacy, Fudan University Shanghai Cancer Center, Shanghai, China. Mixtures of the 2 powders (1:4) were adjusted to a concentration of 1 g/mL in water and stored at 4°C. Guan Sheng Yuan honey was dissolved to a concentration of 0.2 g/mL in the daily drinking water of mice. Red Star (Hongxing) Erguotou liquor was diluted to 50% in water. Pork fat was donated by the Chinese Academy of Sciences (Shanghai, China).
The following antibodies were purchased from BIOTEND (Shanghai, China) and used for immunohistochemistry (IHC) analysis: anti-CD68 (Proteintech, Wuhan, Hubei, China), anti-mouse IL-6 (Proteintech), anti-mouse IL-10 (Signalway Antibody, SAB, College Park, MD), phospho-Stat3 Tyr705 (P-STAT3; Cell Signaling Technology, Danvers, MA).
Establishment of TCM ZHENG Models
We established 4 types of TCM ZHENG models, and C57 mice were randomly divided into equal groups: Con (control), Pi-Xu (Spleen-Deficiency), Shi-Re (Dampness-Heat), and Xue-Yu (Blood-Stasis). The above-mentioned ZHENG models were established as previously reported.12,13 In brief, Pi-Xu (Spleen-Deficiency) was established by feeding mice a water-soluble mixture of mirabilite and rhubarb (0.2 mL per mouse, day 1 to day 7). The Shi-Re (Dampness-Heat) group was developed by lavaging Red Star (Hongxing) Erguotou liquor and pork fat daily (day 1 to day 7). Meanwhile, 200 g/L honey was added in water for daily free drinking. The Xue-Yu (Blood-Stasis) condition was established with a hypodermic injection of 0.01% adrenaline for each mouse (0.13 mg/kg, day 1 to day 7). Mice in the Con group were fed normally.
Establishment of Subcutaneous Tumor Models
Panc02 cells (0.2 mL, 1 × 107/mL) were injected into the right axilla of C57 mice under aseptic conditions. After modeling, the health status of the mice and the formation of tumors were observed every 2 days. When the tumors were formed, the length and width of the tumors in millimeters (mm) were measured 2 times a week, with a and b representing the long diameter and short diameter (mm), respectively. The tumor volume (volume = 1/2ab2, mm3) was measured and recorded. All the mice were euthanized after the maximum diameter of most tumors reached approximately 1.0 cm. The subcutaneous tumors were then immediately resected and weighed.
“ZHENG-First” and “Tumor-First” Models
Tumor-First
The subcutaneous tumor models were established as described above. The next day, all the models were randomly divided into 4 groups to build TCM ZHENG models according to the methods described above, including the Con, Pi-Xu, Shi-Re, and Xue-Yu groups.
ZHENG-First
The TCM ZHENG models were established in accordance with the above-mentioned methods. After 7 days, the subcutaneous models were established according to the methods described above.
Immunohistochemistry
Isolated tumor tissue sections were fixed in 10% formalin and embedded in paraffin. Unstained 3-µm paraffin-embedded tumor sections were then dewaxed and rehydrated in a graded alcohol series for IHC analysis. The sections were immersed in 3% H2O2 methanol for 15 minutes at 37°C to block endogenous peroxidase activity and then boiled in 0.1 mol/L citrate buffer (pH 6.0) for 10 minutes to retrieve antigens. The samples were incubated in 5% normal goat serum to block nonspecific binding. The sections were then incubated with rabbit polyclonal anti-CD68 (1:500), rabbit polyclonal anti-IL-6 (1:200), rabbit anti-IL-10 (1:100), and rabbit monoclonal anti-phospho-Stat3 (Tyr705; 1:400) overnight at 4°C. The next day, the sections were incubated with primary antibody for 1 hour at 37°C and then rinsed 3 times with phosphate-buffered saline. The samples were then incubated with horseradish peroxidase–conjugated goat anti-rabbit secondary antibody for 30 minutes at room temperature. The sections were then color developed with 3′-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin.
All the immunostained sections were evaluated by 3 experienced pathologists with no knowledge of the background of the study. The percentage of positive tumor cells was evaluated in 10 fields with a high-power (×200) microscope, and the values in 5 representative fields (×400 magnification) were recorded. The ratio of the positive CD68 staining area to the total area was calculated to quantify TAM infiltration. The measurement of the expression of IL-6 and IL-10 was conducted as previously described.12 Positive P-STAT3 expression was defined as >25% nuclear staining or greater than the moderate staining intensity of the tumor cells.
Western Blot Analysis
Total proteins were extracted from tumor tissues by grinding in radioimmunoprecipitation assay buffer containing protease inhibitors and phosphatase inhibitors. Then, 20 µg of total protein lysate was separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes. After being blocked in 5% bovine serum albumin or 5% nonfat dry milk at 37°C in TBS-T (tris-buffered saline-T) or PBS-T (phosphate-buffered saline-T) for 1 hour, the membranes were incubated with primary antibodies, including anti-Stat3 (Cell Signaling Technology; 1:1000), anti-phospho-Stat3 (Cell Signaling Technology; 1:2000), and anti-β-actin (Proteintech; 1:1000), at 4°C overnight, and then the appropriate secondary antibodies conjugated to horseradish peroxidase at room temperature for 1 hour.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). TaKaRa PrimeScript RT reagent Kit was used for reverse transcription to obtain cDNA (Table 1). The expression of β-actin and candidate genes was calculated using quantitative real-time polymerase chain reaction (PCR; ABI 7900HT Real-Time PCR system, Applied Biosystems, Foster City, CA).
Table 1.
Primer Sequences.
| Mouse β-actin forward | GATCAAGATCATTGCTCCTCCTG |
| Mouse β-actin reverse | AGGGTGTAAAACGCAGCTCA |
| Mouse STAT3 forward | ACGACCTGCAGCAATACCAT |
| Mouse STAT3 reverse | AACGTGAGCGACTCAAACTG |
| Mouse IL-6 forward | GCCTTCTTGGGACTGATGCT |
| Mouse IL-6 reverse | TGCCATTGCACAACTCTTTTC |
| Mouse IL-10 forward | AGGCGCTGTCATCGATTTCTC |
| Mouse IL-10 reverse | CGGAGAGAGGTACAAACGAGG |
Statistical Analyses
Statistical analyses were performed with independent Student’s t test (2-tailed) or 1-way analysis of variance using SPSS software. Statistical significance was based on P < .05.
Results
The Criteria of Different TCM ZHENG Conditions Based on TCM Theory
The successful establishment of different ZHENG conditions was confirmed by visual observations based on the “Guidelines on the Clinical Research of New Chinese Medicine Drugs,” and these observations were verified to be valid based on a previous study.12 Mice in the Pi-Xu group displayed lassitude, having cold extremities and loose stools. Shi-Re ZHENG was evidenced by the behaviors of poor appetite, heaviness in the limbs, and dry and hard stools. Mice in the Xue-Yu group showed hypoactivity, dark purple and cold tails, and ear margin and subcutaneous ecchymosis. No typical symptoms were found in the control group.
The Occurrence of TCM ZHENG Before and After Tumor Establishment Affected Tumor Growth
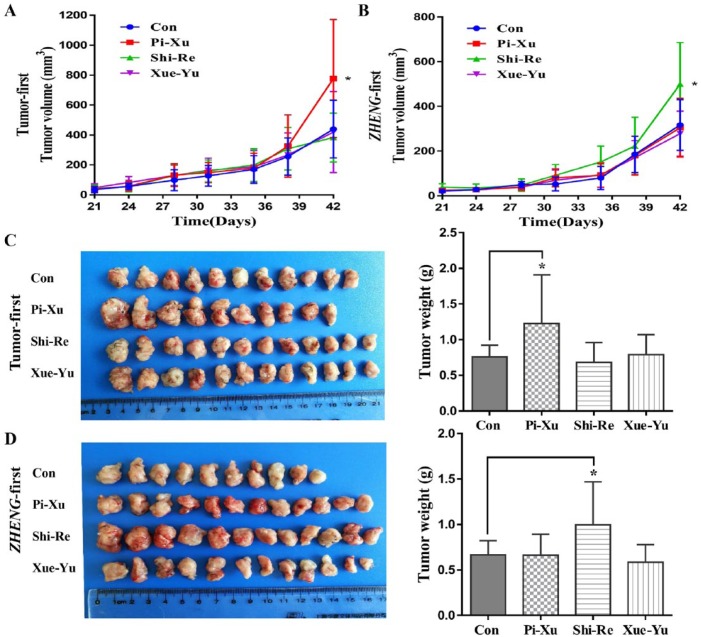
A relationship between the tumor microenvironment and ZHENG conditions was confirmed. The results showed that tumors under different ZHENG conditions exhibited different tumor microenvironments, which may ultimately affect tumor growth.13 However, the effects of TCM ZHENG occurred before pancreatic cancer tumor formation, and whether there are differences between “Tumor-first” and “ZHENG-first” conditions remained unclear. Thus, we compared the tumor growth in the “Tumor-first” (Figure 1A) and “ZHENG-first” (Figure 1B) models. In the “Tumor-first” model, tumor growth in the Pi-Xu group was faster than that in the other groups. However, in the “ZHENG-first” model, the trend in tumor growth was the most obvious in the Shi-Re group. Figure 1C and Figure 1D show photographs of the tumors and tumor weights from the “Tumor-first” and “ZHENG-first” models.
Figure 1.
The trend in tumor growth in the “Tumor-first” and “ZHENG-first” models: (A) In the “Tumor-first” model, the tumor growth in the Pi-Xu group was faster than that in the control group; *P < .05. (B) In the “ZHENG-first” model, tumor growth in the Shi-Re group was significantly faster than that in other groups; *P < .05. Tumor photographs and tumor weights in the (C) “Tumor-first” model group and (D) “ZHENG-first” model group.
The Differences in TAM Expression in the “Tumor-First” and “ZHENG-First” Models
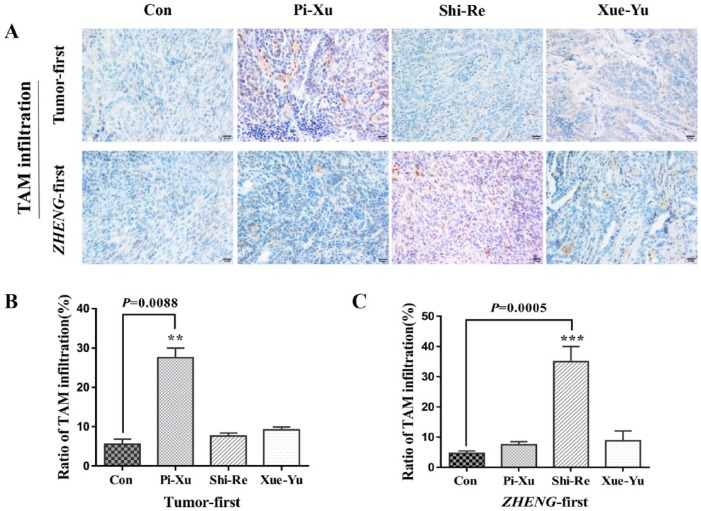
The tumor microenvironment is composed of the extracellular matrix, stromal cells, inflammatory cells, and vessels. There is accumulating evidence that in the tumor microenvironment, the expression of cytokines and the infiltration of TAMs is correlated with tumorigenesis and influences tumor growth once the tumor is established.14,15 To determine the relationship among TAM, TCM ZHENG, and tumor growth, we performed immunohistochemical staining and assessed the ratio of TAM infiltration using a CD68 antibody to examine the levels of TAM expression (“Tumor-first” Figure 2A top and “ZHENG-first” Figure 2A bottom). Consistent with the tumor growth results, we found significantly high TAM expression in the Pi-Xu group in the “Tumor-first” model compared with that in the control group (Figure 2B). However, TAM expression was not significantly different in the Pi-Xu group, but there was a statistical difference between the Shi-Re group and the control group in the “ZHENG-first” model (Figure 2C). The results suggested that increased TAM recruitment under different ZHENG conditions might be related to tumor growth in the “Tumor-first” and “ZHENG-first” models.
Figure 2.
Immunohistochemical analysis with a high-power (400×) microscope to assess TAM expression in subcutaneously transplanted pancreatic cancer tissues in “Tumor-first” and “ZHENG-first” models: (A) TAM expression was evaluated using a CD68 antibody; “Tumor-first” (top) and “ZHENG-first” (bottom). The CD68 positive staining ratio was quantitatively estimated to assess TAM infiltration; *P < .05. (B) “Tumor-first” model and (C) “ZHENG-first” model.
Correlation Between Inflammation and Tumor Growth Under “Tumor-First” and “ZHENG-First” Conditions
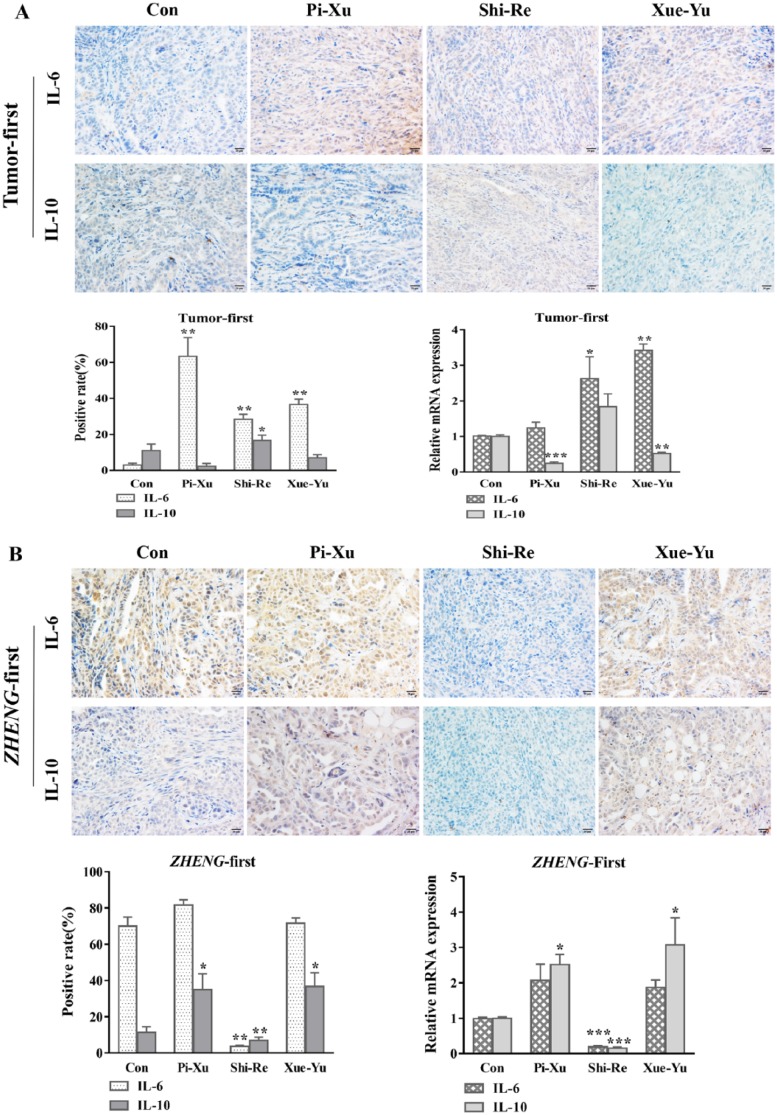
In vivo and in vitro evidence suggests that the IL-6 system plays a positive role in local inflammatory reactions through amplification of leukocyte recruitment.16 Recent studies have provided evidence of an association between inflammation and cancer.17 Researchers found that TAM-derived IL-6 can induce chemoresistance by activating the IL-6R/STAT3/miR-204-5p pathway in CRC cells.18 The expression of the anti-inflammatory type II cytokine IL-10 in TAMs was stimulated via phosphorylation of Smad5 and STAT3. IL-10, in turn, induced the formation of TAMs and suppressed the local antitumor effect.19 We hypothesized that IL-6 and IL-10 contribute to tumor growth under different TAM conditions in these 2 different TCM syndromes. To verify this hypothesis, we evaluated IL-6 and IL-10 expression in tumors under different TCM ZHENG conditions. We performed immunohistochemical staining and real-time PCR to examine the protein and relative mRNA expression of IL-6 and IL-10. In the “Tumor-first” model, the results showed an apparent increase in the IL-6 expression level in the Pi-Xu group (P = .0040), consistent with the observation of increased TAM expression in the Pi-Xu group. Similar results were also found in the Shi-Re and Xue-Yu groups. Conversely, IL-10 exhibited the lowest level in the Pi-Xu group, although the result was not significant (P = .0581). Increased IL-10 expression was observed in the Shi-Re model. No difference in IL-10 expression was found in the Xue-Yu group compared with the control group (P = .1201; Figure 3A). Whereas in the “ZHENG-first” model, we found that IL-10 and IL-6 expression levels were both decreased in the Shi-Re group, which was contrary to the high TAM infiltration. Meanwhile, in the Xue-Yu and Pi-Xu groups, the results showed increased levels of IL-10. There were no differences in IL-6 expression in the Pi-Xu and Xu-Yu groups (Figure 3B). The similar mRNA IL-6 and IL-10 expression levels are also shown in Figure 3.
Figure 3.
mRNA expression and immunohistochemical analysis with a high-power (400×) microscope to examine IL-6 and IL-10 expression in subcutaneously transplanted pancreatic cancer tissues under different TCM ZHENG conditions: (A) “Tumor-first” model and (B) “ZHENG-first” model.
STAT3 Activation in the “Tumor-First” and “ZHENG-First” Models
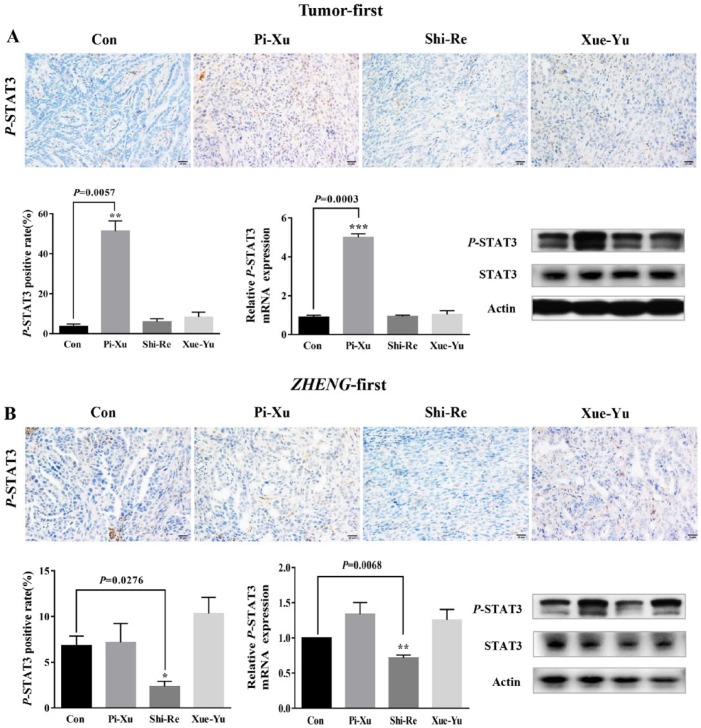
Given the well-known effects of IL-6 and IL-10 on STAT3 activation and their discrepancies under different TAM infiltration levels in the TCM ZHENG models, we asked whether TAMs regulated the IL-6 and IL-10 expression to activate STAT3 and contribute to tumor growth in the “Tumor-first” and “ZHENG-first” models. We tested STAT3 activation (evaluated by phosphorylation of STAT3, designated P-STAT3) using western blotting and IHC. In the “Tumor-first” model, a strong increase in the protein and mRNA expression level of P-STAT3 was observed in the Pi-Xu group; however, there was no significant increase in the Shi-Re and Xue-Yu groups (Figure 4A). Our results suggested that in the “Tumor-first” model, TAMs promoted tumor growth via mediation of STAT3 phosphorylation induced by IL-6 but independent of IL-10. To our surprise, in the “ZHENG-first” model, P-STAT3 was obviously reduced in the Shi-Re group (Figure 4B), which was consistent with the downregulation of IL-6 and IL-10 but contradictory to the high TAM infiltration.
Figure 4.
The expression of P-STAT3 was different in the “Tumor-first” and “ZHENG-first” models. (A) In the “Tumor-first” model, P-STAT3 expression was significantly increased in the Pi-Xu group compared with that in the control group; P < .05. (B) In the “ZHENG-first” model, the Shi-Re group presented a significant decrease in the P-STAT3 mRNA and protein expression levels; P < .05.
Discussion
Even though treatments, including surgery, radiotherapy, chemotherapy, and targeted therapy, for cancer have significantly progressed, they are insufficient for pancreatic cancer due to multidrug resistance, poor prognosis, and low survival.20,21 Increasing evidence has indicated that TCM is effective in prolonging the survival of pancreatic cancer patients.22,23 ZHENG plays a crucial role in guiding clinical TCM treatment. However, the validity of ZHENG has always been questioned because of its undefined molecular mechanisms, and because its differentiation is dependent on the subjective judgments of doctors.24
We have established a novel model of “ZHENG-subcutaneous transplanted tumor” (“Tumor-first”) and explored the potential molecular basis of different ZHENG in the prognosis and biological characteristics of pancreatic cancer.12 However, it is unknown whether the impact of ZHENG on tumor development is different when the actual occurrence of ZHENG occurs before the tumor. In this article, we propose another model of “subcutaneous transplanted tumor ZHENG” (“ZHENG-first”) and discuss tumor progression related to ZHENG in each of the 2 models. As shown in the results, the tumor growth trend in the 2 models was different. Tumors in the Pi-Xu group grew faster in the “Tumor-first” model compared with those in the Shi-Re group in the “ZHENG-first” model. The results suggested different effects of ZHENG conditions on tumor progression. To provide normalized treatment, decisions regarding which patients are suited for removal of damp-heat or spleen-governed nutrition conditions to slow or stop the growth of pancreatic cancer should be based on the results and the underlying mechanism.
TAMs have been confirmed to suppress immune responses and are believed to contribute to tumor progression and invasion.25 TAM-related inflammatory cytokines, such as IL-6 and IL-10 and the transcription factor STAT3, are also involved in this process.26-28 An imbalanced adjustment in IL-6, IL-10, and STAT3 phosphorylation has been regarded as an important pro-inflammatory regulator in the tumor microenvironment and the key anti-inflammatory mediator in tumor immune evasion.29 Recent studies have demonstrated that TAMs can promote tumorigenesis through IL-6/STAT3 signaling or by regulating STAT3 through IL-10 signaling.27,30 In our previous studies, we summarized that the main ZHENGs for pancreatic cancer were Shi-Re, Pi-Xu, and Xue-Yu. In addition, the Shi-Re ZHENG has been found to be correlated with inflammatory conditions.31 Based on the direct or indirect roles of TAMs and related inflammatory factors in cancer, we proposed that the TAM infiltration in the 2 models might be the key to influencing tumor growth.
As shown in this study, the TAM expression levels in the “Tumor-first” Pi-Xu group and the “ZHENG-first” Shi-Re group were increased compared with those in the control group. This was consistent with the fast tumor growth in these 2 groups. Correspondingly, the pro-inflammatory cytokines IL-6 and IL-10 exhibited different responses in the “Tumor-first” and “ZHENG-first” models. In the “Tumor-first” models, tumors in the Pi-Xu group displayed the fastest growth trend, high TAM expression, and high IL-6 and P-STAT3 levels. However, although there were also significant increases in IL-6 and IL-10 in the Shi-Re and Xue-Yu groups, no differences in tumor growth were observed compared with that in the control group. Considering these findings, we proposed that the expression of TAMs in the “Tumor-first” models might be the key to tumor growth promotion induced by IL-6 upregulation to activate STAT3. In the “ZHENG-first” model, the most obvious tumor growth and highest TAM levels were similarly observed in the Shi-Re group. Surprisingly, IL-6, IL-10, and P-STAT3 were all conversely downregulated compared with other groups, which exhibited strong IL-6 staining and increased IL-10 levels. These results indicated that the IL-6/P-STAT3 signaling pathway was not required for TAMs to promote tumor growth under the “ZHENG-first” conditions. There may be other mechanisms by which tumor development is promoted, but this requires additional evidence. Although we have no additional evidence to uncover the specific mechanism underlying those 2 models, the differentiation of the 2 types of models may provide a certain understanding for establishing TCM ZHENG criteria, which could lead to more appropriate clinical treatment.
Deepening our understanding of tumor biology gives us a chance to create a future for precision medicine that provides highly specific, minimally toxic, and dramatically effective treatment for cancer patients.32 However, the undiscovered molecular network underlying ZHENG conditions blocks TCM progress in clinical practice. In our study, we improved the tumor models with ZHENG conditions and provided a special perspective regarding inflammation in tumors and TCM ZHENG. However, elucidating the specific regulatory mechanisms still requires further studies.
Conclusion
In brief, our study showed that TAM infiltration plays an important role in tumor growth under different TCM ZHENG conditions. The sequence in which the tumor and ZHENG conditions occurred had various effects under the “Tumor-first” and “ZHENG-first” conditions, which might be related to the involvement of inflammatory cytokines.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81603444 and 81573752) and the Shanghai Science and Technology Committee Project (16401932700).
References
- 1. Mohammed S, Van Buren GN, 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20:9354-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586-594. [DOI] [PubMed] [Google Scholar]
- 3. Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163-172. [DOI] [PubMed] [Google Scholar]
- 4. Saung MT, Zheng L. Current standards of chemotherapy for pancreatic cancer. Clin Ther. 2017;39:2125-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yauch RL, Settleman J. Recent advances in pathway-targeted cancer drug therapies emerging from cancer genome analysis. Curr Opin Genet Dev. 2012;22:45-49. [DOI] [PubMed] [Google Scholar]
- 6. Chau F, Fung K, Koon C, Lau K, Wei S, Leung P. Bioactive components in herbal medicine experimental approaches. In: Benzie IFF, Wachtel-Galor S. eds. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Boca Raton, FL: CRC Press; 2011. [PubMed] [Google Scholar]
- 7. Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014;12:331-335. [DOI] [PubMed] [Google Scholar]
- 8. Jiang Y, Liu LS, Shen LP, et al. Traditional Chinese medicine treatment as maintenance therapy in advanced non–small-cell lung cancer: a randomized controlled trial. Complement Ther Med. 2016;24:55-62. [DOI] [PubMed] [Google Scholar]
- 9. Chao TH, Fu PK, Chang CH, Chang SN, Mao FC, Lin CH. Evidence-based Chinese Medicine Research Group Prescription patterns of Chinese herbal products for post-surgery colon cancer patients in Taiwan. J Ethnopharmacol. 2014;155:702-708. [DOI] [PubMed] [Google Scholar]
- 10. Bao H, Gao J, Huang T, Zhou ZM, Zhang B, Xia YF. Relationship between traditional Chinese medicine syndrome differentiation and imaging characterization to the radiosensitivity of nasopharyngeal carcinoma. Chin J Cancer. 2010;29:937-945. [DOI] [PubMed] [Google Scholar]
- 11. Jiang M, Zhang C, Zheng G, et al. Traditional Chinese medicine Zheng in the era of evidence-based medicine: a literature analysis. Evid Based Complement Alternat Med. 2012;2012:409568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai HY, Wang P, Feng LY, et al. The molecular mechanisms of traditional Chinese medicine ZHENG syndromes on pancreatic tumor growth. Integr Cancer Ther. 2010;9:291-297. [DOI] [PubMed] [Google Scholar]
- 13. Chen Z, Chen L, Wang P, Dai HY, Gao S, Wang K. Tumor microenvironment varies under different TCM ZHENG models and correlates with treatment response to herbal medicine. Evid Based Complement Alternat Med. 2012;2012:635702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315-325. [DOI] [PubMed] [Google Scholar]
- 17. Liang CM, Chen L, Hu H, et al. Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J Hepatol. 2015;7:1390-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yin Y, Yao S, Hu Y, et al. The immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL-6. Clin Cancer Res. 2017;23:7375-7387. [DOI] [PubMed] [Google Scholar]
- 19. Lee JH, Lee GT, Woo SH, et al. BMP-6 in renal cell carcinoma promotes tumor proliferation through IL-10-dependent M2 polarization of tumor-associated macrophages. Cancer Res. 2013;73:3604-3614. [DOI] [PubMed] [Google Scholar]
- 20. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [DOI] [PubMed] [Google Scholar]
- 21. Nie J, Zhao C, Deng LI, et al. Efficacy of traditional Chinese medicine in treating cancer. Biomed Rep. 2016;4:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang X, Hao J, Zhu CH, et al. Survival benefits of Western and traditional Chinese medicine treatment for patients with pancreatic cancer. Medicine (Baltimore). 2015;94:e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu Y, Xu S, Cai Y, Liu L. Qingyihuaji formula inhibits pancreatic cancer and prolongs survival by downregulating Hes-1 and Hey-1. Evid Based Complement Alternat Med. 2015;2015:145016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Gu Z, Qi X, Zhai X, et al. Study on TCM syndrome differentiation of primary liver cancer based on the analysis of latent structural model. Evid Based Complement Alternat Med. 2015;2015:761565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155-161. [DOI] [PubMed] [Google Scholar]
- 26. Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [DOI] [PubMed] [Google Scholar]
- 27. Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang R, Lu M, Zhang J, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res. 2011;30:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braun DA, Fribourg M, Sealfon SC. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem. 2013;288:2986-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goswami KK, Sarkar M, Ghosh S, et al. Neem leaf glycoprotein regulates function of tumor associated M2 macrophages in hypoxic tumor core: critical role of IL-10/STAT3 signaling. Mol Immunol. 2016;80:1-10. [DOI] [PubMed] [Google Scholar]
- 31. Chen Z, Wang P. Clinical distribution and molecular basis of traditional Chinese medicine ZHENG in cancer. Evid Based Complement Alternat Med. 2012;2012:783923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsimberidou AM, Eggermont AM, Schilsky RL. Precision cancer medicine: the future is now, only better. Am Soc Clin Oncol Educ Book. 2014:61-69. doi: 10.14694/EdBook_AM.2014.34.61. [DOI] [PubMed] [Google Scholar]