Abstract
Objective: This study was designed to assess the feasibility of using the Jaw Dynasplint System as an adjunct to conventional stretching exercises as a preventative measure against trismus in patients undergoing radiotherapy. Methods: Study participants (n = 40) were randomized using a permuted block design to conventional stretching or stretching plus use of the Jaw Dynasplint 3 times per day for 30 minutes. Patients were instructed to record maximum interincisal opening each day as well as logging use of the Jaw Dynasplint. Results: At 6 months after initiation of the preventative regimen, 50% of patients in the Dynasplint arm and 75% in the conventional stretching arm remained on their assigned therapy. Trismus was diagnosed in 2 patients in the control arm and in 4 patients in the Dynasplint arm. Only 25% (95% confidence interval = 11.1, 46.9) of patients in the Dynasplint arm used the device as prescribed. Conclusions: The addition of the Jaw Dynasplint decreased compliance compared with conventional stretching. It is unlikely that the prescribed regimen will prove efficacious as a preventative measure due to low compliance.
Keywords: trismus, Dynasplint, physical therapy, fibrosis, head and neck cancer
Introduction
Limited jaw opening, trismus, is one of a number of changes in oral function often experienced by head and neck cancer patients as a result of cancer therapy. It can result from impairment of any neural, vascular, or muscular part of the body normally responsible for jaw mobility. In the head and neck cancer population, trismus is often the result of tumor invasion, surgical defect, or fibrosis.1-3 Diminished ability to open the mouth can result in malnutrition, speech difficulties, decreased oral hygiene, inability to receive dental treatment, jaw pain, and other adverse effects, and can, therefore, be very debilitating.
The proportion of head and neck cancer patients experiencing trismus has been reported to vary from 5% to 79%,4-10 based on a number of factors including the dose of radiation delivered to the muscles of mastication, tumor site, T stage, and history of surgery.1,4,7,8,11-14 Trismus is traditionally considered to be a late effect of cancer therapy with functional deficits becoming evident in the first year after completing radiation therapy.15 These deficits can rapidly progress to a state of functional impairment.
The most common measurement associated with trismus is maximum interincisal opening (MIO), in which the distance between the incisal edges of a patient’s upper and lower central incisors is measured with the mouth open as widely as possible. MIO as measured by the clinician is considered the gold standard for the assessment of jaw opening, and it has been shown to be highly correlated with patient measurement.16,17 Normal MIO for men is 50 to 60 mm and 45 to 55 mm for women.18 A common definition of trismus related to when patients often experience functional deficits is the following: mild trismus 30 to 35 mm, moderate trismus 25 to 30 mm, and severe trismus <25 mm.18
The most common treatment for trismus is physical therapy consisting of active range of motion (ROM) exercises, hold and relax techniques, and manual stretching. Once trismus has developed, it may be hard to reverse. Thus, prevention with a program of self-care is important for managing this side effect. This includes education about trismus as a side effect of therapy, the need for routine monitoring of jaw ROM, and the routine use of stretching to maintain jaw ROM. A number of adjunctive devices have been studied to determine if they add to standard self-care. These include stacked tongue depressors,18 TheraBite Jaw Motion Rehabilitation System,19-21 and the Jaw Dynasplint System.22-24 A trial that implemented the TheraBite system, a high-load stretching device, as a preventative intervention failed to demonstrate feasibility or efficacy when compared with usual care with no standardized exercise regimen.25 It can be postulated that mechanical trauma from a high-pressure device may augment the damage resulting from the cancer therapy. The Jaw Dynasplint System is a low-load, prolonged-duration stretching device, which has been shown in retrospective studies to improve MIO in post-radiotherapy head and neck cancer patients with established trismus.24 It was, therefore, hypothesized that the Jaw Dynasplint System, if feasible for implementation as a preventative measure, might be more effective.
The primary goal of the study was to assess the feasibility of incorporating the use of the Jaw Dynasplint into a standard program of self-care for the prevention of trismus in head and neck cancer patients undergoing primary or adjuvant radiation.
Materials and Methods
Study Design
This study was designed as a pilot randomized trial between the Jaw Dynasplint and a standard trismus self-care program versus a control arm consisting of a standard trismus self-care program alone. This design allowed for assessment of the dropout rate and compliance with Jaw Dynasplint treatment. The control arm was included primarily to provide an estimate of efficacy and the variability of changes in MIO among patients in both treatment arms for use in powering future efficacy trials.
The Vanderbilt University Institutional Review Board approved this study. Forty head and neck cancer patients at high risk for trismus who were planned for either primary or adjuvant radiation with or without chemotherapy were randomized to a jaw stretching regimen or jaw stretching regimen plus the Jaw Dynasplint between August of 2012 and June 2014. The randomization sequence was generated by the statistician and provided to the study coordinator after the patient was enrolled and had their initial assessment for eligibility. After study enrollment, patients were randomized via a computer-generated, permuted (1:1) block program.
Inclusion and Exclusion Criteria
Eligibility criteria included the following: (1) histologically proven head and neck cancer of any histopathology, (2) concurrent chemoradiotherapy, (3) baseline MIO ≥35 mm, and (4) planned primary or adjuvant radiation treatment with ≥50 Gy delivered to a total volume of at least 2 cc to the mastication muscles, unilaterally or bilaterally, over the entire course of radiation treatment. Exclusion criteria included the following: (1) collagen vascular disorders predisposing to radiation fibrosis and (2) having postsurgical oral health status that precluded the use of the device. All patients signed informed consent prior to participating in study-related activities.
Treatment Arms
Control arm patients were provided written educational material on the importance of jaw ROM, taught exercises to maintain jaw ROM, and provided with interincisal distance measurement cards for self-monitoring. The jaw stretching regimen consisted of stretching the mouth open and laterally for a 30-second hold; moving the jaw in a circle with 5 repetitions in each direction; passive stretching by applying downward pressure on the mandible with the index finger held for 30 seconds; and circular jaw massage for 30 seconds. The control arm recorded a daily MIO measurement from baseline to 6 months posttreatment. If trismus developed at any time during the study period, patients on the control arm (MIO ≥35 mm) were provided with access to the Jaw Dynasplint as a therapeutic modality.
Patients in the device arm were instructed on the above-mentioned jaw stretching regimen plus adjunctive use of the Jaw Dynasplint. The patients utilizing the Jaw Dynasplint were instructed to wear the device for 30 minutes, 3 times a day, during cancer treatment and the early recovery period (3 months posttreatment). All patients completed daily compliance logs during the study period from baseline to 3 months posttreatment, in which they recorded their self-assessed MIO. The Dynasplint arm patients’ logs also included the length of time wearing the device during each of the prescribed treatment sessions and barriers to wearing the device.
Evaluation of Endpoints
Evaluations were performed by study personnel at baseline, weekly during radiation therapy, and at 1, 2, 3, and 6 months posttreatment. The following were completed at baseline and each study visit: Vanderbilt Head and Neck Symptom Survey plus General Symptom Survey version 2.0 (VHNSS v2.0 plus GSS) and MIO measurement by study staff.26-29 Study staff measured MIO 2 times in the seated position by using an interincisal measuring card demarcated in millimeters in increments. Patients were instructed in the use of these cards, and it was demonstrated for them at their baseline exam.
Statistical Methods
Patient demographic and treatment characteristics were summarized using descriptive statistics and compared using χ2 tests of independence (nominal data), Mann-Whitney tests (radiation cycles and dose), and independent t tests (age, education). Feasibility was measured in terms of recruitment, retention, compliance, barriers, and safety. Weekly summaries of the compliance rates were plotted to explore possible critical periods during treatment for which compliance may be expected to drop or recover. Study retention and incidence of trismus were summarized for each of the study groups and compared using the likelihood-ratio statistic. Descriptive and graphical summaries of the weekly measured symptoms (VHNSS v2.0 plus GSS) were generated. Tests of differences between the groups and in patterns of change over the treatment and 6-month posttreatment study period were conducted using mixed-effects generalized linear models. Statistical significance conclusions were based on a type I error rate of .05.
The primary purpose of this study is to assess feasibility by measuring compliance and secondarily to inform the potential efficacy of the Jaw Dynasplint for the prevention of trismus in this population, not to formally test a hypothesis about that efficacy. Given an expected trismus incidence rate of 50%, which was assumed based on literature review of previous studies in high-risk cohorts,4-10 a clinically significant reduction to 25% was proposed to be clinically significant. Given the proposed sample size, the proportion of patients in the treatment arm who fully comply with the prescribed regimen can be estimated with a maximum standard error of 0.11, and a decrease in trismus development to 15% would be required to achieve 80% power with a 1-tailed α = .05.
Results
Patient Characteristics
Key patient characteristics are reported in Table 1.
Table 1.
Patient Characteristics by Study Arm. Average Radiation Is the Average Radiation Dose Over 3 Points in the Muscles of Mastication in cGy.
| Total (N = 40) |
Control (N = 20) |
Intervention (N = 20) |
P | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 57.4 (11.6) | 57.7 (13.4) | 57.0 (9.9) | .735 |
| Education (years) | 13.4 (3.2) | 13.1 (3.4) | 13.7 (3.0) | .384 |
| n (%) | n (%) | n (%) | 1.000 | |
| Gender | ||||
| Female | 4 (10.0) | 2 (10.0) | 2 (10.0) | |
| Male | 36 (90.0) | 18 (90.0) | 18 (90.0) | |
| Race | .214 | |||
| American Indian/Alaskan Native | 1 (2.6) | 0 (0.0) | 1 (5.0) | |
| Black or African American | 2 (5.1) | 2 (10.5) | 0 (0.0) | |
| White | 36 (92.3) | 17 (89.5) | 19 (95.0) | |
| Cancer site | .406 | |||
| Nasopharynx | 2 (5.0) | 2 (10.0) | 0 (0.0) | |
| Oropharynx | 34 (85.0) | 17 (85.0) | 17 (85.0) | |
| Hypopharynx | 1 (2.5) | 0 (0.0) | 1 (5.0) | |
| Salivary gland | 2 (5.0) | 1 (5.0) | 1 (5.0) | |
| Other | 1 (2.5) | 0 (0.0) | 1 (5.0) | |
| Stage | .090 | |||
| II | 2 (5.0) | 2 (10.0) | 0 (0.0) | |
| III | 4 (10.0) | 3 (15.0) | 1 (5.0) | |
| IVA | 29 (72.5) | 11 (55.0) | 18 (90.0) | |
| IVB | 5 (12.5) | 4 (20.0) | 1 (5.0) | |
| Surgery | .736 | |||
| No | 27 (67.5) | 14 (70.0) | 13 (65.0) | |
| Yes | 13 (32.5) | 6 (30.0) | 7 (35.0) | |
| Total treatment | .614 | |||
| Induction + chemoradiation | 16 (40.0) | 7 (35.0) | 9 (45.0) | |
| Chemoradiation | 11 (27.5) | 6 (30.0) | 5 (25.0) | |
| Surgery + chemoradiation | 9 (22.5) | 6 (30.0) | 3 (15.0) | |
| Surgery + induction + chemoradiation | 3 (7.5) | 1 (5.0) | 2 (10.0) | |
| Surgery + induction + chemoradiation + brachytherapy | 1 (2.5) | 0 (0.0) | 1 (5.0) | |
| Median [IQR] (Min, Max) | Median [IQR] (Min, Max) | Median [IQR] (Min, Max) | ||
| Radiation cycles | 6.0 [5-7] (2-7) | 6.0 [5-7] (2,7) | 6.0 [4-7] (2,7) | .910 |
| Average radiation dose (cGy) | 6.058 [4207-6300] (2297, 8867) | 6.043 [3990-6300] (2310, 6380) | 6.116 [5037-6300] (2297, 8867) | .332 |
Abbreviations: IQR, interquartile range; min, minimum; max, maximum.
Feasibility
Recruitment
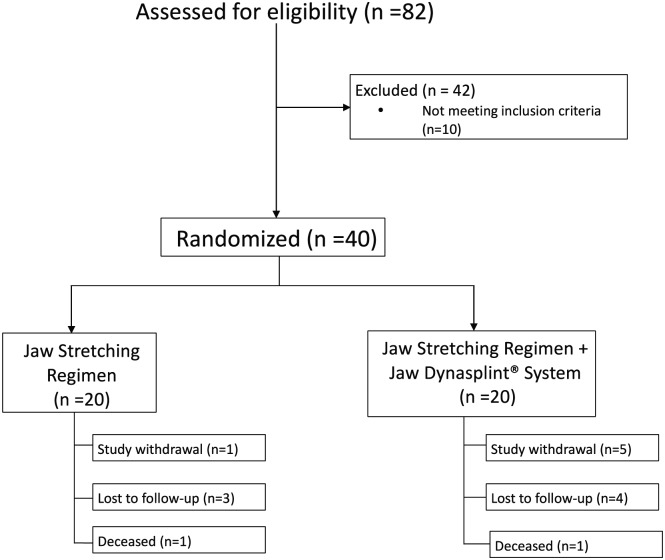
During the accrual period, 82 consecutive patients were invited to participate in the study (Figure 1). Thirty-two declined study participation, and there were 10 screening failures. Of the screening failures, 2 had preexisting mild trismus (MIO <35 mm) and 6 patients were planned to receive radiation doses below the defined dosimetric inclusion criteria. A total of 40 patients were recruited over a period of 14 months.
Figure 1.
Patient enrollment diagram.
Retention
Retention remained relatively high in the control arm throughout the duration of the study. Of the 20 patients enrolled in the control group, 16 patients (80%) remained on study at the completion of radiation. Moreover, 15 of 20 patients (75%) remained on study at 6 months postradiation treatment.
Retention in the device arm was substantially lower: at week 4 of radiation, only 13 of 20 patients (65%) remained in the study, falling to a low of 10 (50%) patients at the end of treatment and 6 months posttreatment, P = .100.
Reasons for study withdrawal included the following: control arm = 1 unwilling to continue study participation, 3 lost to follow-up, and 1 deceased; device arm = 5 unwilling to continue study participation, 4 lost to follow-up, and 1 deceased.
Compliance
Compliance was defined as the number of days completing the daily log of MIO measurements. For the first 7 weeks on study, the control arm patients recorded more MIO than did those in the device arm, P = .023. The median number of days of assessment was 40 in the control arm (interquartile range = 27-44 days, minimum = 0, maximum = 49) compared with 25 in the device arm (interquartile range = 2-38 days, minimum = 0, maximum = 43). Patients in the device arm experienced difficulty adhering to the Jaw Dynasplint regimen. Only 5 of 20, 25% (11.1, 46.9), patients in the device arm were able to comply with use of the device at the prescribed schedule of 30 minutes a day, 3 times a day. After week 3, less than 50% patients reported using the Jaw Dynasplint for any period of time. Barriers to Jaw Dynasplint use included mucositis pain, gagging, fatigue, and device fit issues.
Safety
No adverse events were reported in either study arm.
Efficacy
The overall incidence of moderate and severe trismus (MIO ≤30 mm) was 15% (7.1, 29.1). Two patients in the control arm and 4 patients in the device arm developed trismus (MIO ≤35 mm), odds ratio = 2.72 (0.36, 13.97), P = .372. Trismus was diagnosed during the active treatment and early recovery period in 1 patient in the control arm (MIO = 30 mm) and 4 patients in the device arm (MIO = 25-30 mm). A second patient in the control arm developed trismus (MIO = 22 mm) at 6 months posttreatment.
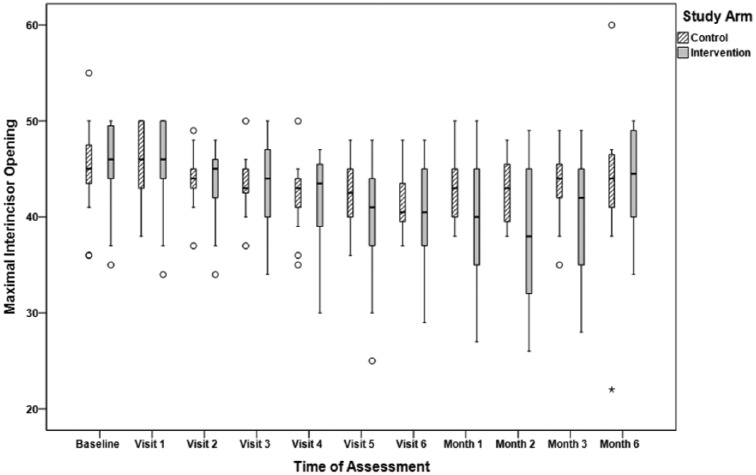
Summaries of the MIO measured by research staff at each study visit are displayed in Figure 2. There was a statistically significant difference between the groups in the patterns of MIO change over treatment and 6 months posttreatment, P = .019. At baseline, MIO was similar for the control arm (median MIO = 45 mm) and the device arm (median MIO = 46 mm). During active treatment, MIO declined in both arms (median MIO at week 7 in both arms, 40.5 mm). By 6 months posttreatment, patients in both arms had returned to near baseline MIO (median, control arm: 44 mm, device arm: 44.5 mm; Table 2).
Figure 2.
Maximum interincisal opening (mm) measured by study personnel.
Table 2.
Maximum Interincisal Opening (MIO) at Key Study Assessment Points by Study Arm. C, Control; I, Intervention.
| Posttreatment (Months) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
1 |
3 |
6 |
|||||
| C | I | C | I | C | I | C | I | |
| N | 19 | 19 | 17 | 13 | 16 | 13 | 15 | 10 |
| Percentile | ||||||||
| 10th | 36.0 | 37.0 | 38.8 | 29.8 | 37.1 | 28.8 | 31.6 | 34.4 |
| 25th | 43.0 | 44.0 | 40.0 | 34.5 | 41.5 | 34.5 | 40.0 | 39.5 |
| 50th | 45.0 | 46.0 | 43.0 | 40.0 | 44.0 | 42.0 | 44.0 | 44.5 |
| 75th | 48.0 | 50.0 | 45.0 | 46.0 | 45.8 | 46.0 | 47.0 | 49.3 |
| 90th | 50.0 | 50.0 | 47.6 | 49.2 | 49.0 | 48.6 | 52.2 | 50.0 |
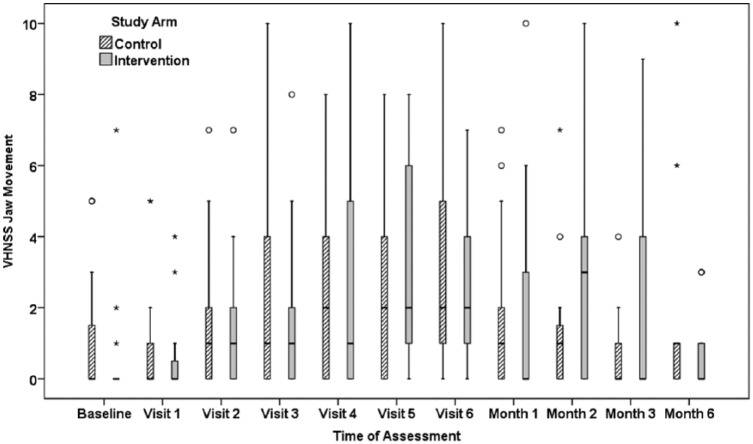
The perception of decreased jaw ROM was assessed with the VHNSS questionnaire item “I have limitations in the ability to open or move my jaw.” The possible range of responses is 0 “None” to 10 “Severe.” The patient reports are summarized in Figure 3. As with the MIO, there was a statistically significant difference between the groups in the patterns of patient reports over the treatment phase and 6 months posttreatment, P = .018.
Figure 3.
Patient-reported limitations in jaw movement via item 49 of the Vanderbilt Head and Neck Symptom Survey.
Discussion
Our study demonstrated that the use of the Jaw Dynasplint for 30 minutes, 3 times a day, during primary or adjuvant radiation in head and neck cancer patients is not feasible as a preventative intervention. Compliance decreased over the course of radiation to the point that only 50% of patients used the Dynasplint for any length of time at the end of treatment. Furthermore, a substantial number of patients declined participation in the study after having it described to them reflecting a baseline apprehension to the commitment to use the device. Among patients randomized to Jaw Dynasplint therapy, the barriers that the patients identified to using the device are common in head and neck cancer patients, and therefore, the lack of compliance is likely to generalize. Thus, further investigation of the Jaw Dynasplint as an adjunctive measure in combination with standard self-care measures will likely be limited in efficacy due to noncompliance. Future studies may choose to examine the preventative efficacy Dynasplint alone versus the standard regimen of stretching to reduce patient burden.
Of note, the incidence of trismus was low in both arms despite the fact that we selected for a high-risk patient population who were planned for ≥50 Gy of radiation to the muscles of mastication. Based on prior reports using cross-sectional data, we anticipated that trismus would develop in between 20% and 60% of patients.4-10 One potential reason for the low trismus rate is that the study concluded 6 months postradiation therapy and that the follow-up duration may have been inadequate. Countering this argument is prospective data would indicate that a significant percentage of patients who develop trismus do so in the early postradiation period.15 A second possible explanation is that the general level of awareness regarding trismus as a long-term complication of head and neck cancer therapy has increased over time. It is now considered standard of care to provide patients with education materials on trismus and preventive jaw stretching exercises as a component of standard of care. The results of this study imply that a systematic approach to education and monitoring may lead to increased awareness among patients of trismus as a late toxicity resulting in increased compliance with standard jaw ROM exercise and resultant low rates of trismus. The low event rate in the standard of care arm further implies that it is unlikely that an additional intervention above and beyond a self-care program consisting of systematic education, careful monitoring, and routine stretching will improve long-term outcomes.
Limitations
It is possible that patients on the Jaw Dynasplint arm either overreported or underreported their use of the device. The measurement of MIO was used as a surrogate marker for compliance in the standard of care arm. It is possible that the patients measured their ROM without actually having completed their stretching exercises. Unlike activities such as walking, for which objective measures are available, there are no available methods to ensure the accurate patient report of jaw exercises. In addition, patients that elected not to participate in the study may have differed from those that did in terms of their risk for trismus and likelihood of compliance.
Conclusions
Patients undergoing head and neck cancer therapy have a tremendous symptom burden to contend with requires complex supportive care regimens. This limits how much inconvenience can be introduced into their daily preventative regimen. This study demonstrates that a stretching regimen as a no-cost intervention is plausible and that compliance can be good when paired with appropriate trismus education. Future investigations into the Jaw Dynasplint or other stretching adjuncts as preventatives should be aware of and not underestimate the impact of the additional time and effort any interventional supplement may require on the part of the already heavily taxed patient.
Acknowledgments
We would like to acknowledge our research nurse, Melissa Adair, RN. Dynasplint Sytems, Inc, provided all Jaw Dynasplint Systems for the clinical trial.
Footnotes
Authors’ Note: Mary S. Dietrich is also affiliated to Vanderbilt University Medical Center, Nashville, TN, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Scott B, Butterworth C, Lowe D, Rogers SN. Factors associated with restricted mouth opening and its relationship to health-related quality of life in patients attending a maxillofacial oncology clinic. Oral Oncol. 2008;44:430-438. [DOI] [PubMed] [Google Scholar]
- 2. Teguh DN, Levendag PC, Voet P, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck. 2008;30:622-630. [DOI] [PubMed] [Google Scholar]
- 3. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38:E1-E10. [DOI] [PubMed] [Google Scholar]
- 4. Kent ML, Brennan MT, Noll JL, et al. Radiation-induced trismus in head and neck cancer patients. Support Care Cancer. 2008;16:305-309. [DOI] [PubMed] [Google Scholar]
- 5. Johnson J, van As-Brooks CJ, Fagerberg-Mohlin B, Finizia C. Trismus in head and neck cancer patients in Sweden: incidence and risk factors. Med Sci Monit. 2010;16:CR278-CR282. [PubMed] [Google Scholar]
- 6. Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35:337-342. [DOI] [PubMed] [Google Scholar]
- 7. Lee R, Slevin N, Musgrove B, Swindell R, Molassiotis A. Prediction of post-treatment trismus in head and neck cancer patients. Br J Oral Maxillofac Surg. 2012;50:328-332. [DOI] [PubMed] [Google Scholar]
- 8. Scott B, D’Souza J, Perinparajah N, Lowe D, Rogers SN. Longitudinal evaluation of restricted mouth opening (trismus) in patients following primary surgery for oral and oropharyngeal squamous cell carcinoma. Br J Oral Maxillofac Surg. 2011;49:106-111. [DOI] [PubMed] [Google Scholar]
- 9. Jeremic G, Venkatesan V, Hallock A, et al. Trismus following treatment of head and neck cancer. J Otolaryngol Head Neck Surg. 2011;40:323-329. [PubMed] [Google Scholar]
- 10. Lindblom U, Garskog O, Kjellén E, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014;53:620-627. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh LC, Chen JW, Wang LY, et al. Predicting the severity and prognosis of trismus after intensity-modulated radiation therapy for oral cancer patients by magnetic resonance imaging. PLoS One. 2014;9:e92561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Molen L, Heemsbergen WD, de Jong R, et al. Dysphagia and trismus after concomitant chemo-intensity-modulated radiation therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol. 2013;106:364-369. [DOI] [PubMed] [Google Scholar]
- 13. Wetzels JW, Merkx MA, de Haan AF, Koole R, Speksnijder CM. Maximum mouth opening and trismus in 143 patients treated for oral cancer: a 1-year prospective study. Head Neck. 2014;36:1754-1762. [DOI] [PubMed] [Google Scholar]
- 14. Pauli N, Johnson J, Finizia C, Andréll P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013;52:1137-1145. [DOI] [PubMed] [Google Scholar]
- 15. Wang CJ, Huang EY, Hsu HC, Chen HC, Fang FM, Hsiung CY. The degree and time-course assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope. 2005;115:1458-1460. [DOI] [PubMed] [Google Scholar]
- 16. Saund DS, Pearson D, Dietrich T. Reliability and validity of self-assessment of mouth opening: a validation study. BMC Oral Health. 2012;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jager-Wittenaar H, Dijkstra PU, Vissink A, van Oort RP, Roodenburg JL. Variation in repeated mouth-opening measurements in head and neck cancer patients with and without trismus. Int J Oral Maxillofac Surg. 2009;38:26-30. [DOI] [PubMed] [Google Scholar]
- 18. Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004;40:879-889. [DOI] [PubMed] [Google Scholar]
- 19. Pauli N, Fagerberg-Mohlin B, Andréll P, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol. 2014;53:502-529. [DOI] [PubMed] [Google Scholar]
- 20. Kamstra JI, Roodenburg JL, Beurskens CH, Reintsema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer. 2013;21:951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buchbinder D, Currivan RB, Kaplan AJ, Urken ML. Mobilization regimens for the prevention of jaw hypomobility in the radiated patient: a comparison of three techniques. Int J Oral Maxillofac Surg. 1993;51:863-867. [DOI] [PubMed] [Google Scholar]
- 22. Stubblefield MD, Manfield L, Riedel ER. A preliminary report on the efficacy of a dynamic jaw opening device (dynasplint trismus system) as part of the multimodal treatment of trismus in patients with head and neck cancer. Arch Phys Med Rehabil. 2010;91:1278-1282. [DOI] [PubMed] [Google Scholar]
- 23. Shulman DH, Shipman B, Willis FB. Treating trismus with dynamic splinting: a cohort, case series. Adv Ther. 2008;25:9-16. [DOI] [PubMed] [Google Scholar]
- 24. Barañano CF, Rosenthal EL, Morgan BA, McColloch NL, Magnuson JS. Dynasplint for the management of trismus after treatment of upper aerodigestive tract cancer: a retrospective study. Ear Nose Throat J. 2011;90:584-590. [DOI] [PubMed] [Google Scholar]
- 25. Loorents V, Rosell J, Karlsson C, Lidbäck M, Hultman K, Börjeson S. Prophylactic training for the prevention of radiotherapy-induced trismus—a randomised study. Acta Oncol. 2014;53:530-538. [DOI] [PubMed] [Google Scholar]
- 26. Cooperstein E, Gilbert J, Epstein JB, et al. Vanderbilt Head and Neck Symptom Survey version 2.0: report of the development and initial testing of a subscale for assessment of oral health. Head Neck. 2012;34:797-804. [DOI] [PubMed] [Google Scholar]
- 27. Jackson LK, Deng J, Ridner SH, Gilbert J, Dietrich MS, Murphy BA. Preliminary testing of a patient-reported outcome measure for recurrent or metastatic head and neck cancer. Am J Hosp Palliat Care. 2016;33:313-320. [DOI] [PubMed] [Google Scholar]
- 28. Kolnick L, Deng J, Epstein JB, et al. Associations of oral health items of the Vanderbilt Head and Neck Symptom Survey with a dental health assessment. Oral Oncol. 2014;50:135-140. [DOI] [PubMed] [Google Scholar]
- 29. Murphy BA, Dietrich MS, Wells N, et al. Reliability and validity of the Vanderbilt Head and Neck Symptom Survey: a tool to assess symptom burden in patients treated with chemoradiation. Head Neck. 2010;32:26-37. [DOI] [PubMed] [Google Scholar]