Abstract
Background: Inonotus obliquus, also known as Chaga, is a parasitic fungus growing on birches and used in traditional medicine (especially by Khanty people) to treat various health problems. In this study, we aimed to quantify the 3 metabolites frequently cited in literature, that is, betulin, betulinic acid, and inotodiol in the Chaga recently discovered in forests located in Normandy (France), and to compare their concentrations with Ukrainian and Canadian Chaga. This study also explores the cytotoxicity of the French Chaga against cancer-derived cells and transformed cells. Methods: A quantification method by HPLC-MS-MS (high-performance liquid chromatography–tandem mass spectrometry) of betulin, betulinic acid, and inotodiol was developed to study the French Chaga and compare the concentration of these metabolites with extracts provided from Chaga growing in Canada and Ukraine. This method was also used to identify and quantify those 3 compounds in other traditional preparations of Chaga (aqueous extract, infusion, and decoction). Among these preparations, the aqueous extract that contains betulin, betulinic acid, and inotodiol was chosen to evaluate and compare its cytotoxic activity toward human lung adenocarcinoma cells (A549 line) and human bronchial epithelial cells (BEAS-2B line). Results: French Chaga contains betulin and betulinic acid at higher levels than in other Chaga, whereas the concentration of inotodiol is greater in the Canadian Chaga. Moreover, the results highlighted a cytotoxic activity of the Chaga’s aqueous extract after 48 and 72 hours of exposure with a higher effect on cancer-derived cells A549 than on normal transformed cells BEAS-2B (P = 0.025 after 48 hours of exposure and P = 0.004 after 72 hours of exposure).
Keywords: Inonotus obliquus, cytotoxicity, cancer, chromatography, betulin, betulinic acid, inotodiol, quantification, traditional medicine
Introduction
Inonotus obliquus is a parasitic Polyporus from the Hymenochetaceae family. This fungus infects hardwood trees, mostly those from the genus Betula (birches), and to a lesser extent, those from the genera Quercus (oaks), Populus (poplars), Alnus (alders), Fagus (ashes), and Acer (maples).1
It was first identified and described by Persoon2 (1801), who named it Boletus obliquus. Then, it was renamed Polyporus obliquus by Fries3 (1830), followed by Quélet4 (1888), who called it Poria obliqua (under the bark of dry Fagus). In 1927, Bourdot and Galzin5 called it Xanthochrous obliquus, and its current name, Inonotus obliquus, was given by Pilàt6,7 (1936, 1942), who studied it thoroughly. Chaga has oblique pores—the origin of its species name obliquus.
Currently, this fungal species has only been described in the northern hemisphere. We can particularly find it in Canada, in the north of the United States of America, in Kazakhstan, in Siberia, in Ukraine, in Japan, in South Korea, in China, as well as in Europe (mostly in the northern and eastern parts of the continent). Its description in France is more recent, the first one dating from 1953 in Seine-et-Marne under the name of Xanthochrous obliquus8; it has also been found and described even more recently in Normandy. Chaga has been used since the 12th century in Eastern Europe. From historical chronicles, it is known that Kiev Prince (Knyaz) Vladimir Monomakh had a lip tumor and got rid of the disease thanks to treatment with Chaga mushroom.9 The Khanty people, an ethnic group from Siberia formerly called the Ostyaks,10 also used it in traditional medicine for different therapeutic indications: as an anthelminthic, as an antitubercular, to cure digestive disorders (gastritis, ulcers, etc), or even to prevent cardiac or hepatic illnesses. They used the crushed asexual form of the Chaga in several ways: by infusion, inhalation, or maceration in water of the charcoal obtained after burning to make body soap, which was used as an antiseptic.11
In the middle of the 20th century, it was still used in Siberia for its properties by Russian farmers and workers too poor to buy tea: they crushed it and drank it as an infusion.
This use of the Chaga in Siberian gulags is mentioned in Alexandre Soljénitsyne’s book Le Pavillon des cancéreux (Cancer Ward).12 Soviet health authorities noticed a decrease of the incidence of cancer cases in this population and assumed that the consumption of this infusion was a protective factor against cancer. In 1955, the USSR Ministry of Health recognized the therapeutic interest of I obliquus used as a decoction and wrote it down in the Soviet Pharmacopeia under the name of Befunginum.
The extracts of I obliquus have been used in China, Korea, Japan, Russia, and the Baltics for their favorable effects on lipid metabolism and cardiac function, as well as for antibacterial, anti-inflammatory, antioxidant, and antitumor activities.13
Inonotus obliquus extracts were found to inhibit hepatitis C virus14 and human immunodeficiency virus15 and demonstrated strong antioxidant and immunostimulatory activities in vitro.16,17 At the same time, animal studies revealed that aqueous extracts of I. obliquus exhibited anti-inflammatory effects in experimental colitis18-21 and promoted lipid metabolism.22 The mushroom has the ability to increase peroxisome proliferator-activated receptors γ transcriptional activities, which are expected to be therapeutic targets for dyslipidemia and type 2 diabetes.23
Its biological activities explain why it is used as an adjuvant in oncology, especially in anticancer chemotherapies in Asian pharmacopoeias.
The chemical analysis of Chaga in scientific literature revealed several compounds such as polysaccharides, triterpenes, and polyphenols, which might be responsible for most of the therapeutic effects previously mentioned.24 A tetracyclic triterpene called inotodiol produced following the lanosterols biosynthetic pathway elicited the interest of the international scientific community.25-29 Inotodiol has antiproliferative properties, demonstrated in vitro with human lung adenocarcinoma cells (A549) cancer-derived cells or HeLa.30,31
In addition, 2 other components derived from birch are frequently described in Chaga: betulin (or betulinol) and betulinic acid.32 Several species of birch are, indeed, used in traditional medicine with a very wide geographical distribution. The spectrum of pharmacological properties associated with their uses is important: antimicrobial, antidiabetic, hepatoprotective, antiarthritic, and anticancer activities.32 These last 2 activities were the most studied, especially from betulin and betulinic acid. In traditional medicine, the use of birch against rheumatism is reported, for example, in Bosnia-Herzegovina and Lebanon.33,34 From an experimental point of view, the study by Gründemann et al35 confirms the anti-inflammatory effect of the extract of Betula pendula by its action on the lymphocytes. Different species belonging to the genus Betula have also been tested to evaluate their anticancer potential. The compounds betulin and betulinic acid were tested in vitro on different models of cancer cells (cutaneous, ovarian, and pulmonary) demonstrating their antiproliferative potential.36-38
Our work is the first experimental approach on Chaga collected in France. This contribution aims, on the one hand, to develop a quantification method of betulin, betulinic acid, and inotodiol in Chaga extracts, and on the other hand, to evaluate and compare the biological activity of these extracts toward A549 line and human bronchial epithelial cells (BEAS-2B line).
Materials and Methods
Collection and Identification of Chaga (Inonotus obliquus)
Chaga can be described as an irregular cracked, black-brownish, hard, brittle fungus, looking like charcoal on the outside, with a diameter ranging from 10 to 20 cm; inside it is brown in color. The microscopic examination shows a monomitic structure with brown hyphae and a thick partition with a diameter from 2.5 to 6-7 µm. These hyphae are however separated but without loops.39,40
The fungal infestation results from a contamination of the duramen of the host trees by basidiospores via unhealed injuries, which have left this matrix uncovered. The asexual form grows as long as the tree lives and causes a fibrous white rot of the central cylinder of the tree, degrading the cellulose, hemicellulose, and lignin. The sexual form (fruiting body) appears between the bark and the sapwood as a yellowish crust turning brown 2 to 12 years after the death of the host tree.41,42 It shows oblique and elongated pores upholstered by a hymenium with bi- to tetrasporic basidium without basal loop. It is this sexual form that releases elliptic to globular, smooth, yellowish basidiospores measuring from 8 to 10 µm × 5 to 7.5 µm ensuring the dissemination of the fungus.43
Asexual forms of I. obliquus were respectively purchased in Canada (Gaspésie Sauvage Produits Forestiers Inc), harvested in Ukraine (the Karpatsky National Park, Ivano-Frankivsk region), and in France (the Forest of Grimbosq, Normandy, France). These forms resembling blackish growths (Figure 1), with a circumference from 15 to 28 cm and measuring about 10 m, were harvested to a height from 0.8 to 2.5 m on trunks of birch (Betula pendula).
Figure 1.

Asexual form of Inonotus obliquus (Chaga) on birch trunk.
Preparation of the Extracts
Chaga was dried in a desiccator (Drying Device Dönex SIGG AG 1978) for 5 days at 35°C. The dry fungal material was crushed and then sprayed with Blender BB90E (Waring) before sieving to 2 mm to prepare aqueous, cyclohexane, and ethyl acetate extracts. The extractions were performed in the darkness at room temperature (20°C).
Cyclohexane Extract
The powder (180 g) was first stirred in contact with cyclohexane (1 L) for 90 minutes on an orbital agitator at 180 rpm. After filtration on Whatman No. 3 paper the recovered solid residue was extracted again with cyclohexane (500 mL) for 2 hours with the same stirring system and then filtered on Whatman No. 3 filter paper. The 2 cyclohexanic extracts were pooled and evaporated with Rotavapor at 40°C until the obtainment of a yellow residue of 2.6294, 2.4744, and 2.5166 g, respectively, for the Canadian, French, and Ukrainian Chaga.
Ethyl Acetate Extract
The dried solid residue was then contacted with ethyl acetate (1 L) for 4 days on an orbital agitator at 180 rpm. At the end of these 4 days, filtration was carried out in 3 steps: on carded cotton, on Büchner, and on filter paper Whatman No. 3.
Extracts were evaporated with Rotavapor at 40°C until the obtainment of a yellow powdery residue of 0.9837, 0.9248, and 0.9315 g, respectively, for the Canadian, French, and Ukrainian Chaga.
Aqueous Extract
The powder (100 g) was stirred on contact with ultrapure water (500 mL) for 22 hours at room temperature (20°C) on an orbital agitator at 180 rpm. Filtration was then carried out on Whatman No. 3 paper in order to obtain 250 mL of aqueous extract. A 100 mL aliquot of this extract was concentrated with Rotavapor at 40°C until a volume of 40 mL was obtained.
Decoction by Khanty Method.11
The asexual form was cut in small pieces from 5 to 10 g then put in boiling water for 15 minutes. We used carded cotton and filter paper Whatman No. 3 for filtration.
Infusion According to the Canadian Method (Gaspésie Sauvage Produits Forestiers Inc)
We put 3 chunks (from 5 to 10 g) in 1 L of cold water and let it rest for 30 minutes, then we heated it without boiling it for 30 additional minutes. We used carded cotton and filter paper Whatman No. 3 for filtration.
Before their use, all extracts obtained were stored in the refrigerator at +4°C and protected from light.
Quantification by High-Performance Liquid Chromatography Coupled to a Mass Spectrometer (HPLC-ESI-QTOF MS/MS)
Reagents and Materials
Acetonitrile (ULC-MS grade) and acetone (pesticides grade) were purchased from Biosolve Chimie (Dieuze, France), while formic acid (98% purity) and ethyl acetate (HPLC HiPerSolv Chromanorm grade) were purchased from VWR (Radnor, PA). Reverse osmosis water (HPLC grade) prepared using a Millipore water purification system was used for all the preparations. The internal standard (Atrazine D5; 99% purity) was obtained from Dr Ehrenstörfer. Betulin (≥97.5% purity) and betulinic acid (≥97.5% purity) standards were purchased from Sigma Aldrich (Saint-Louis, MO), while inotodiol standard (≥95% purity) was purchased from Chemfaces (Hubei, China). All the solutions were filtered through a 0.45 µm PVDF Millipore Millex HV (Merck-Millipore, Billerica, MA).
Standard Solutions and Samples Preparation
Standard stock solutions of atrazine D5, betulin, betulinic acid, and inotodiol at a concentration of 1000 mg/L were prepared in ethyl acetate (2 mg of powder in 2 mL of ethyl acetate). A working solution containing inotodiol, betulin, betulinic acid standards at a concentration of 10 mg/mL was prepared in acetone (50 µL of each stock solution qs 5 mL of acetone). We also prepared a working solution of our internal standard (atrazine D5) at a concentration of 500 µg/L in acetonitrile (5 µL of stock solution in 10 mL of acetonitrile). The range calibration made with these working solutions was from 0.001 to 5 mg/L.
Ethyl acetate and cyclohexane dry extracts were taken up in 10 mL (for the French Chaga) or 30 mL of acetonitrile (for the Canadian and Ukrainian Chagas), passed through ultrasound for 15 minutes, and then filtered through PVDF 0.45 µm filters. The cyclohexane extract was then diluted to 10% and the ethyl acetate extract to 20% (for the French Chaga) or to 1% (for the Canadian and Ukrainian Chagas) in a solution containing acetonitrile, distilled water, and the internal standard (atrazine D5). The water extract, infusion, and decoction were filtered through PVDF 0.45 µm filters and then diluted to 20% (water extract and infusion) or 50% (decoction) in a solution containing acetonitrile, distilled water, and the internal standard (atrazine D5).
HPLC-ESI-QTOF MS/MS Analysis
A HPLC analysis was applied on an Agilent 1290 Infinity LC instrument (Agilent, Santa Clara, CA) consisting of a binary pump, a thermostatted autosampler, and a column compartment. The samples were separated on Waters Acquity UPLC BEH C18 15 µmm × 2.1 mm × 1.7 µm (Waters, Milford, MA). The mobile phase was a stepwise gradient of water (containing 0.01% formic acid, v/v) and acetonitrile (containing 0.01% formic acid, v/v; 0 minute, 97:3; 4 minutes, 70:30; 12minutes, 30:70; 15 minutes, 5:95; 17 minutes, 5:95; and 20 minutes, 97:3). The column temperature was set at 40°C and the flow rate was 0.45 mL/min. The HPLC system was connected to an Agilent 6470 MS-MS triple quadrupole (Santa Clara, CA) equipped with an ESI interface using the following operation parameters: capillary voltage, 3.5 kV ((+) ESI mode); nebulizer, 40 psig; drying gas (nitrogen) flow rate, 10.0 L/min; sheath gas flow rate, 10.0 L/min; gas temperature, 350°C; sheath gas temperature, 350°C; and V charging, 500 V. The multiple reaction monitoring transitions used for the 3 target analytes and internal standards are shown in Table 1. The quantification data were processed with Agilent Mass Hunter Quantitative Workstation Software Version B.07.01 (Agilent Technologies).
Table 1.
Multiple Reaction Monitoring Transitions Used for the 3 Target Analytes and Internal Standards.
| Compound Name | Precollision Ion Mass | MS1 | Produced Ions | MS2 Res | Dwell | Frag (V) | Collision Energy (V) | Cell Acc (V) | Polarity | Retention Time |
|---|---|---|---|---|---|---|---|---|---|---|
| Betulinic acid | 439.3 | Unit | 95 | Unit | 20 | 100 | 48 | 7 | Positive | 14.545 minutes |
| 81.1 | 100 | 48 | Positive | |||||||
| Atrazine D5: ISTD | 221.1 | Unit | 179.1 | Unit | 20 | 115 | 16 | Positive | 7.419 minutes | |
| 69.1 | 115 | 40 | Positive | |||||||
| Betulin | 425.3 | Unit | 95 | Unit | 20 | 100 | 48 | Positive | 14.781 minutes | |
| 81 | 100 | 48 | Positive | |||||||
| Inotodiol | 425.3 | Unit | 246.9 | Unit | 20 | 100 | 12 | Positive | 15.66 minutes |
Abbreviation: MS, mass spectrometer.
Method Validation
The limits of detection, limits of quantification, regression equation, and correlation coefficient of calibration curves (r²) for each standard are reported in Table 2.
Table 2.
LOD, LOQ, Regression Equation, and Correlation Coefficient of Calibration Curves.
| Cyclohexane Extract | Ethyl Acetate Extract | Aqueous Extract | Infusion | Decoction | ||
|---|---|---|---|---|---|---|
| Betulinic acid | LOD (µg/L) | 0.3 | 0.3 | 0.3 | 1.7 | 0.7 |
| LOQ (µg/L) | 1 | 1 | 1 | 5 | 2 | |
| Regression equation/correlation coefficient | French Chaga (February 8, 2016), dilution
range = 1-1000 µg/L: y = (−6.09 ×
10−8) × x2 +
(2.52 × 10−4) × x + (6.58 ×
10−4)/r2 =
0.9998 Canadian/Ukrainian Chaga (December 23, 2016), dilution range = 1-500 µg/L: y = (−2.19 × 10−7) × x2 + (4.55 × 10−4) × x + (3.61 × 10−5)/r2 = 0.9994 |
|||||
| Betulin | LOD (µg/L) | 0.3 | 0.3 | 0.3 | 1.7 | 0.7 |
| LOQ (µg/L) | 1 | 1 | 1 | 5 | 2 | |
| Regression equation/correlation coefficient | French Chaga (February 2, 2016), dilution
range = 1-5000 µg/L: y = (−3.44 ×
10−9) × x2 +
(6.56 × 10−5) × x + (3.62 ×
10−4)/r2 =
0.9998 Canadian/Ukrainian Chaga (December 23, 2016), dilution range = 1-1000 µg/L: y = (−3.55 × 10−8) × x2 + (1.77 × 10−4) × x + (5.11 × 10−5)/r2 = 0.9989 |
|||||
| Inotodiol | LOD (µg/L) | 1.7 | 1.7 | 1.7 | 8.3 | 3 |
| LOQ (µg/L) | 5 | 5 | 5 | 25 | 10 | |
| Regression equation/correlation coefficient | French Chaga (February 2, 2016), dilution
range = 10-5000 µg/L: y = (−1.52 ×
10−10) × x2 +
(1.30 × 10−5) × x + (2.53 ×
10−4)/r2 =
0.9998 Canadian/Ukrainian Chaga (December 23, 2016), dilution range = 10-5000 µg/L: y = (−6.63 × 10−10) × x2 + (2.51 × 10−5) × x + (5.60 × 10−4)/r2 = 0.9992 |
|||||
Abbreviations: LOD, limits of detection; LOQ, limits of quantification.
Cytotoxicity Assay
The A549 cells (human alveolar epithelial cells derived from an adenocarcinoma) having a doubling time of 24 hours were cultured in 96-well microplates (BD Falcon) in a DMEM medium (Gibco) supplemented with 1% bicarbonate solution at 7.5% (Gibco) and 10% decomplemented fetal calf serum.
The BEAS-2B cells (immortalized human bronchial epithelial cells) having a doubling time of 26 hours were cultured in 96-well microplates (BD Falcon) in 500 mL of BEBM medium (Gibco) supplemented with 2 mL bovine pituitary extract, 0.5 mL of hydrocortisone, 0.5 mL of human epidermal growth factor, 0.5 mL of epinephrine, 0.5 mL of transferrin, 0.5 mL of insulin, 0.5 mL of retinoic acid, and 0.5 mL of triiodothyronine to obtain BEGM medium.
Each well was seeded 24 hours before exposure with 10 000 cells for A549 cells line and 15 000 cells for BEAS-2B cells line suspended in 200 µL of medium, and then the microplates are incubated in a stove at 37°C in a 5% CO2 atmosphere.
Before dilution, the aqueous extract was passed over a hydrophilic filter with a PES membrane with a porosity of 0.22 µm. The cells were then exposed to 6 different dilutions of aqueous extract (expressed in %, vol/vol): 25%, 10%, 5%, 2%, 1%, and 0.5%. Eight replicates were made by dilution and exposure duration: 24, 48, and 72 hours.
After the end of the exposure time, the cells were stained with sulforhodamine B and the absorbance reading was performed by spectrophotometry at 570 and 655 nm. From the obtained absorbance means, the percentage of cell growth inhibition was calculated for each concentration.
Statistical Analysis
Student’s t test was used to compare cytotoxicity on A549 and BEAS-2B cells. P < 0.05 was considered as statistically significant. Analyses were conducted using the SAS version 9.4.
Results
Quantification of Metabolites by HPLC-ESI-QTOF MS/MS
We first used organic extracts made from French Chaga to develop the method of detection and quantification of the 3 metabolites searched in this study (betulin, betulinic acid, and inotodiol).
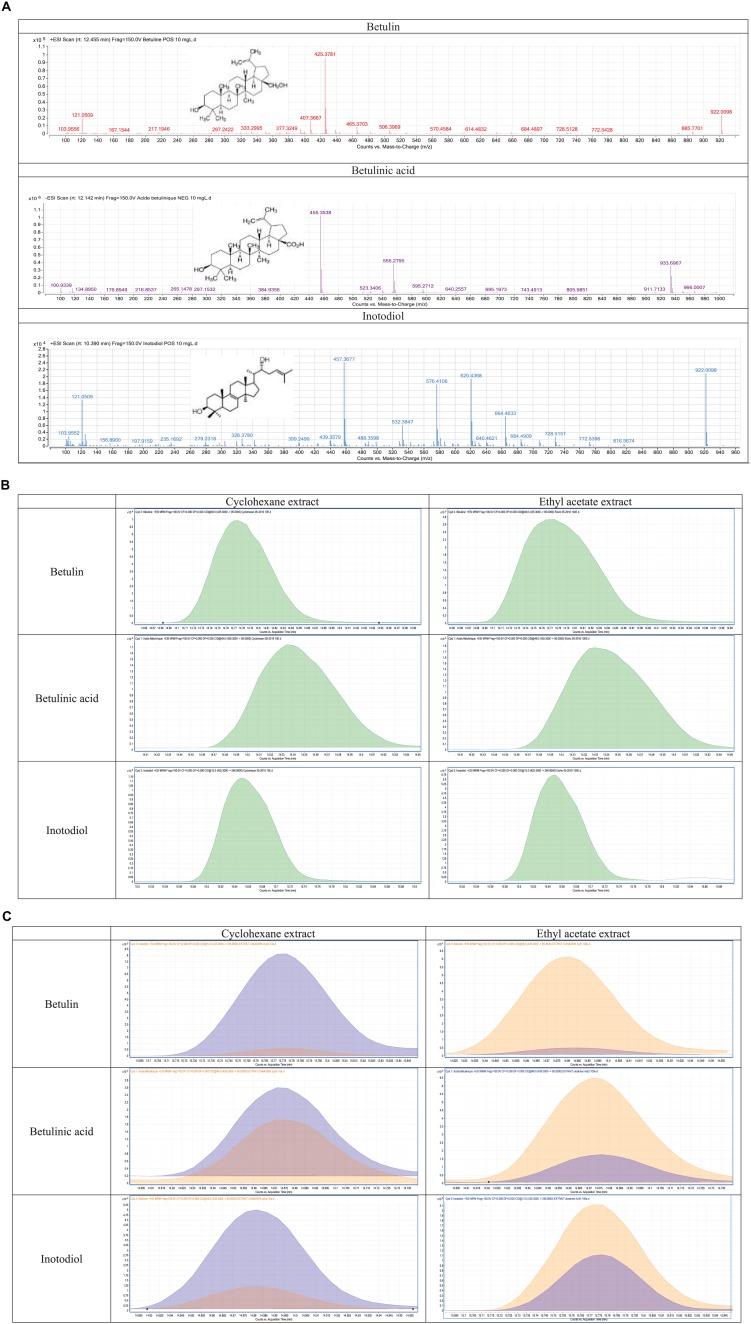
An analysis of the chromatograms by extracted ion chromatograms allowed to demonstrate the presence of betulin, betulinic acid, and inotodiol by searching for their masses in cyclohexane and ethyl acetate extracts. Figure 2A to C shows the mass spectra and the chromatograms obtained for these 3 metabolites in the different organic extracts.
Figure 2.
(A) Mass spectra of betulin, betulinic acid, and inotodiol. (B) Chromatograms of betulin, betulinic acid, and inotodiol in extracts of French Chaga. (C) Chromatograms of betulin, betulinic acid, and inotodiol obtained in extracts of Ukrainian (blue) and Canadian Chaga (orange).
Then, we applied this method of detection and quantification to other preparations of French Chaga, that is, an aqueous extract, an infusion, and a decoction. Betulin, betulinic acid, and inotodiol were found (to a lesser extent than in organic extracts) in the aqueous extract, but not in the infusion or in the decoction (Table 3).
Table 3.
Quantification of Betulin, Betulinic Acid, and Inotodiol in Different Preparations of Chaga.
| Preparations | Betulin (mg/L) | Betulinic Acid (mg/L) | Inotodiol (mg/L) | |
|---|---|---|---|---|
| Canadian Chaga | Cyclohexane extract | 0.15 | 0.12 | 8.27 |
| Ethyl acetate extract | 13.45 | 5.12 | 464.86 | |
| Ukrainian Chaga | Cyclohexane extract | 0.78 | 0.14 | 41.37 |
| Ethyl acetate extract | 0.88 | 1.12 | 147.56 | |
| French Chaga | Cyclohexane extract | 6.48 | 0.37 | 52.70 |
| Ethyl acetate extract | 1100 | 52.92 | 373.02 | |
| Aqueous extract | 0.23 | 0.16 | 11.70 | |
| Infusion | <0.005a | <0.005a | <0.005a | |
| Decoction | <0.002a | <0.002a | <0.002a |
The values are less than limits of quantification.
We also searched for these 3 metabolites in organic extracts prepared from the Canadian and Ukrainian Chaga to compare their concentration depending on the geographical origin of the samples. The results presented in Table 3 show that there are greater concentrations of birch metabolites in French Chaga and more inotodiol in Canadian Chaga. It is important to note, however, that the difference in concentration of these metabolites may be due not only to environmental factors (climate, host tree, etc) but also to the conservation technique and the lapse of time between harvesting and the production of the extracts.
Biological Activity of the Aqueous Extract From French Chaga
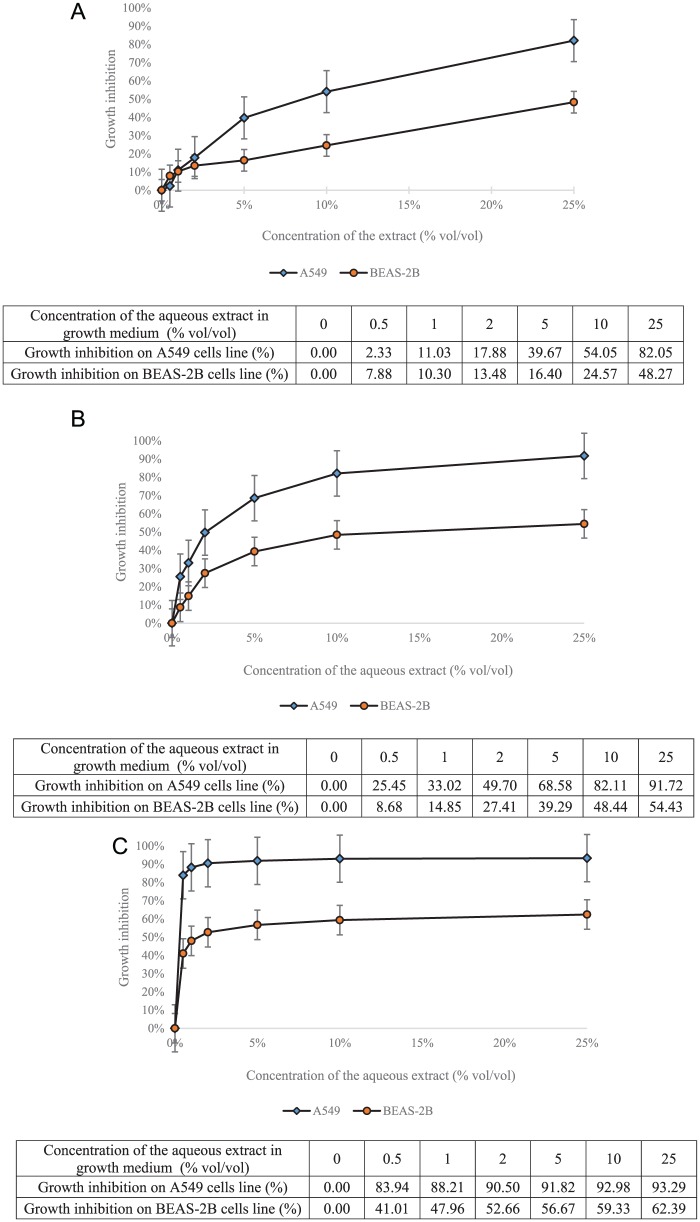
Figure 3A to C shows that cytotoxic activity exists for aqueous extract and was greater on cancerous cells than on normal transformed cells.
Figure 3.
(A) Cytotoxicity of the aqueous extract from French Chaga on A549 and BEAS-2B cells lines after 24 hours of exposure. (B) Cytotoxicity of the aqueous extract from French Chaga on A549 and BEAS-2B cells lines after 48 hours of exposure. (C) Cytotoxicity of the aqueous extract from French Chaga on A549 and BEAS-2B cells lines after 72 hours of exposure.
The cytotoxicity on A549 and BEAS-2B cells was characterized by a dose-dependent and time-dependent effect. Our results showed that after 48 and 72 hours of exposure, the cytotoxic activity was significantly reduced or lesser on the BEAS-2B cells than on the A549 cells (P = 0.025 after 48 hours of exposure and P = 0.004 after 72 hours of exposure). These observations underline the greater sensibility of highly proliferative cells compared with normal ones, which could be kept in mind for therapeutic uses.
Discussion and Conclusions
The identification of betulin and betulinic acid in all fungal extracts can be considered as a signature of the link between the parasitic fungus and its plant host. Indeed, these 2 metabolites, known to be present in the birch bark, are also concentrated in the Chaga. This observation has previously been made with other plant-fungus associations such as pine and Polyporus pinicola.44,45
Our study confirms the presence of inotodiol in the French Chaga. This secondary metabolite has previously been identified in extracts obtained from Chaga samples from China, Finland, Thailand, and Russia.24,46,47 More generally, I. obliquus is characterized by the presence of several lanostane-type triterpenes, in particular inonotsuoxide A, inotodiol, trametenolic acid, and lanostérol.48
The absence of inotodiol in infusion and decoction could be because of the concentrations below the limits of quantification of our method. This could also suggest that the properties attributed to this fungus in traditional medicine are not because of the metabolites we have sought to assay, but rather to the more polar molecules and/or lower molecular masses found in Chaga. Indeed, several studies have already demonstrated other types of molecules such as polysaccharides,49 melanin complexes,50 lignin derivatives,51 or polyphenols such as gallic acid52 in aqueous extracts.
For the cytotoxicity tests, we have chosen to use the aqueous extract, because of the following:
The presence of compounds known for their cytotoxic activity: inotodiol, betulin, and betulinic acid.31,53,54
The presence of water-soluble compounds at the origin of properties recognized in traditional medicine.
The safety of water used as a solvent in cell culture.
The cytotoxic activity of Chaga extracts appears to be related to its diversity of active secondary metabolites. Compounds such as betulin and betulinic acid are known for their anticancer activity.38 Lanostanes such as inotodiol are also studied for their cytotoxic effects.48 Thus, compounds present in the aqueous extract could explain or at least partly account for these effects.
The aqueous extract of Chaga showed antiproliferative activity on different cellular models. For example, Mazurkiewicz et al55 demonstrated the action of an extract of Polish Chaga on A549 lung cells as in our study. Antiproliferative activity has also been demonstrated in melanoma cells,56 hepatic cancer cells,57 as well as in sarcoma cells.58
Chung et al59 showed that different fractions from extracts of Russian Chaga showed cytotoxicity on various cellular models including A549 cells. These fractions corresponded to lanostanes including inotodiol. The study by Zhao et al46 also shows the efficacy of certain lanostanes such as inonotsutriol against A549 cancer cells.
In conclusion, the analysis of the organic extracts of Chaga revealed birch compounds such as betulin, betulinic acid, and a characteristic fungal molecule inotodiol. The Chaga recently discovered in the forests of Normandy contains inotodiol as do those collected in Canada and Ukraine, but our quantification method showed that the geographical origin of the fungus has an impact on the concentration of these metabolites. The biological results confirm a cytotoxic activity of the French Chaga on normal transformed BEAS-2B cells but to a far lesser extent than on lung cancer cells (A549). These observations allow us to consider the therapeutic interest of the Chaga, its chemical complexity, and to emphasize the interest of continuing to investigate mycotherapy potential by associating both chemical and biological approaches.
Acknowledgments
We would like to thank Pr Boris Czerny (ERLIS EA4254, UNICAEN) for his help in the collection of Chaga. We would also like to acknowledge Margaret Dearing for improving the English of the article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Ligue Nationale Contre le Cancer (Comité de la Manche).
ORCID iD: David Garon  https://orcid.org/0000-0003-3545-6641
https://orcid.org/0000-0003-3545-6641
References
- 1. Ryvarden L, Gilbertson RL. European Polypores. Part 1 Abortiporus-Lindtneria. Oslo, Norway: Fungiflora; 1993. [Google Scholar]
- 2. Persoon CH. Synopsis Methodica Fungorum. Pars Secunda. Göttingen, German: Henricum Dieterich; 1801. [Google Scholar]
- 3. Fries EM. Systema mycologicum, sistens fungorum ordines, genera et species, huc usque cognitas, quas ad normam methodi naturalis. Gryphiswaldae: Sumptibus Ernesti Mauritii; 1821. [Google Scholar]
- 4. Quélet L. Flore Mycologique de la France et des Pays Limitrophes. Paris, France: Octave Doin; 1888. [Google Scholar]
- 5. Bourdot H (Abbé), Galzin A. Hyménomycètes de France (Hétérobasidés-Homobasidiés gymnocarpes). Paris, France: Société Mycologique de France; 1927. [Google Scholar]
- 6. Kavina K, Pilàt A. Atlas des Champignons d’Europe Tome III Polyporaceae 1. Praha, Slovakia: Pilat & Kavina; 1936. [Google Scholar]
- 7. Pilàt A. Atlas des champignons d’Europe III Polyporaceae 2. Praha, Slovakia: Pilat & Kavina; 1942. [Google Scholar]
- 8. Doignon P. Les polypores du massif de Fontainebleau, Bulletin de la Société des Naturalistes Parisiens. Cahiers des Naturalistes. 1953;53-57. [Google Scholar]
- 9. Perevedentseva L. Use of wild-growing mushrooms for therapeutic purposes in the Perm Territory, Russia. J Environ Sci Eng. 2013;2:236-242. [Google Scholar]
- 10. de La Harpe JF. Abrégé de l’histoire générale des voyages, tome onzième. Paris, France: Ménard et Desenne, fils; 1825. [Google Scholar]
- 11. Saar M. Fungi in Khanty folk medicine. J Ethnopharmacol. 1991;31:175-179. [DOI] [PubMed] [Google Scholar]
- 12. Soljenitsyne AI. Le Pavillon des Cancéreux. Cartonne–1. Paris, France: Presses Pocket; 1980. [Google Scholar]
- 13. Shashkina MY, Shashkin PN, Sergeev AV. Chemical and medicobiological properties of chaga (review). Pharmaceut Chem J. 2006;40:560-568. [Google Scholar]
- 14. Shibnev VA, Mishin DV, Garaev TM, Finogenova NP, Botikov AG, Deryabin PG. Antiviral activity of Inonotus obliquus fungus extract towards infection caused by hepatitis C virus in cell cultures. Bull Exp Biol Med. 2011;151:612-614. [DOI] [PubMed] [Google Scholar]
- 15. Shibnev VA, Garaev TM, Finogenova MP, Kalnina LB, Nosik DN. Antiviral activity of aqueous extracts of the birch fungus Inonotus obliquus on the human immunodeficiency virus [in Russian]. Vopr Virusol. 2015;60:35-38. [PubMed] [Google Scholar]
- 16. Kim YO, Han SB, Lee HW, et al. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci. 2005;77:2438-2456. [DOI] [PubMed] [Google Scholar]
- 17. Won DP, Lee JS, Kwon DS, Lee KE, Shin WC, Hong EK. Immunostimulating activity by polysaccharides isolated from fruiting body of Inonotus obliquus. Mol Cells. 2011;31:165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi SY, Hur SJ, An CS, et al. Anti-inflammatory effects of Inonotus obliquus in colitis induced by dextran sodium sulfate. J Biomed Biotechnol. 2010;2010:943516. doi: 10.1155/2010/943516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim HG, Yoon DH, Kim CH, et al. Ethanol extract of Inonotus obliquus inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J Med Food. 2007;10:80-89. [DOI] [PubMed] [Google Scholar]
- 20. Mishra SK, Kang JH, Kim DK, Oh SH, Kim MK. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J Ethnopharmacol. 2012;143:524-532. [DOI] [PubMed] [Google Scholar]
- 21. Park YM, Won JH, Kim YH, Choi JW, Park HJ, Lee KT. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J Ethnopharmacol. 2005;101:120-128. [DOI] [PubMed] [Google Scholar]
- 22. Lee JH, Hyun CK. Insulin-sensitizing and beneficial lipid-metabolic effects of the water-soluble melanin complex extracted from Inonotus obliquus. Phytother Res. 2014;28:1320-1328. [DOI] [PubMed] [Google Scholar]
- 23. Joo JI, Kim DH, Yun JW. Extract of Chaga mushroom (Inonotus obliquus) stimulates 3T3-L1 adipocyte differentiation. Phytother Res. 2010;24:1592-1599. [DOI] [PubMed] [Google Scholar]
- 24. Ma L, Chen H, Dong P, Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013;139:503-508. [DOI] [PubMed] [Google Scholar]
- 25. Kahlos K, Hiltunen R. Identification of some lanostane type triterpenes from Inonotus obliquus. Acta Pharma Fennica. 1983;92:220. [Google Scholar]
- 26. Kahlos K, Kahlos L, Hiltunen R. Antitumor tests of inotodiol from the fungus Inonotus obliquus. Acta Pharma Fennica. 1986;95:173-177. [Google Scholar]
- 27. Kahlos K, Kangas L, Hiltunen R. Antitumor activity of some compounds and fractions from an n-hexane extract of Inonotus obliquus in vitro. Acta Pharma Fennica. 1987;96:33-40. [Google Scholar]
- 28. Kahlos K, Kangas L, Hiltunen R. Antitumor activity of triterpenes in Inonotus obliquus. Planta Med. 1986;6:554. [DOI] [PubMed] [Google Scholar]
- 29. Kahlos K, Kangas L, Hiltunen R, Schantz MV. The antitumor activity of some extracts and compound isolated from Inonotus obliquus. Farmaceutish tudschrift voor Belgie. 1984;61:305-306. [Google Scholar]
- 30. Zhong XH, Wang LB, Sun DZ. Effects of inotodiol extracts from Inonotus obliquus on proliferation cycle and apoptotic gene of human lung adenocarcinoma cell line A549. Chin J Integr Med. 2011;17:218-223. [DOI] [PubMed] [Google Scholar]
- 31. Zhao LW, Zhong XH, Yang SY, Zhang YZ, Yang NJ. Inotodiol inhabits proliferation and induces apoptosis through modulating expression of cyclinE, p27, bcl-2, and bax in human cervical cancer HeLa cells. Asian Pac J Cancer Prev. 2014;15:3195-3199. [DOI] [PubMed] [Google Scholar]
- 32. Rastogi S, Pandey MM, Rawat KSA. Medicinal plants of the genus Betula—traditional uses and a phytochemical-pharmacological review. J Ethnopharmacol. 2015;159:62-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Broza SK, Dobeš C, Klatte-Asselmeyer V, Saukel J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J Ethnopharmacol. 2010;131:33-55. [DOI] [PubMed] [Google Scholar]
- 34. El Beyrouthy M, Arnold N, Delelis-Dusollier A, Dupont F. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J Ethnopharmacol. 2008,120:315-334. [DOI] [PubMed] [Google Scholar]
- 35. Gründemann C, Gruber CW, Hertrampf A, Zehl M, Kopp B, Huber R. An aqueous birch leaf extract of Betula pendula inhibits the growth and cell division of inflammatory lymphocytes. J Ethnopharmacol. 2011;136:444-451. [DOI] [PubMed] [Google Scholar]
- 36. Dehelean CA, Soica C, Ledeţi I, et al. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem Cent J. 2012;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drag M, Surowiak P, Drag-Zalesinska M, Dietel M, Lage H, Oleksyszyn J. Comparison of the cytotoxic effects of birch bark extract, betulin and betulinic acid towards human gastric carcinoma and pancreatic carcinoma drug-sensitive and drug-resistant cell lines. Molecules. 2009;14:1639-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fulda S. Betulinic acid for cancer treatment and prevention. Int J Mol Sci. 2008;9:1096-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernicchia A. Polyporaceae s.l. Fungi europaei, 10. Aalassio, Italy: Candusso; 2005. [Google Scholar]
- 40. Breitenbach J, Kränzlin F. Champignons de Suisse. Tome 2: Champignons sans lames (Hétérobasidiomycètes; Aphyllophorales; Gastéromycètes). Lucerne, Switzerland: Mycologia; 1996. [Google Scholar]
- 41. Campbell WA, Davidson RW. A Poria as the fruiting stage of the fungus causing the sterile conks on Birch. Mycologia. 1938;30:553-560. [Google Scholar]
- 42. Zabel RA. Basidiocarp development in Inonotus obliquus and its inhibition by stem treatments. Forest Sci. 1976;22:431-437. [Google Scholar]
- 43. Lee MW, Hur H, Chang KC, Lee TS, Ka KH, Jankovsky L. Introduction to distribution and ecology of sterile conks of Inonotus obliquus. Mycobiology. 2008;36:199-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cambie RC. Betulin from Polyporus pinicola. N Z J Sci. 1978;21:565-567. [Google Scholar]
- 45. Cole RJ, Schweikert MA. Handbook of Secondary Fungal Metabolites. Vol 2 London, England: Academic Press; 2003. [Google Scholar]
- 46. Zhao F, Mai Q, Ma J, et al. Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia. 2015;101:34-40. [DOI] [PubMed] [Google Scholar]
- 47. Liu C, Zhao C, Pan HH, et al. Chemical constituents from Inonotus obliquus and their biological activities. J Nat Prod. 2014;77:35-41. [DOI] [PubMed] [Google Scholar]
- 48. Ríos JL, Andújar I, Recio MC, Giner RM. Lanostanoids from fungi: a group of potential anticancer compounds. J Nat Prod. 2012;75:2016-2044. [DOI] [PubMed] [Google Scholar]
- 49. Fan L, Ding S, Ai L, Deng K. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohydr Polym. 2012;90:870-874. [DOI] [PubMed] [Google Scholar]
- 50. Mazurkiewicz W. Analysis of aqueous extract of Inonotus obliquus. Acta Pol Pharm. 2006;63:497-501. [PubMed] [Google Scholar]
- 51. Wang Q, Mu H, Zhang L, Dong D, Zhang W, Duan J. Characterization of two water-soluble lignin metabolites with antiproliferative activities from Inonotus obliquus. Int J Biol Macromol. 2015;74:507-514. [DOI] [PubMed] [Google Scholar]
- 52. Glamočlija J, Ćirić A, Nikolić M, et al. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom.” J Ethnopharmacol. 2015;162:323-332. [DOI] [PubMed] [Google Scholar]
- 53. Foo JB, Yazan SL, Tor YS, et al. Induction of cell cycle arrest and apoptosis by betulinic acid-rich fraction from Dillenia suffruticosa root in MCF-7 cells involved p53/p21 and mitochondrial signaling pathway. J Ethnopharmacol. 2015;166:270-278. [DOI] [PubMed] [Google Scholar]
- 54. Yim NH, Jung YP, Kim A, Kim T, Ma JY. Induction of apoptotic cell death by betulin in multidrug-resistant human renal carcinoma cells. Oncol Rep. 2015;34:1058-1064. [DOI] [PubMed] [Google Scholar]
- 55. Mazurkiewicz W, Rydel K, Pogocki D, Lemieszek MK, Langner E, Rzeski W. Separation of an aqueous extract Inonotus obliquus (Chaga). A novel look at the efficiency of its influence on proliferation of A549 human lung carcinoma cells. Acta Pol Pharm. 2010;67:397-406. [PubMed] [Google Scholar]
- 56. Youn MJ, Kim JK, Park SY, et al. Potential anticancer properties of the water extract of Inonotus obliquus by induction of apoptosis in melanoma B16-F10 cells. J Ethnopharmacol. 2009;121:221-228. [DOI] [PubMed] [Google Scholar]
- 57. Youn MJ, Kim JK, Park SY, et al. Chaga mushroom (Inonotus obliquus) induces G0/G1 arrest and apoptosis in human hepatoma HepG2 cells. World J Gastroenterol. 2008;14:511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen C, Zheng W, Gao X, et al. Aqueous extract of Inonotus obliquus (Fr) pilat (hymenochaetaceae) significantly inhibits the growth of sarcoma 180 by inducing apoptosis. Am J Pharmacol Toxicol. 2007;2:10-17. [Google Scholar]
- 59. Chung MJ, Chung CK, Jeong Y, Ham SS. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing sarcoma-180 cells. Nutr Res Pract. 2010;4:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]