Abstract
Objectives. Triphala is a herbal medicine that has been widely used for treating a variety of ailments. This study aims to systematically analyze the antitumor effects of Triphala on gynecological cancers. Methods. The antineoplastic activities of Triphala on gynecological cancers were analyzed using network pharmacology-based strategies. Afterward, the human ovarian cancer cell line SK-OV-3, cervical cancer cell line HeLa, and endometrial cancer cell line HEC-1-B were selected for experimetal valification. Results. Network pharmacology analysis suggested that Triphala could comprehensively intervene in proliferation and apoptosis through diverse signaling pathways, mainly including MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53. The Cell Counting Kit 8 (CCK-8) assay illustrated that Triphala was able to inhibit cell proliferation with half inhibition concentration (IC50) values of 98.28 ± 13.71, 95.56 ± 8.94, and 101.23 ± 7.76 µg/mL against SK-OV-3, HeLa, and HEC-1-B cells, respectively. The ELISA experiment demonstrated that Triphala was capable of promoting programmed cell death, with dosage correlations. The antiproliferative and proapoptotic activities were confirmed by flow cytometric analysis using Ki67 antibody and Annexin V/propidium iodide (PI) dual staining. Western blotting revealed a decrease in expression levels of phospho-Akt, phospho-p44/42, and phospho-NF-κB p56 in cells administered Triphala, which indicated that the possible mechanism could involve downregulation of MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53 signaling pathways, as was predicted. Conclusion. Triphala holds great promise for treating gynecological cancers. Although the favorable pharmacological properties have been preliminarily investigated in this study, further studies are still needed to uncover the sophisticated mechanism of Triphala in cancer therapy.
Keywords: Triphala, gynecologic cancer, mechanism, network pharmacology, systems biology
Introduction
Triphala, or ‘Bras Bu gSum Thang in Tibetan, has been extensively prescribed and well recognized as one of the most important herbal formulae by both Ayurvedic and Tibetan medical practitioners for its broad spectrum of beneficial effects, such as longevity, rejuvenation, hypocatharsis, blood purification, antifebrile and antimicrobial nature, and others.1-3 Based on the central concept of Tibetan medical theory, the human body is composed of Nyipa gSum (3 humors), and health only exists when the constitutional elements are well balanced. According to the fundamental Tibetan medical text rGyud bZhi,4 the genesis and progression of tumor results from the imbalance of the 3 principles or humors, and Triphala is able to restore and maintain the homeostasis of the 3 humors. Therefore, Triphala is often used to treat benign and malignant tumors, either as a sole remedy or as a basic recipe in many antineoplastic formulations.4
Triphala is composed of the fruity parts of 3 chebulic myrobalans—namely, Terminalia chebula Retz, Terminalia bellerica (Gaertn) Roxb, and Phyllanthus emblica Linn, in a ratio of 1:1:1 or 2:1:1,5 each of which contains various structurally diverse chemicals with therapeutic potentials. It has been demonstrated that the main bioactive chemical constitutes are polyphenols, such as tannins, flavonoids, phenolic acids and glycosides, as well as other aromatic acids and carbohydrates, saponins, sterols, alkaloids, amino acids, fatty acids, and others.6,7 Although there is a lack of specific data to elucidate the antineoplastic effect of Triphala against gynecological tumors, emerging studies have reported that a few of its constituents (gallic acid, ellagic acid, chebulic acid, and their derivatives, flavonoids, ascorbic acid, and others) have such properties.8,9 Because the anticancer mechanism of Triphala is a concerted pharmacological intervention of multiple components and targets,10,11 it is necessary to apply the network pharmacological-based strategy in this study as an effective and efficient approach for understanding the network regulatory effects of herbal formulae.12,13
Materials and Methods
Data Collection
The chemical candidates were collected from the available literature related to both Triphala and its ingredients. The bidimensional chemical structures were either obtained from NCBI PubChem (http://pubchem.ncbi.nlm.nih.gov/) and ChEMBL (https://www.ebi.ac.uk/chembl/) databases or charted with ISIS/Draw 2.5 (MDL Information Systems Inc, San Leandro, CA). The downloaded chemical structures were then converted into steric structures and exported in mol2 format with hydrogen atoms. Gasteiger charges were added by using Open Babel 2.4.1.14-16 Afterward, the mol2 format structural files were uploaded to PharmMapper server (http://59.78.96.61/pharmmapper/) using the default setting for potential targets screening, based on an inverse docking approach.17 The associations between the predicted targets and female genital organ cancers were identified through the DGA database (http://dga.nubic.northwestern.edu).18 The top 10 gynecological cancer–related target candidates of each compound of Triphala were chosen for further studies, based on the fit score. The official names of the encoding genes of the predicted targets were obtained from the UniProt database (http://www.uniprot.org/).19
Bioinformatics Analysis
The biological properties and pathway enrichment of Triphala were analyzed manually. Briefly, the molecular functions of the potential target candidates were obtained from GeneCards database (http://www.genecards.org/).20 The principal regulatory activities against gynecological malignancies were roughly classified into 3 categories (cell growth and proliferation, cell death and apoptosis, cell migration and invasion), whereas the key signaling pathways included MAPK/ERK signaling, PI3K/Akt/mTOR signaling, NF-κB/p53 signaling, TGF-β/Smad signaling, IL6/Jak/Stat signaling, Wnt/β-Catenin signaling, and Notch/Jagged signaling. The gene enrichment of each aforementioned biological activity and signaling pathway was calculated individually. The interaction between the compounds, targets, and effects of Triphala against gynecological cancers were analyzed, and the interaction network was drawn by Cytoscape 3.5.1.21
Preparation of Medicine Extract
The finely powdered Triphala containing 3 equal proportions of myrobalans (Dabur India Ltd, Alwar, India; batch number: AL1675) was extracted by 70% methanol aqueous solutions (sample weight to solvent volume ratio of 1/10) with stirring. The extract solution was centrifuged at 4000 rpm for 15 minutes at room temperature, then filtered through a 0.45-µm membrane filter (Merck Millipore Ltd, Cork, Ireland) to remove particulate matter. The solvent of filtrate was removed by rotary evaporation followed by freeze drying. The achieved extract powder was weighed and stored at −20°C and was dissolved in complete medium at different concentrations and filtered with a 0.22-µm membrane filter (Merck Millipore) before being applied to the cells.
Cell Culture
Human ovarian carcinoma cell line SK-OV-3, cervical carcinoma cell line HeLa, and endometrial carcinoma cell line HEC-1-B were provided by China Infrastructure of Cell Line Resource. SK-OV-3 cells were cultured in McCoy’s 5A medium (Corning Inc, Corning, USA); HeLa cells were grown in RPMI 1640 medium (Corning); and HEC-1-B cells were cultured in DMEM medium (Corning). All mediums were supplemented with 10% fetal bovine serum (Corning) and 1% penicillin-streptomycin (Beyotime Biotechnology Inc, Nantong, China). The cells were incubated at 37°C under a humidified atmosphere of 5% carbon dioxide.
Cell Counting Kit (CCK-8) Assay for Proliferation Activity
CCK-8 (Dojindo Inc, Kumamoto, Japan) assay was performed to estimate the proliferation inhibitory activity of Triphala according to the manufacturer’s specification. Briefly, the gynecological cells were seeded in 96-well plates separately at a density of 1 × 104 cells per well and incubated with complete medium for 24 hours; then, they were cultured with gradient concentrations (25-150 µg/mL) of Triphala extract for an additional 48 hours. After the drug intervention, the tetrazolium salt-based CCK-8 solution was added and reacted for another 3 hours. The optical densities (ODs) were measured at 450 nm with a microplate reader (Beijing Pulang New Technology Co, Beijing, China), and the percentage of antiproliferation was calculated. The half maximal inhibition concentration (IC50) values were calculated using SPSS Statistics 19.0 software (IBM, Chicago, IL) by probit analysis.
Enzyme-Linked Immunosorbent Assay (ELISA) Assay for Apoptosis Analysis
The apoptosis induction activity of Triphala was examined with Cell Death Detection ELISA Plus kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instruction. Briefly, the cells were seeded and cultured using the same method described above. After the drug intervention, the cells were collected and resuspended in lysis buffer, and the lysate was centrifuged to remove the intact nuclei. The supernatant containing cytoplasmic histone-associated DNA fragments was transferred into a streptavidin-coated microplate and incubated with immunoreagent and substrate for quantitative immunoassay. The OD value was determined at 405 nm using a microplate reader, and the fold increase of DNA fragmentation, reflecting the amount of programmed cell deaths, was calculated, as Absorbance of treated cells/Absorbance of negative control cells.
Flow Cytometric Analysis
Flow cytometry assays were processed using monoclonal antibody Ki-67 and Annexin V/propidium iodide (PI) double staining, respectively, to evaluate the degree of proliferation and apoptosis. Briefly, the gynecological cancer cells were seeded into 6-well plates at a density of 5 × 105 cells per well and incubated with Triphala at respective IC50 concentrations for 48 hours, harvested by trypsinization, and washed and resuspended in cell staining buffer. For proliferation analysis, the cells were fixed, and the nuclear membrane was permeabilized using Foxp3/Transcription Factor Staining Buffer Set (eBioscience Inc, San Diego, CA) before staining with anti-Ki67 antibody (BioLegend Inc, San Diego, CA) at 4°C for 1 hour. For apoptosis detection, the cells were incubated using FITC Annexin V Apoptosis Detection Kit with PI (BioLegend) at room temperature for 15 minutes. Afterward, the fluorescent staining was detected and analyzed using a flow cytometer (BD Biosciences, San Jose, CA), and the mean fluorescent intensity for Ki-67 was calculated using Flowjo VX software (Tree Star Inc, Ashland, OR).
Western Blotting Assay
The gynecological cancer cells were cultured in 10-cm petri dishes, grown to approximately half confluence, and then treated with Triphala at respective IC50 concentrations for 48 hours. The cultured cells were harvested by centrifugation and fractionated using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Biotechnology Inc, Nantong, China) following the manufacturer’s instruction with supplement of protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma-Aldrich Corp, St Louis, MO). The protein concentrations were determined using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). The total or nuclear proteins were separated by SDS-PAGE electrophoresis, transferred to a nitrocellulose membrane, and then incubated with phospho-Akt, phospho-p44/42, and phospho-NF-κB p56 (Cell Signaling Technology, Danvers, MA) monoclonal antibodies correspondingly. Afterward, secondary antibodies (Abcam) were attached, and expression levels of proteins were detected by chemiluminescence using Pierce ECL Plus Western Blotting Substrate (Thermo Fisher Scientific).
Statistical Analysis
All data were represented as means ± SDs of a minimum of 3 independent experiments. Statistical analyses were carried out by 1-way ANOVA, with the least-significant difference post hoc multiple comparison tests, via SPSS Statistics 19.0 software. A P value of <.05 was considered to be statistically significant.
Results
Prediction of Potential Targets
A total of 50 major components with anticancer properties were obtained from the available literature and then analyzed through a pharmacophore mapping approach performed by an online PharmMapper tool. Using the DGA database, the output results were screened based on their correlation with gynecological cancers and then sorted by normalized fit scores. The 55 top-ranked gynecological cancer–related proteins were identified as principal potential targets of Triphala (Table S1; supplementary material available at http://journals.sagepub.com/home/ict/supplemental-data).
Functional and Molecular Analysis
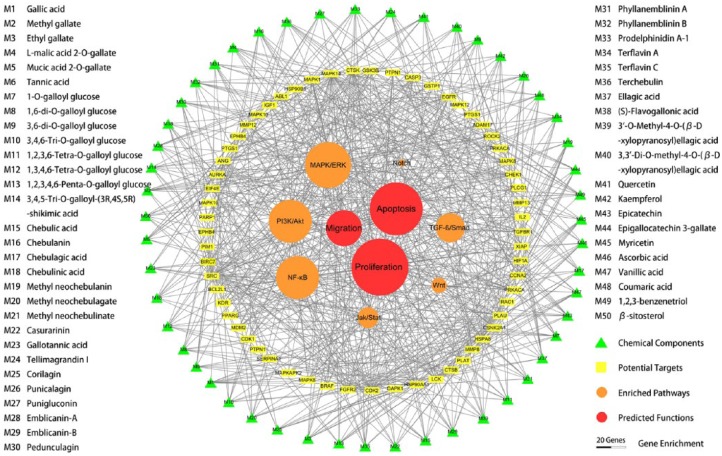
The potential targets were annotated to cellular functions and molecular signaling pathways, and the gene enrichment was calculated. A total of 41 genes were associated with cell growth and proliferation, 38 genes with cell death and apoptosis, and 27 genes with cell migration and invasion. Among them, the enrichment scores of genes related with cell proliferation and apoptosis were at above average levels. Furthermore, 33 genes were involved in MAPK/ERK signaling pathways, 29 genes in PI3K/Akt/mTOR signaling pathways, 29 genes in NF-κB/p53 signaling pathways, 21 genes in TGF-β/Smad signaling pathways, 15 genes in IL6/Jak/Stat signaling pathways, 11 genes in Wnt/β-Catenin signaling pathways, and 5 genes in Notch/Jagged signaling pathways (Table S2). Among these pathways, the gene enrichment scores of MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53 signaling pathways were above the mean value. The results suggested that the predominant pharmacological effects of Triphala against gynecological cancers may involve proliferation inhibition and apoptosis induction of tumor cells, through regulating MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53 signaling pathways. The drug-target-effect interaction network was visualized by Cytoscape software and is shown in Figure 1.
Figure 1.
Prediction of the biological functions and mechanism of Triphala against gynecological cancers based on a network pharmacological approach. The drug-target-effect interaction network showed that 50 candidate compounds of Triphala (green triangles) were predicted to have 55 major protein targets (yellow squares), which could participate in 3 primary molecular pathways (orange circles) and lead to 2 principal cancer activities (red circles). The diameters of circles indicate the number of associated proteins.
Validation of Antiproliferative Activities
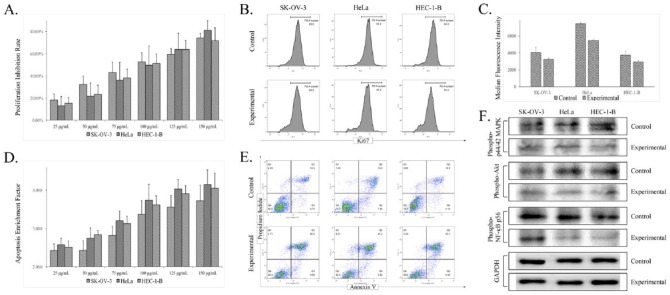
The antiproliferation properties of Triphala were examined by CCK-8 and flow cytometry–based Ki-67 assays. The CCK-8 assay showed that Triphala significantly inhibited the growth of all 3 gynecological tumor cell lines in a concentration-dependent manner (Figure 2A). The IC50 values against human ovarian cancer SK-OV-3 cells, cervical cancer HeLa cells, and endometrial cancer HEC-1-B cells were 98.28 ± 13.71, 95.56 ± 8.94, and 101.23 ± 7.76 µg/mL, respectively. Meanwhile, the antiproliferative effect of Triphala was also confirmed by flow cytometry assay, which showed that the expression of proliferation-related antigen Ki-67 in different cancer cells were decreased (Figure 2C).
Figure 2.
Validation of the biological activities and molecular pathways of Triphala against different gynecological cancer cell lines in vitro. Dose-dependent cell growth inhibition and apoptosis induction were observed using CCK-8 (A) and ELISA (D) assays. The antiproliferatory and proapoptotic effects were also detected by flow cytometry assays using proliferation–associated antibody Ki-67 (B and C) and Annexin V/PI (E). Western blotting (F) further demonstrated that the level of phospho-Akt, phospho-p44/42, and phospho-NF-κB p56 decreased in different gynecological cancer cell lines, which suggested that the antitumor mechanism of Triphala is related to MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53 signaling pathways, as predicted.
Abbreviations: CCK, Cell Counting Kit; ELISA, enzyme-linked immunosorbent assay; PI, propidium iodide.
Validation of Proapoptotic Activities
The proapoptotic effects of Triphala were analyzed by ELISA and Annexin V/PI flow cytometric assays. The ELISA assay demonstrated that the apoptotic nucleosomal DNA release was gradually elevated significantly after exposure to increasing concentrations of Triphala (Figure 2D), which indicated that Triphala could induce apoptosis of SK-OV-3, HeLa, and HEC-1-B cells in a dose-dependent manner. Furthermore, the flow cytometric assay illustrated that the proportion of Annexin V–positive/PI-positive cells increased markedly (Figure 2E), which further confirmed the apoptosis promoting activity of Triphala on different gynecological cancer cells in vitro.
Investigation of Molecular Pathways
The regulation of cellular signaling pathways predicted in the functional and molecular analyses was validated by western blotting assay. Results illustrated that compared with the control group (no treatment), the expression levels of phospho-Akt, phospho-p44/42, and phospho-NF-κB p56 in SK-OV-3, HeLa, and HEC-1-B cells treated with Triphala were decreased, indicating that the actual antineoplastic mechanism of Triphala could be related to modulation of MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53 signaling pathways.
Discussion
Despite advances in diagnosis and treatment, gynecological cancers are still one of the most commonly diagnosed cancers and leading causes of cancer-related death among women worldwide. It has been suggested that traditional medicines, as therapeutic supplements or substitutes for conventional medications, have been frequently used by women with gynecological malignancies in order to improve treatment outcomes and reduce side effects,22 even if the safety and efficacy of these experience-based medicines are not always evidence based.23,24 Because traditional herbal formulae always contain numerous chemical ingredients with diverse pharmacological activities, the pharmaceutical studies on traditional medicines based on conventional “one drug, one target” concept are extremely resource intensive, time-consuming, and therefore, inefficient.25 Consequently, the clinical effectiveness and pharmacological mechanisms of most traditional herbal medicines remain to be further elucidated. Contrary to the “one drug, one target” concept, a “multiple components, network target” concept supports the application of network pharmacology.12,25,26 Studies also showed that network pharmacology has already been successfully used for predicting bioactivities of traditional medicines and is considered a time-saving and cost-effective approach.27-29
Triphala, as a nontoxic therapeutic herbal remedy, has been clinically used for thousands of years in the treatment of various diseases, such as cancers and immune system disorders.30 Growing numbers of in vitro and in vivo studies have confirmed that Triphala possesses potent antineoplastic activities against different cancers, including breast, colon, melanoma, pancreas, and prostate malignancies, by inhibition of proliferation and metastasis and promotion of apoptosis.31-35 Increased activation of ERK and p53 have been observed in pancreatic tumor cells that were administered Triphala,34 whereas suppressed expression of c-Myc and Cyclin D1 were also found in colon cancer and colon cancer stem cells,32 which demonstrates that Triphala could exert its anticancer effects through the MAPK/ERK and Wnt/β-Catenin signaling pathways. In this study, the anticancer functions of Triphala against gynecological cancers and the possible mechanisms were predicted using network pharmacology strategies. The results of analyses illustrated that Triphala possesses comprehensive antitumor activities through modulation of multiple important cancer-related signaling pathways. Subsequently, the major hypothesized cellular functions and molecular pathways were examined in several common cell lines of gynecological tumors. Results of in vitro experiments confirmed that Triphala could inhibit tumor cell proliferation and promote apoptosis by regulation of MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53 signaling pathways. The consistency between the prediction and experimental validation further indicated that network pharmacology is a reliable and practical method that could be used on multicomponent drugs, including traditional herbal formulae.
However, some limitations still existed in this study. First, because of the time and outlay budgetary limits, only major antitumor activities and key molecules that are involved in the putative signaling pathways were investigated in this study. For instance, 23 proteins (CDK2, SRC, MAPK14, PLAU, CTSK, HSPA8, HSP90AA1, ANG, CHEK1, CTSB, LCK, CCNA2, PRKACA, MMP12, XIAP, AURKA, BIRC7, EGFR, CASP3, FGFR2, MAPK1, MMP8, and EPHB4) targeted by Triphala components were predicted to have hit numbers that were more than average; nevertheless, the actual interactions between these proteins remain to be discovered. Second, although several cancer-related cellular processes and signaling pathways with higher enrichment scores were investigated, the results of which were roughly in accordance with other previous studies,31-35 some other cellular features (invasion and metastasis activities, and TGF-β/Smad, IL6/Jak/Stat, Wnt/β-Catenin, and Notch/Jagged pathways) with lower enrichment scores were not investigated in this study. It has been widely recognized that the biological effects of herbal medicines can be only interpreted by networked interactions among components and targets, suggesting that these unstudied biological features may be as important as the already investigated biological properties for the pharmacological effects of Triphala.12,36,37 Third, many researchers have suggested that the radioprotective, chemoprotective, immunomodulatory, and antivirus activities also play important roles in carcinogenesis38-42; however, this topic is beyond the research scope of this study. Thus, more studies are still needed for further clarifying the antineoplastic mechanism of Triphala.
Conclusion
It can be concluded that Triphala possesses significant proliferative inhibitory effects and apoptotic induction effects against female reproductive cancers. The molecular mechanism can be associated with the downregulation of MAPK/ERK, PI3K/Akt/mTOR, and NF-κB/p53 signaling pathways and also possibly related with TGF-β/Smad, IL6/Jak/Stat, Wnt/β-Catenin, and Notch/Jagged pathways. However, the complicated molecular mechanism of its antitumor effects remains to be further elucidated in future studies.
Acknowledgments
The authors would like to thank the Ministry of Finance of the People’s Republic of China (No. 1981420400011) and China Postdoctoral Science Foundation (No. 2017M610812) for financially supporting this work.
Footnotes
Authors’ Note: Yuhang Zhao and Min Wang contributed equally to the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by Ministry of Finance of the People’s Republic of China (No. 1981420400011) and China Postdoctoral Science Foundation (No.2017M610812).
ORCID iD: Xianda Hu  https://orcid.org/0000-0002-3916-6054.
https://orcid.org/0000-0002-3916-6054.
References
- 1. Peterson CT, Denniston K, Chopra D. Therapeutic uses of Triphala in Ayurvedic medicine. J Altern Complement Med. 2017;23:607-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olennikov DN, Kashchenko NI, Chirikova NK. In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: a case of Padma Hepaten formulation. Nutrients. 2015;7:8456-8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jagetia GC, Malagi KJ, Baliga MS, Venkatesh P, Veruva RR. Triphala, an Ayurvedic rasayana drug, protects mice against radiation-induced lethality by free-radical scavenging. J Altern Complement Med. 2004;10:971-978. [DOI] [PubMed] [Google Scholar]
- 4. Gonpo YY, Mao J, Ma S, et al. Si bu yi xu. Shanghai, China: Shanghai Scientific & Technical Publishers; 2012. [Google Scholar]
- 5. Ginsburg I, Koren E, Horani A, et al. Amelioration of hepatic fibrosis via Padma Hepaten is associated with altered natural killer T lymphocytes. Clin Exp Immunol. 2009;157:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhatnagar S, Rani A, Kumari R. Therapeutic potential of Triphala against human diseases. Int J Pharm Sci Rev Res. 2015;31:5-13. [Google Scholar]
- 7. Pfundstein B, El Desouky SK, Hull WE, et al. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry.2010;71:1132-1148. [DOI] [PubMed] [Google Scholar]
- 8. Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71-78. [DOI] [PubMed] [Google Scholar]
- 9. Belapurkar P, Goyal P, Tiwari-Barua P. Immunomodulatory effects of Triphala and its individual constituents: a review. Indian J Pharm Sci. 2014;76:467-475. [PMC free article] [PubMed] [Google Scholar]
- 10. Variya BC, Bakrania AK, Patel SS. Emblica officinalis (amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol Res. 2016;111:180-200. [DOI] [PubMed] [Google Scholar]
- 11. Saleem A, Husheem M, Härkönen P, Philaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 2002;81:327-336. [DOI] [PubMed] [Google Scholar]
- 12. Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11:110-120. [DOI] [PubMed] [Google Scholar]
- 13. Chandran U, Mehendale N, Tillu G, et al. Network pharmacology of Ayurveda formulation Triphala with special reference to anti-cancer property. Comb Chem High Throughput Screen. 2015;18:846-854. [DOI] [PubMed] [Google Scholar]
- 14. Kim S, Thiessen PA, Bolton EE, et al. PubChem substance and compound databases. Nucleic Acids Res. 2016;44:D1202-D1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaulton A, Hersey A, Nowotka M, et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017;45:D945-D954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Ouyang S, Yu B, et al. PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010;38:W609-W614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng K, Xu W, Zheng J, et al. The Disease and Gene Annotations (DGA): an annotation resource for human disease. Nucleic Acids Res. 2013;41:D553-D560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Apweiler R, Bairoch A, Wu CH, et al. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2004;32:D115-D119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fishilevich S, Zimmerman S, Kohn A, et al. Genic insights from integrated human proteomics in GeneCards. Database (Oxford). 2016;2016:baw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chase DM, Gibson SJ, Sumner DA, Bea JW, Alberts DS. Appropriate use of complementary and alternative medicine approaches in gynecologic cancers. Curr Treat Options Oncol. 2014;15:14-26. [DOI] [PubMed] [Google Scholar]
- 23. Kuo YT, Chang TT, Muo CH, et al. Use of complementary traditional Chinese medicines by adult cancer patients in Taiwan: a nationwide population-based study [published online June 1, 2017]. Integr Cancer Ther. doi: 10.1177/1534735417716302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eschiti VS. Lesson from comparison of CAM use by women with female-specific cancers to others: it’s time to focus on interaction risks with CAM therapies. Integr Cancer Ther. 2007;6:313-344. [DOI] [PubMed] [Google Scholar]
- 25. Tao W, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145:1-10. [DOI] [PubMed] [Google Scholar]
- 26. Zhang B, Wang X, Li S. An integrative platform of TCM network pharmacology and its application on a herbal formula, Qing-Luo-Yin. Evid Based Complement Alternat Med. 2013;2013:456747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Liu Z, Li C, et al. Drug target prediction based on the herbs components: the study on the multitargets pharmacological mechanism of qishenkeli acting on the coronary heart disease. Evid Based Complement Alternat Med. 2012;2012:698531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sucher NJ. The application of Chinese medicine to novel drug discovery. Expert Opin Drug Discov. 2013;8:21-34. [DOI] [PubMed] [Google Scholar]
- 29. Tang J, Aittokallio T. Network pharmacology strategies toward multi-target anticancer therapies: from computational models to experimental design principles. Curr Pharm Des. 2014;20:20-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baliga MS. Triphala, Ayurvedic formulation for treating and preventing cancer: a review. J Altern Complement Med. 2010;16:1301-1308. [DOI] [PubMed] [Google Scholar]
- 31. Sandhya T, Mishra KP. Cytotoxic response of breast cancer cell lines, MCF 7 and T 47 D to Triphala and its modification by antioxidants. Cancer Lett. 2006;238:304-313. [DOI] [PubMed] [Google Scholar]
- 32. Vadde R, Radhakrishnan S, Reddivari L, et al. Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/cyclin D1 and elevation of Bax/Bcl-2 ratio. Biomed Res Int. 2015;2015:649263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Birla N, Das PK. Phytochemical and anticarcinogenic evaluation of Triphala powder extract, against melanoma cell line induced skin cancer in rats. Pharm Biol Eval. 2016;3:366-370. [Google Scholar]
- 34. Shi Y, Sahu RP, Srivastava SK. Triphala inhibits both in vitro and in vivo xenograft growth of pancreatic tumor cells by inducing apoptosis. BMC Cancer. 2008;8:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russell LH, Jr, Mazzio E, Badisa RB, et al. Differential cytotoxicity of triphala and its phenolic constituent gallic acid on human prostate cancer LNCap and normal cells. Anticancer Res. 2011;31:3739-3745. [PMC free article] [PubMed] [Google Scholar]
- 36. Lu K, Chakroborty D, Sarkar C, et al. Triphala and its active constituent chebulinic acid are natural inhibitors of vascular endothelial growth factor—a mediated angiogenesis. PLoS One. 2012;7:e43934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sivasankar S, Lavanya R, Brindha P, Angayarkanni N. Aqueous and alcoholic extracts of Triphala and their active compounds chebulagic acid and chebulinic acid prevented epithelial to mesenchymal transition in retinal pigment epithelial cells, by inhibiting SMAD-3 phosphorylation. PLoS One. 2015;10:e0120512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takauji Y, Miki K, Mita J, et al. Triphala, a formulation of traditional Ayurvedic medicine, shows protective effect against X-radiation in HeLa cells. J Biosci. 2016;41:569-575. [DOI] [PubMed] [Google Scholar]
- 39. Dhanalakshmi S, Devi RS, Srikumar R, Manikandan S, Thangaraj R. Protective effect of Triphala on cold stress-induced behavioral and biochemical abnormalities in rats. Yakugaku Zasshi. 2007;127:1863-1867. [DOI] [PubMed] [Google Scholar]
- 40. Srikumar R, Parthasarathy JN, Devi SR. Immunomodulatory activity of Triphala on neutrophil functions. Biol Pharm Bull. 2005;28:1398-1403. [DOI] [PubMed] [Google Scholar]
- 41. Phetkate P, Kummalue T, U-Pratya Y, Kietinun S. Significant increase in cytotoxic T lymphocytes and natural killer cells by triphala: a clinical phase I study. Evid Based Complement Alternat Med. 2012;2012:239856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Srikumar R, Parthasarathy NJ, Shankar EM, et al. Evaluation of the growth inhibitory activities of Triphala against common bacterial isolates from HIV infected patients. Phytother Res. 2007;21:476-480. [DOI] [PubMed] [Google Scholar]