Abstract
Background: The blood-brain barrier (BBB) is the greatest challenge in the treatment of intracranial malignant tumors. Objective: The aim of this study is to determine the role of borneol in opening the BBB and elucidate the underlying mechanisms. Materials and Methods: Twenty Sprague-Dawley (SD) rats were randomized into borneol group intragastrically administered with 10% borneol corn oil (2 mL/kg) and control group. After 30 minutes, 2% Evans blue (4 mL/kg) was injected. Thirty minutes later, brain tissue was analyzed using the Evans blue standard curve. Another 40 SD rats were randomized into high-, medium-, and low-dose borneol groups and a control group. Each rat in the experimental groups was intragastrically administered with 10% borneol corn oil (2 mL/kg, 1.25 mL/kg, and 0.5 mL/kg, respectively). The control group was injected with corn oil of 1.25 mL/kg. After 30 minutes, the rats were killed, and the brain tissues were collected. The expression of occludin, occludens-1, nitric oxide synthase, P-glycoprotein, and intercellular cell adhesion molecule-1 (ICAM-1) was detected by immunohistochemy. Results: The concentration of Evans blue in the borneol group was higher than in the control group (P < .05). The mean density of ICAM-1 expression was higher in the experimental group than in the control group (P < .05). In contrast, significant differences of positive area and total density of ICAM-1 were shown only between the high-dose group and the control group (P < .05). Conclusion: Borneol can open the BBB, which might be related with the increased expression of ICAM-1.
Keywords: borneol, blood-brain barrier, ICAM-1, SD rats, traditional Chinese medicine
Introduction
Intracranial malignant tumor is one of the most common diseases of the nervous system, causing increased intracranial pressure, compression of the brain tissue, and resulting in life-threatening dysfunction of the nervous system. Primary intracranial malignant tumor accounts for about 1.9% of the adult malignancies, of which the most common pathological type is glioma.1 Other malignant tumors eventually appear as brain metastases in about 20% to 30% of cases. Metastatic lung cancer is most common in intracranial malignant tumors.2
Chemotherapy is an important method in the treatment of intracranial malignant tumors. The blood-brain barrier (BBB) is the greatest challenge in treating intracranial malig-nant tumors. How to improve drug permeability across the BBB is a research hotspot. Borneol, molecular formula C10H18O, is a commonly used traditional Chinese medicine in China. It tastes hard, slightly cold, and affects heart, spleen, and lung. Its functions include aromatic resuscitation and promoting increased drug distribution. It can enhance the permeability of various membranes including skin, mucosa, and the BBB.3 Zhang et al4 evaluated the effect of oral administration of borneol on brain-targeted delivery of nanoparticles. Co-incubation of brain capillary endothelial cells with borneol and nanoparticles could significantly enhance the cellular uptake. Recent studies showed that borneol could promote penetration of methotrexate, nimustine, and other drugs through the BBB, increasing drug concentration in the brain.5 The purpose of this study is to further elucidate the role of borneol in the opening of the BBB and delineate the underlying molecular mechanism.
Materials and Methods
Animals and Reagents
This study was conducted in accordance with the institutional guidelines for the care and use of laboratory animals set by Zhejiang Chinese Medical University (Zhejiang, China). Sixty male Sprague-Dawley (SD) rats (300 ± 20 g) were provided by the Laboratory Animal Center of Zhejiang Chinese Medical University (Hangzhou, China). Corn oil and borneol were obtained from Hangzhou Jiu-zhou Pharmacy (Hangzhou, China). Evans blue powder was purchased from Shanghai Kexing Company (Shanghai, China). Secondary antibodies to occludin, occludens-1 (ZO-1), nitric oxide synthase (NOS), P-glycoprotein (P-gp), and intercellular cell adhesion molecule-1 (ICAM-1) were purchased from Beijing Zhongshan Gold Bridge Biological Technology Co, Ltd (Beijing, China). Diaminobenzidine was supplied by Beijing Zhongshan Gold Bridge Biological Technology Co, Ltd (Beijing, China).
Fluorescent Spectrophotometry for Establishment of Evans Blue Standard Curve
An amount of 0.010 g Evans blue was dissolved with formamide in a 100-mL volumetric flask and adjusted to a concentration of 100 µg/mL. Aliquots of 0.25 mL, 0.5 mL, 1 mL, 2 mL, 4 mL, 6 mL, and 8 mL were transferred into a 10-mL volumetric flask and diluted with formamide, respectively. The absorbance (a) was read at 620 nm using 970CRT fluorescent spectrophotometer (Shanghai Sanke Company, Shanghai, China). The regression equation was computed by the absorbance (a) to Evans blue concentration (b) in the solution through zero setting using formamide.
Fluorescent Spectrophotometry for Detection of Evans Blue Content
Twenty male SD rats were randomly divided into a borneol group (10 rats) and a control group (10 rats). The rats in the borneol group were administered with 10% borneol corn oil solution (2 mL/kg) intragastrically, and the rats in the control group were administered with the same dose of corn oil. Thirty minutes later, a 2% Evans blue solution (4 mL/kg) was injected into the rats by tail vein. After 30 minutes, the rats were sacrificed by cervical dislocation, and brain tissue of each (0.3 g) was taken and weighed accurately. Physiological saline (0.9%) was added to the brain tissue at a ratio of 1:19, homogenized, and centrifuged at 4000 rpm/min for 20 minutes. A 0.2-mL supernatant was withdrawn using a pipette and detected using fluorescent spectrophotometry with rat brain homogenate serving as a control for the absorbance. According to the standard curve, the content of EB (ng/mL) in brain tissue was determined.
Immunohistochemistry for Detection of Occludin, ZO-1, NOS, P-gp, and ICAM-1
Forty male SD rats were randomly divided into groups of high-, medium-, and low-dose borneol groups (30 rats) along with a control group (10 rats). The rats were fasted overnight before the experiment, weighed in the morning on the day of experiment. The experimental groups of rats were intragastrically administered with 10% borneol corn oil at the dose of 2 mL/kg, 1.25 mL/kg, and 0.5mL/kg, while the control group was administered with 1.25 mL/kg corn oil. Thirty minutes later, the rats were killed and the brain tissue was extracted into a bottle with formalin fixed liquid and labeled as 1 to 40. The number of specimens in each group was 9, due to failure to obtain the predetermined brain tissue from one rat in each group.
Samples were heated in an oven for 2 hours at 60°C, dewaxed, distilled with water for 2 minutes, followed by high-pressure steam distillation. After blocking with a peroxidase in 3% H2O2 solution for 10 minutes, washing 3 times with phosphate-buffered saline (PBS) for 5 minutes, the mixture was supplemented with a primary antibody and incubated at 37°C for 60 minutes. After PBS washing in the same manner, secondary antibodies to occludin, ZO-1, NOS, P-gp, and ICAM-1, respectively, were added. Occludin and ZO-1 were diluted to 1:50, while NOS, P-gp, and ICAM-1 were diluted to 1:100. After incubation at 37°C for 60 minutes in PBS, samples were washed 3 times with PBS for 5 minutes. Each section was colored by diaminobenzidine for 1 to 2 minutes.
Each slice was photographed microscopically, and the images were analyzed by IPP6.0 image analysis software. The positive area, mean density, and total density were measured.
Statistics
The statistical software SPSS 17 was used for statistical analysis. The values were expressed as the mean ± square error (). The Student’s t test was used to compare the differences between the 2 groups, and P < .05 denoted significant difference. The single factor variance analysis was used to compare the groups.
Results
Standard Curve of Evans Blue
The regression equation—y = a + b * x, with a = 0.00064, b = 0.09425, r2 = 0.99991—showed good linear relationship between Evans blue concentration from 0.05 to 4.5 µg/mL and absorbance values (Figure 1).
Figure 1.
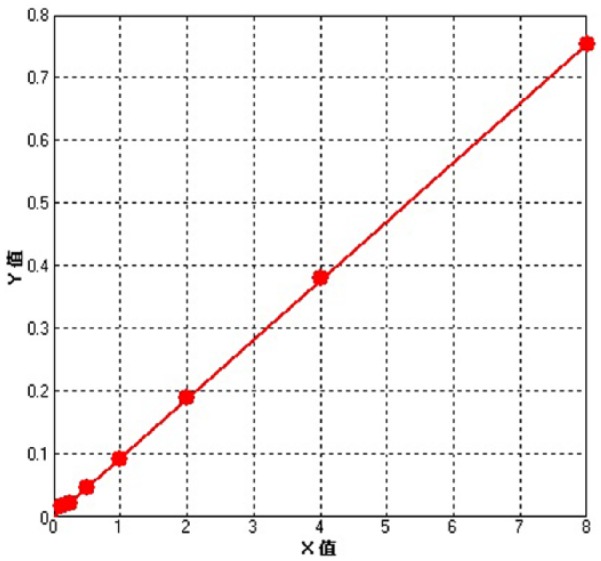
Standard curve of Evans blue.
Evans Blue Content
The optical density value and Evans blue content in the borneol group were higher than in the control group (P < .05; Table 1).
Table 1.
Optical Density and Evans Blue Content in Experimental and Control Groups.
| Groups | n | Optical Density |
Content (µg/mL) |
||
|---|---|---|---|---|---|
| P | P | ||||
| Control group | 10 | 0.0870 ± 0.0164 | 0.9908 ± 0.1639 | ||
| Borneol group | 10 | 0.1350 ± 0.0206 | <.05 | 1.4710 ± 0.2057 | <.05 |
Expression of Occludin, ZO-1, NOS, P-gp, and ICAM-1
No significant difference was observed in the expression of occludin among the various borneol groups or between the different experimental groups and the control group in positive area, mean density, and total density (P > .05; Table 2 and Figure 2). Obvious differences of ZO-1 expression appeared between the experimental and the control groups in mean density (P < .05). However, there was no significant difference in mean density among the 3 different experimental groups (P > .05). No significant difference was shown in ZO-1 expression among the different borneol groups or between the experimental borneol groups and the control group in positive area (P > .05) and total density (P > .05; Table 3 and Figure 2).
Table 2.
Comparison of Occludin Expression.
| Groups | n | Positive Area (µm2) () | Mean Density () | Total Density () |
|---|---|---|---|---|
| Control group | 9 | 35655.00 ± 22156.226 | 0.2412 ± 0.0283 | 1.0368 ± 6.9716 |
| High group | 9 | 28076.78 ± 17744.036** | 0.2293 ± 0.0118** | 7.6383 ± 5.1534** |
| Medium group | 9 | 29668.33 ± 15516.837** | 0.2276 ± 0.0139** | 8.1874 ± 4.6320** |
| Low group | 9 | 25405.40 ± 10894.482** | 0.2246 ± 0.0154** | 6.9441 ± 3.1402** |
P < .05, **P > .05 versus control group.
Figure 2.
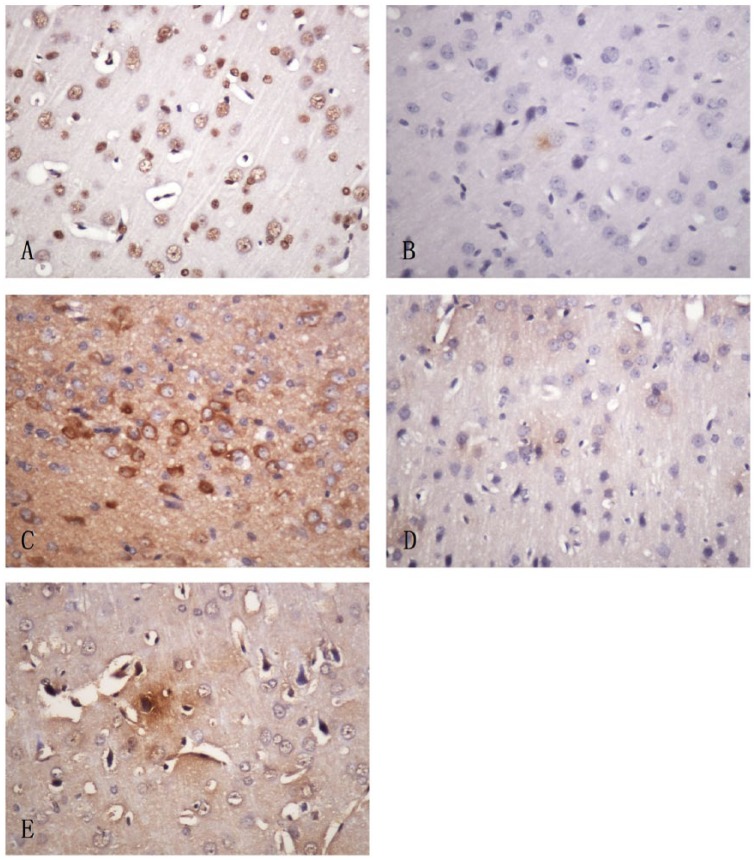
The expression of occludin, ZO-1, NOS, P-gp, and ICAM-1 detected by immunochemistry.
(A) Occluding, mainly located in the nucleus of glial cells and neurons. (B) ZO-1, mainly located in the cytoplasm of glial cells and neurons. (C) NOS, mainly located in the cytoplasm of neurons and vascular endothelial cells. (D) P-gp, mainly located in the cytoplasm of neurons. (E) ICAM-1, mainly located in the cytoplasm of vascular endothelial cells.
Table 3.
ZO-1 Expression in Different Groups.
| Groups | n | Positive Area (µm2) () | Mean Density () | Total Density () |
|---|---|---|---|---|
| Control group | 9 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| High group | 9 | 2780.22 ± 5367.739** | 0.07 ± 0.071* | 472.74 ± 914.655** |
| Medium group | 9 | 5323.11 ± 7703.858** | 0.11 ± 0.118* | 1324.52 ± 2263.061** |
| Low group | 9 | 2371.56 ± 3768.895** | 0.07 ± 0.088* | 476.61 ± 773.918** |
P < .05, **P > .05 versus control group.
There was no significant difference in NOS expression between the experimental groups and the control group in terms of positive area, mean density, and total density (P > .05). Meanwhile, no significant differences occurred among the different borneol groups (P > .05; Table 4 and Figure 2).
Table 4.
Comparison of Nitric Oxide Synthase Expression.
| Groups | n | Positive area (µm2) () | Mean Density () | Total Density () |
|---|---|---|---|---|
| Control group | 9 | 105965.44 ± 120981.123 | 0.1719 ± 0.0023 | 2.0543 ± 2.4546 |
| High group | 9 | 128179.00 ± 179867.052** | 0.1428 ± 0.0838** | 2.6384 ± 3.8538** |
| Medium group | 9 | 70409.00 ± 105006.001** | 0.1540 ± 0.0580** | 1.4647 ± 2.2926** |
| Low group | 9 | 74895.56 ± 118527.556** | 0.1752 ± 0.0077** | 1.5686 ± 2.6899** |
P > .05 versus control group.
No apparent differences were seen in P-gp expression among the different experimental groups or between the experimental groups and the control group in positive area, mean density, and total density (P > .05; Table 5 and Figure 2).
Table 5.
Comparison of P-Glycoprotein Expression.
| Groups | n | Positive Area (µm2) () | Mean Density () | Total Density () |
|---|---|---|---|---|
| Control group | 9 | 6.44 ± 19.333 | 0.02 ± 0.065 | 1.39 ± 4.171 |
| High group | 9 | 70.33 ± 159.949** | 0.08 ± 0.095** | 30.678 ± 10.226** |
| Medium group | 9 | 18.78 ± 55.213** | 0.06 ± 0.096** | 9.923 ± 3.308** |
| Low group | 9 | 1.78 ± 3.833** | 0.04 ± 0.087** | 0.708 ± 0.236** |
P > .05 versus control group.
As for the expression of ICAM-1, Table 6 and Figure 2 show that there was a significant difference between high-, medium-, and low-dose borneol groups and the control group in mean density (P < .05), and more significant especially for the high-dose group. However, significant difference occurred only between the high-dose borneol group and the control group in positive area and total density (P < .05). And significant difference was observed between the high-dose borneol group and the low-dose group in total density (P < .05). However, neither in positive area nor mean density was significant difference seen between the different borneol groups (P > .05).
Table 6.
Comparison of Intercellular Cell Adhesion Molecule-1 Expression.
| Groups | n | Positive area (µm2) () | Mean Density () | Total Density () |
|---|---|---|---|---|
| Control group | 9 | 540.78 ± 968.002 | 0.1371 ± 0.1041 | 1.3090 ± 2.3879 |
| High group | 9 | 54815.78 ± 75885.329* | 0.2117 ± 0.0281* | 1.3545 ± 1.6164* |
| Medium group | 9 | 20072.22 ± 14032.406** | 0.2115 ± 0.0265* | 5.7597 ± 4.0004** |
| Low group | 9 | 13294.33 ± 7282.652** | 0.1980 ± 0.0127* | 4.0023 ± 2.3294** |
P < .05, **P > .05 versus control group.
Discussion
The BBB is the most important barrier in brain-targeted delivery. In 1885, Ehrlich found that intravenously injected dye could stain most organs except the brain.6,7 Therefore, methods to test whether the BBB is opened became a new field of study. In some experiments,8,9 Evans blue became a common method to test whether the BBB is opened or not. In brain tumors, drug distribution to brain tumor is more restricted than in peripheral tumors. For example, Taskar et al found the lapatinib concentration in lung metastasis tissue is 5.15-fold higher than that in brain metastasis, in their metastatic breast tumor-bearing mouse model.10 Chen et al found that borneol opened the BBB, promoted diffusion via pinocytosis by increasing the number and volume of pinosomes.11 Jin et al found that borneol increased the permeability of the blood-eye barrier, by altering the distribution of transmembrane proteins claudin-5 and occludin in blood-optic nerve.12
This study further investigated the role of borneol in transport across the BBB and determined the underlying mechanisms. Data show that Evans blue served as an effective marker in evaluating the permeability across BBB.13 Evans blue is an azo dye that has a very high affinity for serum albumin. Because of this, it can be useful in physiology in estimating the proportion of body water contained in blood plasma. It is used to assess the permeability of the BBB to macromolecules. Because serum albumin cannot cross the barrier and virtually all Evans blue is bound to albumin, normally the neural tissue remains unstained.14 When the BBB has been compromised, albumin-bound Evans blue enters the central nervous system. Evans blue was also used to evaluate the role of borneol in opening BBB in this study, and the results were consistent with previous study.15
BBB’s tight junction is located in the adjacent microvascular endothelial cells of the brain, comprising the transmembrane protein family of claudins, occludin, and the surrounding membrane proteins including ZO-1s and adhesion molecules among others, maintaining the structural integrity of BBB cells. ZO-1 and occludin are both protein biomarkers of tight junctions. Their expression induces the opening of closed connections and increases the cellular permeability of BBB.16 Incubating lexiscan with bEnd.3 cell monolayers could decrease the transendothelial cell electrical resistance and diminish the expression of occludin, caudin-5, and ZO-1, which were all essential for keeping tight junction integrity.17 Gao et al18 found the expression of ZO-1 in untreated bEnd.3 monolayers was continuously aligned on the cell-cell interface, while conjugating 16 of lexiscan molecules onto 1 dendrimer (Den-Reg16) treatment led to discontinued expression even in the absence of ZO-1, resulting in 17.6-fold increase of permeability of the model drug and 68% reduction of transendothelial cell electrical resistance. Downregulation of protein biomarkers opens the tight junctions and increases the permeability of BBB.
Our results indicate that the expression of occludin located in the brain microvascular endothelial cells is not altered after borneol administration. The average density of ZO-1 in the borneol groups was higher than in the control group. However, no significant differences in positive area or total density were seen between the experimental and the control groups. These results suggested that occludin and ZO-1 may not play a major role in borneol-mediated opening of the BBB.
The NOS catalyzes the NO synthesis from L-arginine. NO opens the BBB.19 The study of Banks showed the pathway of Ca2+-NOS-NO and VEGF-PI3K-Akt-eNOS-NO are not the only 2 pathways to activate NO on the BBB; astrocytes can also induce endothelial cell synthesis by releasing tumor necrosis factor.20
P-gp is a transmembrane glycoprotein with a relative molecular weight of 170 kD. It is encoded by the multidrug-resistance gene and belongs to the superfamily of ATP-binding cassette transporter protein. P-gp is an ATP-dependent efflux pump, which eliminates intracellular drugs to decrease their cellular concentrations. Studies suggested that the opening of the BBB by borneol reduces the expression of P-gp in the BBB,21 which was inconsistent with our study findings.
ICAM-1 belongs to the immunoglobulin superfamily of adhesion molecules. ICAM-1 on the surface of brain microvascular endothelial cell mainly regulates adhesion and migration of leukocytes. A number of free radicals and cytotoxins are released when leukocytes adhere to the vascular endothelium, following endothelial cell damage and BBB opening.22 Yun’s study showed that bloodletting puncture treatment could reduce water content of brain and the permeability of the BBB caused by ischemic stroke through the model of permanent middle cerebral artery occlusion. It was demonstrated that the expression levels of ICAM-1 and VEGF were downregulated.23 That means the suppression of expression levels of ICAM-1 can attenuate BBB disruption, protecting cerebral microvessel structure. Another study found the same results through the mouse model of experimental autoimmune encephalomyelitis. It proved that resveratrol can protect the integrity of the BBB in experimental autoimmune encephalomyelitis mice by repressing adhesion proteins ICAM-1 and vascular cell adhesion molecule-1.24
Our study suggests that the average density of ICAM-1 in high-, medium-, and low-dose borneol groups was higher than in the control group, and the positive area and the total density in the high-dose group are also higher than in the control group. We believe that borneol enhances the expression of ICAM-1 on the BBB. The increased expression of ICAM-1 probably promotes the adhesion and migration of leukocytes and increases the release of free radicals and cell toxins after adherence to the vascular endothelial cells, which aggravates the endothelial cell damage and promotes the opening of the BBB. Our study suggests that borneol can increase the expression of ICAM-1, and then lead to the opening of BBB, which is represented by the content of Evans blue dye. The increase of the expression of ICAM-1 and the opening of the BBB in our experiment are in accordance with Kong’s study in which the reduction of ICAM-1 decreased the opening of the BBB.22 Taken together, our findings demonstrated that borneol can open the BBB and might be related with the increased expression of ICAM-1. The mechanism of how ICAM-1 opens the BBB needs to be further verified and will be our next study.
The BBB can prevent the entry of drugs into the brain. The ability of borneol to open the BBB could be used for tumor treatment, repair of brain injury, treatment of brain infections, and chronic diseases of nervous system. The prospects of application of borneol would be of great significance.25
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Natural Science Foundation of China (81202806), Zhejiang Provincial Natural Science Foundation of China (No. Y2110004), Zhejiang Province Traditional Medical Science Fund Project of China (No. 2014ZB021, No. 2015ZA037), and the 1022 Talent Training Program of Zhejiang Cancer Hospital.
ORCID iDs: Tao Wu  https://orcid.org/0000-0003-4821-5740
https://orcid.org/0000-0003-4821-5740
Qiaoyuan Cheng  https://orcid.org/0000-0002-2862-2886
https://orcid.org/0000-0002-2862-2886
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [DOI] [PubMed] [Google Scholar]
- 2. Alexandru D, Bota DA, Linskey ME. Epidemiology of central nervous system metastases. Prog Neurol Surg. 2012;25:13-29. [DOI] [PubMed] [Google Scholar]
- 3. Bhatia SP, McGinty D, Letizia CS, Api AM. Fragrance material review on l-borneol. Food Chem Toxicol. 2008;46(suppl 11):S81-S84. [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Han L, Qin J, Lu W, Wang J. The use of borneol as an enhancer for targeting aprotinin-conjugated PEG-PLGA nanoparticles to the brain. Pharm Res. 2013;30:2560-2572. [DOI] [PubMed] [Google Scholar]
- 5. Xin HL, He XR, Li W, Zhou ZD, Zhang S, Wang GJ. The effect of borneol on the concentration of meropenem in rat brain and blood. J Asian Nat Prod Res. 2014;16:648-657. [DOI] [PubMed] [Google Scholar]
- 6. Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood-brain barrier. Int J Nanomedicine. 2014;9:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eyal S, Hsiao P, Unadkat JD. Drug interactions at the blood-brain barrier: fact or fantasy? Pharmacol Ther. 2009;123:80-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miao XM, Cheng SX, Yang Z, et al. Therapeutic bloodletting at Jing-well points combine hypothermia attenuated acute cerebral edema after traumatic brain injury in rats [in Chinese]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2015;31:249-253. [PubMed] [Google Scholar]
- 9. Chen X, Zhao Z, Chai Y, Luo L, Jiang R, Zhang J. The incidence of critical-illness-related-corticosteroid-insufficiency is associated with severity of traumatic brain injury in adult rats. J Neurol Sci. 2014;342:93-100. [DOI] [PubMed] [Google Scholar]
- 10. Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29:770-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen YM, Wang NS. Effect of borneol on the intercellular tight junction and pinocytosis vesicles in vitro blood-brain barrier model [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:632-634. [PubMed] [Google Scholar]
- 12. Jin D, Wang F, Qu L, et al. The distribution and expression of claudin-5 and occludin at the rat blood-optic nerve barrier after borneol treatment. Mol Biol Rep. 2011;38:913-920. [DOI] [PubMed] [Google Scholar]
- 13. Do J, Foster D, Renier C, et al. Ex vivo Evans blue assessment of the blood brain barrier in three breast cancer brain metastasis models. Breast Cancer Res Treat. 2014;144:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hawkins BT, Egleton RD. Fluorescence imaging of blood-brain barrier disruption. J Neurosci Methods. 2006;151:262-267. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Q, Wu D, Wu J, et al. Improved blood-brain barrier distribution: effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J Ethnopharmacol. 2015;162:270-277. [DOI] [PubMed] [Google Scholar]
- 16. Jia W, Martin TA, Zhang G, Jiang WG. Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Res. 2013;33:2353-2359. [PubMed] [Google Scholar]
- 17. Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci. 2011;31:13272-13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao X, Qian J, Zheng S, et al. Overcoming the blood-brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS Nano. 2014;8:3678-3689. [DOI] [PubMed] [Google Scholar]
- 19. Su YF, Lin CL, Lee KS, et al. A modified compression model of spinal cord injury in rats: functional assessment and the expression of nitric oxide synthases. Spinal Cord. 2015;53:432-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275-292. [DOI] [PubMed] [Google Scholar]
- 21. Yu B, Ruan M, Dong X, Yu Y, Cheng H. The mechanism of the opening of the blood-brain barrier by borneol: pharmacodynamics and pharmacokinetics combination study. J Ethnopharmacol. 2013;150:1096-1108. [PubMed] [Google Scholar]
- 22. Kong QX, Wu ZY, Chu X, Liang RQ, Xia M, Li L. Study on the anti-cerebral ischemia effect of borneol and its mechanism. Afr J Tradit Complement Altern Med. 2013;11:161-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu N, Wang Z, Chen Y, et al. The ameliorative effect of bloodletting puncture at hand twelve Jing-well points on cerebral edema induced by permanent middle cerebral ischemia via protecting the tight junctions of the blood-brain barrier. BMC Complement Altern Med. 2017;17:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang D, Li SP, Fu JS, Zhang S, Bai L, Guo L. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J Neurophysiol. 2016;116:2173-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437-449. [DOI] [PubMed] [Google Scholar]