Abstract
Background: Pediatric cancer patients experience different psychological processes during hospitalization that may regulate the immune response and affect recovery and response to cancer treatment. In this study, we aimed to examine the feasibility of longitudinal testing of psychophysiological parameters of stress and fatigue in pediatric osteosarcoma patients hospitalized for chemotherapy submitted to clown intervention; and to investigate whether changes in the levels of biomarkers are associated with psychological stress and fatigue levels in these patients after the clown intervention. Methods: A pretest-posttest quasi-experimental pilot study was conducted at the pediatric oncology inpatient unit in a comprehensive cancer care center in Brazil including children and adolescents with osteosarcoma hospitalized for chemotherapy. Eight saliva samples were collected, comprising 4 at baseline (pre-intervention) and 4 after the clown intervention (+1, +4, +9, and +13 hours post-awakening). Salivary cortisol, α-amylase (sAA), cytokines, and matrix metalloproteinase-9 (MMP-9) levels were determined using high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits. Stress and fatigue were measured by Child Stress Scale–ESI and PedsQL Multidimensional Fatigue Scale respectively. Bivariate association analysis between stress and fatigue scores and biomarker levels were investigated using nonparametric statistics. Effect sizes were calculated for each outcome variable. Results: Six pediatric osteosarcoma patients were enrolled with no missing data. No significant effects sizes were observed for psychophysiological outcomes. Effect sizes ranged from 0.54 (cortisol) to 0 (interleukin-1β [IL-1β]). Decreasing overall trends were observed for cortisol levels for all 6 pediatric osteosarcoma patients over time. In addition, a similar pattern of tumor necrosis factor–α (TNF-α) levels over time was found for all 6 patients. Patients with metastatic osteosarcoma showed a linear trend for a decrease in MMP-9 levels between 1 and 9 hours after the clown intervention and restoration to basal levels after 13 hours. Conclusions: The results of this pilot study suggest that it is feasible longitudinally measure psychophysiological outcomes in the pediatric osteosarcoma inpatients for chemotherapy. Clown intervention merits further study as a way to reduce stress as well as fatigue, since that the stress and cytokines measurements are feasible based on our work.
Keywords: psychological stress, cancer-related fatigue, biomarkers, pediatric inpatients, osteosarcoma, clown intervention.
Introduction
Osteosarcoma is a relatively rare (0.2% of all neoplasms), highly malignant tumor, with a global incidence of approximately 1 to 3 cases/million a year.1,2 Its incidence increases with age throughout childhood, being most common during adolescence.1,2 Landmark studies have demonstrated improved outcomes in patients submitted to neoadjuvant and adjuvant chemotherapy, and patients with localized cancer have achieved 70% long-term survival.3-5 However, symptom cluster management efforts have not developed in a pace similar to that of development of new therapies in pediatric oncology.6,7
Pediatric cancer patients experience multiple physical and psychoneurological symptoms (e.g., cancer-related fatigue [CRF], stress, pain, anxiety) with a high level of symptom distress experienced mainly during hospitalization for chemotherapy.8 CRF is the most prevalent symptom experienced during treatment of childhood cancer and provides a foundation for exploring symptom associations and interactions.9 Pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor–α (TNF-α) have been implicated in the pathophysiology of CRF and altered sleep patterns.10 Also, the psychological stress experienced in the course of cancer treatment causes different behaviors in pediatric patients and negatively affects their quality of life (QoL). In addition, as treatment evolves, it suppresses important facets of the immune response such as the activity of natural killer (NK) cells and the proliferation of T cells.11 A few studies have explored the biological mechanisms of symptom clusters in adult cancer patients, with a primary focus on serum cytokines,12-15 but similar research in pediatric oncology is still lacking. The convergence of subjective and objective measures could lead to the development of personalized strategies for the management of cancer symptom clusters.6,16
Psychological stress associated with cancer development leads to disturbances/disruption in the hypothalamic-pituitary-adrenal (HPA) axis and suppresses important neuroimmunoendocrine pathways.12,14,17 Interventions aimed at attenuating the physiological changes related to chronic stress favor the “recovery” of the immune system and, consequently, induce alterations in neuroendocrine and immune factors that increase immunological surveillance during cancer treatment.12 In an attempt to alleviate some of the cancer-related symptoms, pediatric oncology patients can take advantage of nonpharmacological interventions,18 including clown intervention, which may be an advantageous approach to reduce stress in pediatric cancer patients.
Clown intervention began in North America in the 1980s and, since then it has become very well accepted and praised in hospital wards, especially pediatric ones. Indeed, it is thought to play an important complementary role in pediatric care and recovery.19-22 Recently, 2 meta-analyses confirmed that clown intervention has a positive effect on pediatric patient and parent outcomes.23,24 This hypothesis is supported by studies showing that clown intervention enhances emotional and behavioral responses.25,26 Despite this recognition, few studies have investigated the molecular mechanisms that mediate the positive health outcomes of clown intervention. To the best of our knowledge, only 2 studies27,28 conducted with hospitalized children with different acute pathologies receiving clown intervention have used biomarker levels (salivary cortisol) as a variable to assess stress outcome. These studies found lower levels of salivary cortisol following clown intervention compared with preintervention levels.
Based on the knowledge that psychological processes may regulate the immune response and that patient well-being can significantly affect recovery and response to cancer treatment,29 in this pilot study we aimed (1) to examine the feasibility of longitudinal testing of psychophysiological parameters of stress and fatigue in pediatric osteosarcoma patients hospitalized for chemotherapy submitted to clown intervention and (2) to investigate whether changes in the levels of biomarkers are associated with psychological stress and fatigue levels in these patients after the clown intervention.
Methods
Design and Participants
This pilot study used a pretest-posttest quasi-experimental design.30 Clown intervention is part of the routine of the hospital where the study was conducted. The study evaluated the effect of clown intervention (independent variable) on psychological stress and cancer-related fatigue levels (dependent variables) with nonrandom allocation of the subjects.
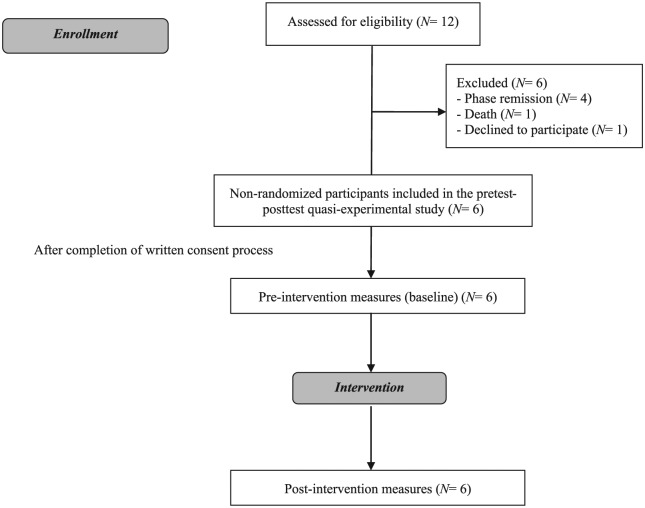
We recruited 12 children and adolescents with osteosarcoma between August 2015 and August 2016 among consecutive attendants at the Pediatric Oncology Department of the Ribeirão Preto Medical School Hospital at University of São Paulo (HCFMRP/USP), Ribeirão Preto, Brazil. Of the patients invited to be part of the study, 6 met all eligibility criteria and agreed to participate (Figure 1).
Figure 1.
Diagram for participant flow.
To be eligible for the study participants had to be pediatric oncology patients, either gender, aged 6 to 15 years, diagnosed with osteosarcoma and hospitalized for chemotherapy at HCFMRP/USP. All participants were receiving chemotherapy for osteosarcoma during the study data collection. In addition, participants had to be awake, aware, willing to participate, and able to understand the study design, answer the scales, and communicate verbally, in reading and writing in Portuguese.
Patients with other chronic/autoimmune diseases or somatic and/or mental illnesses; patients receiving radiotherapy; patients receiving palliative care; patients using antidepressants and/or mood-altering drugs; patients with potential coulrophobia (fear of clowns); patients with active infectious conditions (eg, febrile neutropenia); patients in the immediate postoperative period; and patients with oral lesions and/or dental cavities that could affect saliva collection were excluded from the study.
The study was approved by the Institutional Review Board at Ribeirão Preto College of Nursing, University of São Paulo, Brazil (reference 815.213/2014). After study procedures had been fully explained, written informed consent was obtained from one or both parents, and assent of the child (affirmative agreement) was collected for children 6 years of age or older.
Procedures
Experimental Procedure
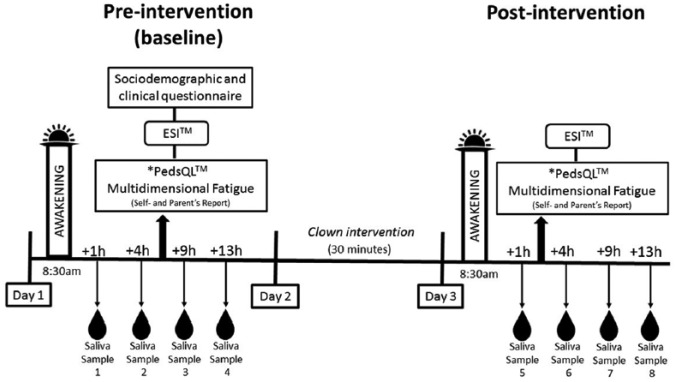
Because this is a pretest-posttest study, all patients served as their own controls before and after the intervention over a 3-day period. The experimental scheme is shown in Figure 2.
Figure 2.
Experimental scheme.
Each participant received 1 session of the clown intervention and provided a total of 8 saliva samples over a 3-day period (4 samples collected at baseline/pre-intervention and 4 samples at post-intervention). All saliva samples were collected each day at the same time for all participants to maintain comparability among participants and to avoid that differences would be due to normal daily fluctuations in biomarkers.
Pre- and post-intervention samples were collected at +1, +4, +9, and +13 hours post-awakening (8:30 am), that is, at 9:30 am, 12:30 pm, 5:30 pm, and 9:30 pm, respectively, according to international recommendations and previous studies on the dosage of cortisol31 and to better reflect the daily profile of α-amylase.32-34
To minimize external influence of clinical procedures on the measurements, in all sample collection time points, osteosarcoma pediatric patients had no invasive procedures or any other acute stresses in the last hour before sample collection and underwent preparation for saliva collection.
The preparation for saliva collection consisted of not ingesting any food or drinks 1 hour before the procedure and not brushing the teeth or using mouthwash before collection. After preparation had been completed, participants were requested to refrain from swallowing briefly (for 30 seconds) and then “drooling” the saliva from the mouth as much as possible directly into the collection device.
Intervention
Clown intervention was performed by undergraduate and graduate students from the University of São Paulo (USP) who were members of Companhia do Riso (The Laugh Company). Established in 1996, Companhia do Riso is an extension project that serves the 3 pillars of the University of São Paulo (teaching–research–extension) and whose aim is to lift the moods of children/adolescents during hospitalization as well as their families and staff. Companhia do Riso clowns seek to parody the medical routine using humor to help children adapt to the hospital environment, the intimidating medical jargon and procedures, and to defuse states of anxiety. Thus, using clown intervention in the pediatric setting may assist in the recovery process and help minimize distress, which are the main purpose of Companhia do Riso.35
Children accompanied by their parents interacted simultaneously with 2 volunteer clowns in the pediatric oncology ward for approximately 30 minutes at a time. In this period, the clowns performed several activities while adapting their techniques to the best of their ability to each patient’s age and psychological condition. The 2 clowns arranged common play sessions with patients in their ward and used various methods for entertaining the children such as singing, dancing, magic tricks, gags, puppets, games, mostly using improvisation and their distinctive humor and charisma. Each clown has his or her own identity and style, with specific practical and theoretical background.35 Before the intervention, the volunteers participated in specific training sessions focused on practical work situations to develop theatrical and artistic/clown competences in addition to social, psychological, and pedagogical skills. Importantly, the aim of this study was to evaluate the effect of clown intervention on psychological stress and fatigue levels as a whole, and not a specific technique of the clown art. All clowns who participated in the sessions had been working in that hospital for at least 2 years. Regular meetings were held for the entire duration of the study to discuss any problems and difficulties that may have arisen during data collection. Moreover, once a week, the Companhia do Riso coordinator attended the sessions to supervise the work.
Measures
Clinical/Medical Assessment
Sociodemographic data (age, gender, level of education, family income) and clinical variables (body weight, height, body mass index, body surface area, vital signs, complete blood count at baseline (as a routine hospital procedure), time since diagnosis, therapeutic modality, time since hospitalization, time since initiation of chemotherapy, presence of metastases, chemotherapy protocol, use of corticosteroids; and experience of coulrophobia were recorded on day 1 between 12:30 pm and 5:30 pm (+4 and +9 hours post-awakening). All information was collected from medical records.
Stress Measurement
The Child Stress Scale (Escala de Estresse Infantil, ESI) is derived from the Child Stress Symptoms Inventory and was validated to Portuguese language and Brazilian population by 2 psychologists.36 The ESI is used to evaluate stress in children aged 6 to 14 years and includes 4 dimensions of stress (physical, psychological, psychological with depressive components, and psychophysiological), enabling us to determine which type of stress reaction is more frequent and in which dimension it is stronger. The ESI is based on questions that were answered by the child or adolescent using a 4-point Likert-type scale in the form of a circle that must be filled in (painted). Each quarter of the filled circle corresponds to one point. The scoring procedure is based on cutoff points for each of the four dimensions with higher scores indicating higher stress levels.36 Scoring was performed by a psychologist blinded to whether the data were from pre- or post-intervention. The ESI has been validated in Brazil, with high internal consistency (Cronbach’s α = .90) and high correlation between the dimensions (Spearman’s correlation, rs = .73).36 The ESI is applicable for a maximum period of up to 1 month of the stressor event and used to determine small changes in a short period of time as in our case. Moreover, the ESI has been shown to hold the attention of the children and adolescents, helping them to reflect on their feelings and symptoms, thus allowing for a reliable measurement of their stress levels.36 ESI was administered on day 1 (baseline) between 12:30 pm and 5:30 pm (+4 and +9 hours post-awakening) and on day 3 (post-intervention) between 9:30 am and 12:30 pm (+1 and +4 hours post-awakening).
Fatigue Measurement
The Pediatric Quality of Life Inventory (PedsQL) Multidimensional Fatigue Scale (MFS) includes three dimensions that evaluate general fatigue, fatigue and sleep/rest, and cognitive fatigue.37 The three dimensions use a 5-point Likert-type scale ranging from 0 (“never”) to 4 (“almost always”). For scoring, items are reversed scored and linearly transformed to a 0-100 score, with higher scores indicating lower fatigue levels. The PedsQL is a globally recognized instrument whose reliability has been demonstrated in other studies.37 In Brazil, this instrument has been recently validated for the pediatric oncology population and its reliability as measured by the internal consistency of items and dimensions was acceptable (Cronbach’s α between .70 and .90 for both self and proxy versions).38 Factorial analysis showed that the original structure of the PedsQL has been retained in the Brazilian version.38 The choice of this instrument for our investigation was based on three reasons. First, children and adolescents should always be the primary source of information on their fatigue,39 unless they are unable or unwilling to report because of issues related to their disease or treatment.40 Second, the parents’ perspective can be valid, especially with younger children where parents have closer contact and caregiving roles with the child. Moreover, the impact of the disease in the entire family often leads to a closer interaction between family members, who participate more closely in the child’s disease experience.40 Third, the PedsQL MFS is an effective and reliable tool for measuring fatigue worldwide,37 which allowed the comparison of our findings with international outcomes. Hence, a valid and reliable instrument for measuring fatigue in Portuguese allowed us to better assess this symptom in this particular context.38 PedsQL MFS (self and proxy versions) was administered on the same days and times as the ESI.
Biological Samples and Laboratory Methods
A total of 8 nonfasting saliva samples were collected over a 3-day period (4 at baseline and 4 at post-intervention) for measurement of biomarkers including cortisol, salivary α-amylase (sAA), pro- and anti-inflammatory cytokines, and matrix metalloproteinase-9 (MMP-9). Saliva samples (2 mL) were collected from each participant using the passive drool technique, which is considered the gold standard for obtaining many components of saliva. Participants were instructed to drool through a sterile saliva collection aid (Salimetrics, LCC, State College, PA, USA) to collect saliva directly into a cryovial, which retains saliva contaminants. After saliva collection, the samples were taken to the Laboratory of Genomics and Immunobiology at USP, Brazil. Saliva samples were aliquoted into polypropylene microtubes (Eppendorf), centrifuged for 15 minutes at 3000 rpm and 5°C, and stored at −80°C for later batch analysis. Saliva samples were run in duplicates (50 μL each) along with standards and controls in all assays.
Basal HPA axis and sympathetic nervous system activities were measured by assessment of salivary cortisol and sAA, respectively. Under normal circumstances, salivary cortisol peaks at waking, then steadily declines throughout the day, whereas sAA sharply drops at waking and then steadily increases throughout the day.32,33,41 sAA activity was measured as described by Rohleder et al.42,43 In short, 20 μL of standards and diluted saliva (1:625) were incubated with 80μl of substrate reagent (Salimetrics, LCC) in a water bath at 37°C for 90 seconds. After a second incubation (5 minutes) and measurement, absorbance changes were recorded and converted to sAA concentrations using a linear regression calculated for each microplate (inter- and intra-assay coefficients of variation <10%). Free cortisol levels in saliva were measured in duplicate using a commercially available chemiluminescence assay (Salimetrics LLC). Inter- and intra-assay variation was less than 10%.
Salivary cortisol, sAA (Salimetrics LLC), cytokines (BD Biosciences, San Diego, CA, USA), and MMP-9 (R&D, Minneapolis, MN, USA) concentrations in saliva were measured using high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits for human samples following the manufacturers’ instructions.
Area under the curve (AUC) was calculated for salivary cortisol and sAA to measure their respective total daily production based on the log-trapezoidal method.44
Statistical Analysis
Statistical analyses were performed using R 3.2.3 “Wooden Christmas Tree” for Windows (R Development Core Team, 2016) and GraphPad Prism version.6.0 (GraphPad Software, Inc, San Diego, CA, USA). Descriptive analyses were conducted to characterize the samples. Bivariate association analyses were performed to compare psychological and fatigue stress scores and biomarkers concentrations before and after the intervention using the nonparametric paired Wilcoxon’s signed rank test. Effect sizes using Cohen’s d was calculated for each variable.45 Significance level was kept at .05 for all tests, with the understanding that this is an exploratory study (as opposed to confirmatory) and accordingly, cautious interpretation of the results will be performed.
Results
Sample Characteristics
Six pediatric patients (3 girls and 3 boys) with osteosarcoma were included in the study and there were no missing values for any of the measures for all participants. The mean age of the patients was 12.3 years (standard deviation [SD] = 3.32 years), 50% were Caucasian and had completed primary school. Sociodemographic and clinical data are shown in Table 1. None of the patients had coulrophobia. In addition, there were no deviations from the collection protocol.
Table 1.
Sociodemographic and Clinical Characteristics of Pediatric Patients With Osteosarcoma (N = 6).
| Variable | Value |
|---|---|
| Sociodemographic characteristic | |
| Age, years, mean (SD) [min-max] | 12.33 (3.32) [6-15] |
| Gender, female, n (%) | 3 (50.0) |
| Caucasian, n (%) | 3 (50.0) |
| Education, n (%) | |
| Preschool | 1 (16.7) |
| Primary school | 3 (50.0) |
| Secondary school | 2 (33.3) |
| Clinical characteristic | |
| Body weight, kg, mean (SD) [min-max] | 45.47 (14) [19-60] |
| Body mass index, kg/m2, mean (SD) [min-max] | 19.39 (4.12) [14.37-26.31] |
| Body surface area, m2, mean (SD) [min-max] | 1.35 (0.29) [0.76-1.58] |
| Lung metastases, n (%) | |
| No | 2 (33.3) |
| Yes | 4 (66.6) |
| Chemotherapy protocol, n (%) | |
| Cisplatin, dexrazoxane, and doxorubicin | 3 (50.0) |
| Methotrexate with leucovorin | 3 (50.0) |
| Dexamethasone use, n (%) | |
| On | 3 (50.0) |
| Off | 3 (50.0) |
Psychological Stress and Fatigue Symptoms
Both psychological stress and fatigue levels measured by the ESI and PedsQL MFS, respectively, remained unchanged following clown intervention (pre-/post-intervention 30.8 (13.7) vs 26 (14.8), P = .15 and 73.7 (15.1) vs 73.9 (16.7), P = .84, respectively; Table 2). Parents and patients agreed on the responses to the 3 dimensions of the PedsQL MFS, as reflected by the Cohen’s κ coefficient index (κ = 0.8).
Table 2.
Scoring of PedsQL Multidimensional Fatigue Scale and Child Stress Scale (Escala de Estresse Infantil, ESI) Dimensions in Pediatric Patients With Osteosarcoma at Pre-intervention (Baseline) and Post-intervention.
| Instrument | Preintervention (N = 6), Mean (SD) | Postintervention (N = 6), Mean (SD) | P a |
|---|---|---|---|
| PedsQL dimensions (self-report) | |||
| General fatigue | 78.4 (14) | 77.7 (21) | >.999 |
| Sleep/rest fatigue | 66.4 (9.1) | 66.4 (16.8) | .84 |
| Cognitive fatigue | 76.3 (29.8) | 77.7 (21) | .78 |
| Total PedsQL fatigue score | 73.7 (15.1) | 73.9 (16.7) | .84 |
| PedsQL dimensions (parent’s report)b | |||
| General fatigue | 77 (3.4) | 63.1 (17.7) | .14 |
| Sleep/rest fatigue | 63.1 (16.7) | 55.5 (13.6) | .34 |
| Cognitive fatigue | 89.5 (10.1) | 90.2 (11.9) | >.999 |
| Total PedsQL fatigue score | 76.6 (8) | 69.6 (9.9) | .093 |
| ESI domains | |||
| Physical reactions | 6.3 (5.7) | 5.3 (3.2) | .52 |
| Psychological reactions | 11.6 (4.2) | 11.1 (6.7) | >.999 |
| Psychological reactions with depressive component | 6 (4.5) | 4.8 (5.1) | .40 |
| Psychophysiological reactions | 6.8 (4.1) | 4.6 (2.2) | .28 |
| Total ESI score | 30.8 (13.7) | 26 (14.8) | .15 |
Abbreviations: ESI, Escala de Estresse Infantil (Child Stress Scale); PedsQL, Pediatric Quality of Life Inventory.
P values based on Wilcoxon’s signed rank test.
The concordance index of the parents and children responses for the PedsQL Multidimensional Fatigue Scale dimensions were not significantly different at both pre- and postintervention (P > .05).
Salivary Cortisol, sAA, Cytokines, and MMP-9 Trajectories Following Clown Intervention
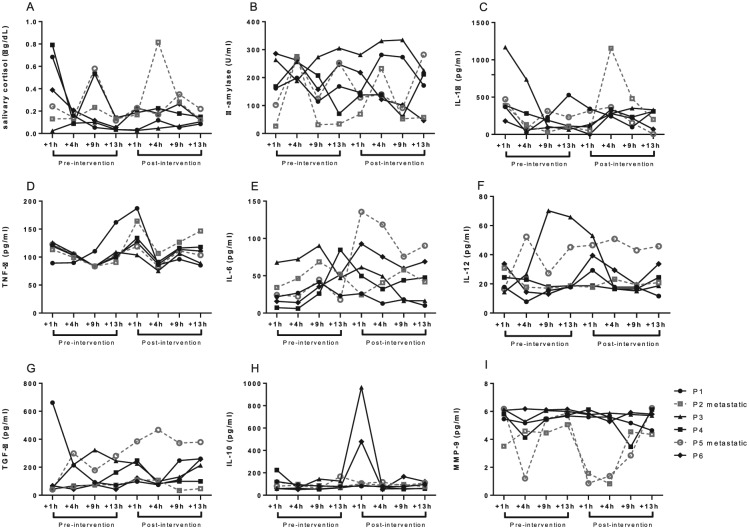
Figure 3 shows the pattern of biomarkers trajectories on clown intervention per patient at pre-intervention (baseline/day 1) and at post-intervention (day 3). Salivary cortisol and sAA AUCs were calculated for baseline (pre-intervention) and post-intervention. No significant changes were found for either salivary cortisol AUC (P = .39) or sAA AUC (P = .58). Although no significant changes were found comparing pre and post-intervention, decreasing overall trends were observed for cortisol levels for all 6 pediatric patients with osteosarcoma over time. No trends were observed for sAA levels with regard to longitudinal changes (Figure 3B).
Figure 3.
(A) Salivary cortisol, (B) salivary α-amylase, (C-F) pro-inflammatory cytokines, (G and H) anti-inflammatory cytokines, and (I) matrix metalloproteinase-9 trajectories in children and adolescents with osteosarcoma on clown intervention (N = 6). The graphs show the biomarkers trajectories per patient at preintervention (baseline/day 1) and at postintervention (day 3). Pediatric patients with metastatic osteosarcoma are represented in the graph by dashed lines. Full lines show pediatric patients with nonmetastatic osteosarcoma. Samples were collected +1, +4, +9, and +13 hours post-awakening (8:30 am), that is, at 9:30 am, 12:30 pm, 5:30 pm, and 9:30 pm, respectively.
Among the cytokines, no trends were found in IL-1β, IL-6, IL-12p70, TGF-β, and IL-10 along the collection time points (Figure 3C and E-H, respectively). However, as shown in Figure 3D, all 6 patients exhibited a similar pattern of TNF-α levels over time, which consisted in a shortly increase after 1 hour of intervention and then restoration to baseline levels following more 3 hours of measurement (+4 hours postintervention).
Furthermore, MMP-9 levels remained stable over time in pediatric patients with nonmetastatic osteosarcoma. Meantime, patients with metastatic osteosarcoma showed a trend for a decrease in MMP-9 levels between 1 and 9 hours after the clown intervention and restoration to basal levels after 13 hours (Figure 3I).
Effect Sizes of the Outcomes
Calculated effect sizes are shown in Table 3. Effect sizes ranged from 0.54 (cortisol) to 0 (IL-1β). With the current sample size of 6 patients, medium effects were observed for (cortisol, MMP-9 and perceived fatigue from the parents) and small effects for others outcome variables.
Table 3.
Effect Sizes for Each Outcome Variable.
| Variables | z-testa | P | Effect Size (d)b |
|---|---|---|---|
| Total PedsQL fatigue score (self-report) | 0.19 | .84 | 0.05 |
| Total PedsQL fatigue score (parents report) | 1.67 | .09 | 0.48 |
| Total ESI score | 1.41 | .15 | 0.40 |
| Salivary cortisol AUC | 1.86 | .05 | 0.54 |
| Salivary α-amylase AUC | 0.19 | .84 | 0.05 |
| IL-1β | 0.00 | >.999 | 0 |
| TNF-α | 0.77 | .43 | 0.22 |
| IL-6 | 0.57 | .56 | 0.16 |
| IL-12p70 | 0.19 | .84 | 0.05 |
| TGF-β | 0.40 | .68 | 0.11 |
| IL-10 | 0.57 | .56 | 0.16 |
| MMP-9 | 1.77 | .08 | 0.50 |
Abbreviations: ESI, Escala de Estresse Infantil (Child Stress Scale); PedsQL, Pediatric Quality of Life Inventory; IL, interleukin; TNF-α, tumor necrosis factor–α; TGF-β, transforming growth factor–β; MMP-9, matrix metalloproteinase-9; AUC, area under curve.
Cohen’s d effect size calculator for z-test. For the single-sample z-test, Cohen’s d is calculated by subtracting the population mean (before intervention) from the sample mean (after the intervention), and then dividing the result by the population’s standard deviation.
Effect sizes using Cohen’s d were calculated for each variable.45 Interpreting Cohen’s d: d = 0.2 small effect—mean difference is 0.2 SD; d = 0.5 medium effect—mean difference is 0.5 SD; d = 0.8 large effect—mean difference is 0.8 SD.
Discussion
Feasibility
The results of this study suggest that it is feasible to longitudinally measure psychophysiological outcomes in the pediatric osteosarcoma inpatients for chemotherapy. Children and adolescents with osteosarcoma hospitalized for chemotherapy adhered to the saliva collection protocol, and there was no missing questionnaires or saliva sample data. Furthermore, a low attrition rate (7%) was observed over the 12-month observation period. This low attrition rate and no missing data suggest that the instruments and specimen collection were not overly burdensome for pediatric osteosarcoma inpatients and their parents. The feasibility of this pilot study supports replicating this study with a larger sample to test hypotheses.
Psychologic Outcomes
We did not find evidence that the outcomes measured by the ESI and PedsQL MFS, respectively, changed following clown intervention. Contrary to our results, one study that evaluated the impact of a humor therapy program on stress levels in pediatric inpatients with different diagnoses showed that the children in the intervention group presented lower scores on the Parker test—a validated scale for evaluating emotional stress, than children in the nonintervention group.28 In addition, other 2 studies46,47 reported that hospitalized children and adolescents who received clown intervention had an increase in self-reported psychological well-being and an improvement in emotional responses, compared with those in the control group. Probably, the differences found between our results and the others may be partly justified by methodological issues that include study instruments used, design and sample. It is also important to consider the stage of treatment, the type of the chemotherapy protocol and the age of cancer diagnosis as variables that may mediate the relationship with the psychological stress and fatigue.18
Physiologic Outcomes
In this study, although there were no significant effects sizes observed for parameters related to salivary cortisol or sAA, linear trends were found for cortisol levels for all 6 pediatric osteosarcoma patients over time, which was observed to be decreased after clown intervention. These results are consistent with the well-characterized cortisol circadian cycle.48-50 Like other hormones, the circadian rhythm of cortisol is already known, floating during the day according to a predictable cycle and demonstrating variations (day light and dark). It is normally secreted by the adrenal gland in short bursts, with 15 to 30 pulses throughout a day.48 Under normal circumstances, salivary cortisol peaks at waking then steadily declines throughout the day,48-50 whereas sAA sharply drops at waking and then steadily increases throughout the day.32,33
It is well established in the literature that salivary cortisol and sAA are acceptable as noninvasive measures of stress because they correlate with respective plasma levels, which are excellent indicators of the secretion pattern of these molecules.48 Cortisol is used as a biomarker of endocrine stress response and adrenal cortical function41,48,49,50 and reflects the activity of the HPA axis, whereas sAA activity is considered a marker of the sympathetic activity of the autonomic nervous system.32,33 Recent reports have shown lower cortisol levels after clown intervention in pediatric patients in acute conditions27,28 but to the best of our knowledge this is the first study to demonstrate these results in pediatric patients with osteosarcoma.
Abnormal cortisol release in cancer patients may contribute to chronic inflammation.41 Hence, several types of nonpharmacological interventions have been used as targets and have been shown to reduce some of the stressors in pediatric cancer patients.18,51 Although longitudinal analysis of markers such as pro- and anti-inflammatory cytokines did not reveal any significant changes when compared baseline and post-intervention measurements, all 6 patients exhibited a similar pattern of TNF-α levels over time. Salivary pro-inflammatory cytokine levels have been implicated in inflammation and stress in numerous diseases, including cancer.52-55 Pro-inflammatory cytokines, especially TNF-α, IL-1β, and IL-6, have been classically associated with CRF.10,15,47,56 Increased levels of fatigue scores have been correlated with increased of TNF-α levels.10,14,15,57 Also, a previous systematic review and meta-analysis of the impact of psychological stress on inflammatory markers found robust effects for increases in TNF-α after episodes of acute stress.58 In our study, we have found a trend of an increase of TNF-α salivary levels after 1 hour of intervention followed by a restoration to baseline post 4 hours of intervention, which suggest that this nonpharmacological intervention might mediate the changes in cytokine levels that are involved in the pathophysiology of cancer-related fatigue, particularly, in a short term time.
It is noteworthy that some studies of pediatric cancer populations have reported alterations in proinflammatory cytokines that coincide with periods of increased stress,56,59,60 whereas other studies on cancer patients have not observed such trends.61-63 This discrepancy may occur due to differences in timing and techniques of measurements, in addition to the variability in cytokine release by multiple tissues, random fluctuations, and transient cytokine responses to changes in mood and dietary intake.58
Regarding MMP-9 concentration pattern, we observed that pediatric patients with metastatic osteosarcoma showed MMP-9 levels decreasing shortly on clown intervention. Osteosarcoma is a tumor highly metastatic and resistant to chemotherapy.3 In addition, it is known that MMP-9 expression is considered a valuable predictor of survival in some types of cancer, including osteosarcoma, and elevated levels of MMP-9 have been correlated to poor prognosis.64-66 Also, some experimental studies have suggested that stress can increase the risk of tumor progression and metastasis.65,67,68
Limitations
This pilot study represents an area of study in its initial stage and had limitations. First, it was a single-center study with only 6 participants, in which the external validity was not fully guaranteed. Also, as a result, longitudinal variations of some physiologic parameters such as cytokines and endocrine biomarkers may not have been detectable. Second, a quasi-experimental, pretest–posttest design was used instead of a randomized controlled trial design, mainly due to the nature of the intervention and the place where the study was held, which prevented that subjects be assigned randomly. A third limitation is the lack of a control group. The use of an age- and gender-matched control group consisting of medically healthy children would enable direct comparisons across psychophysiological parameters of stress, while controlling for potentially confounding factors. Fourth, the independent observers who administered the ESI and PedsQL MFS were not blind to the experimental conditions. Last, due to the sample size, other confounding factors (eg, hospitalization time, psychological stress due to the hospitalization process, quality of sleep on the night of the intervention) that may influence the relationship between the dependent variables (stress and fatigue responses) and the independent variable (clown intervention) could not be controlled by multivariate analysis.
Even though the pilot study reported here has limitations, it focused on a specific tumor type, making the sample somewhat homogenous. Because of the challenges of conducting randomized controlled trials with large samples when a specific type of cancer diagnosis is scarce or when the study encompasses a certain treatment phase, many studies involving pediatric cancer patients have investigated a range of tumors simultaneously.18,69
Directions for Future Research
The effect sizes generated by testing the constructs presented in Table 3 can be used to power future hypothesis-testing studies of pediatric patients’ perceived stress and fatigue and physiologic responses to stress and fatigue in the pediatric oncology setting involving nonpharmacological interventions. Indeed, for more than 30 years in the scientific literature, attention has repeatedly been drawn to the necessity of effect sizes reports.45,70 Although the value of P provides the probability of obtaining statistical significance, it does not inform on the clinical or practical importance of the results.71-74 In addition, Wilkinson and the American Psychological Association Task Force on Statistical Inference, in 1999, had already published guidelines for research published in psychology stating that research should always present effect sizes for primary outcomes, emphasizing that reporting and interpreting effect sizes in the context of previously reported effects is essential to good research.75 In particular, effect size has been reported by researchers in pilot studies76 as being important and usually a requirement to justify future studies in larger samples.74
Although so far this study is the first to report psychophysiological outcomes of pediatric osteosarcoma inpatients submitted to clown intervention, its limitations preclude statements regarding causality. A larger, prospective, multi-institutional, comparison group-controlled study of outcomes for this pediatric cancer patients would permit correlation analyses between psychological and physiologic outcomes and reduce potential biases. Simultaneous short- and long-term monitoring of the clown intervention could aid in interpretation of psychophysiological outcomes. Finally, other predictors of stress and fatigue responses should be evaluated in future studies with a larger sample size to minimize bias and enable a more detailed investigation using regression models and adjusting for covariates of physiological responses in pediatric cancer patients (e.g., initial distress level, child’s coping style, chemotherapy protocol, and presence of metastases).
It is noteworthy that the assessment of symptom clusters is a hallmark of oncology practice. Moreover, there is great potential to advance cancer research by evaluating psychoneurological symptom clusters via nonpharmacologic intervention and their interactions with biological pathways and developmental changes in symptom severity over time.7,77
Conclusions
The results of this pilot study suggest that it is feasible longitudinally measure psychophysiological outcomes in the pediatric osteosarcoma inpatients for chemotherapy. In addition, a trend was found for salivary cortisol and TNF-α levels for all pediatric patients on clown intervention. Furthermore, patients with metastatic osteosarcoma showed a pattern in MMP-9 levels, which decreased shortly after the clown intervention.
Over recent years, there has been a rapid expansion of clown intervention programs in the pediatric setting. Despite the popularity of this type of intervention, few studies have systematically investigated its effects using potential biomarkers. Importantly, our study expanded the range of health care settings previously investigated by others to include chronic diseases, that is, pediatric cancer patients. Our findings suggest that clown intervention merits further study as a way to reduce stress as well as fatigue, since that the stress and cytokines measurements are feasible based on our work.
Footnotes
Author Contributions: LCJC, GPS, and RAGL contributed substantially to the design of the study and data collection; LCJC collected the data; LCLJ, DSCS, LCV, and JCS, conducted the lab experiments and analyses and contributed to discussion of the manuscript. JBA conducted the statistical analyses. All authors interpreted the data and have contributed to writing, discussion and revised the manuscript critically. All authors have given final approval of the version of the article to be published. LCJC and RAG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to thank the Coordination of Improvement of Higher Education Personnel–CAPES, Brazil, which supported this research with regular doctoral scholarship to Luís Carlos Lopes-Júnior as well as his Doctoral Fellowship/Internship at the University of Alberta (UofA), Edmonton, Alberta, Canada by the Doctoral “Sandwich” Program Abroad–PDSE/CAPES (Process number: BEX 9321/14-4).
ORCID iD: Luis C. Lopes-Júnior  https://orcid.org/0000-0002-2424-6510
https://orcid.org/0000-0002-2424-6510
References
- 1. Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [DOI] [PubMed] [Google Scholar]
- 3. Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemo therapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600-1606. [DOI] [PubMed] [Google Scholar]
- 4. Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452-2458. [DOI] [PubMed] [Google Scholar]
- 5. Bernthal NM, Federman N, Eilber FR, et al. Long-term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high-grade, operable osteosarcoma. Cancer. 2012;118:5888-5893. [DOI] [PubMed] [Google Scholar]
- 6. Miaskowski C, Dodd M, Lee K, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J Pain Symptom Manage. 2010;40:531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopes-Júnior LC, Olson K, de Omena Bomfim E, Pereira-da-Silva G, Nascimento LC, de Lima RA. Translational research and symptom management in oncology nursing. Br J Nurs. 2016;25:S12, S14, S16 passim. [DOI] [PubMed] [Google Scholar]
- 8. Ruland CM, Hamilton GA, Schjødt-Osmo B. The complexity of symptoms and problems experienced in children with cancer: a review of the literature. J Pain Symptom Manage. 2009;37:403-418. [DOI] [PubMed] [Google Scholar]
- 9. Barsevick AM, Irwin MR, Hinds P, et al. ; National Cancer Institute Clinical Trials Planning Meeting. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(suppl):S48-S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chandwani KD, Ryan JL, Peppone LJ, et al. Cancer-related stress and complementary and alternative medicine: a review. Evid Based Complement Alternat Med. 2012;2012:979213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumor biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9:414-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDonald PG, O’Connell M, Lutgendorf SK. Psycho-neuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodologicl innovations. Brain Behav Immun. 2013;30(suppl):S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887-899. [DOI] [PubMed] [Google Scholar]
- 16. Momani TG, Mandrell BN, Gattuso JS, West NK, Taylor SL, Hinds PS. Children’s perspective on health-related quality of life during active treatment for acute lymphoblastic leukemia: an advanced content analysis approach. Cancer Nurs. 2015;38:49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321-328. [DOI] [PubMed] [Google Scholar]
- 18. Lopes-Júnior LC, Bomfim EO, Nascimento LC, Nunes MD, Pereira-da-Silva G, Lima RA. Non-pharmacological interventions to manage fatigue and psychological stress in children and adolescents with cancer: an integrative review. Eur J Cancer Care (Engl). 2016;25:921-935. [DOI] [PubMed] [Google Scholar]
- 19. Oppenheim D, Simonds C, Hartmann O. Clowning on children’s wards. Lancet. 1997;350:1838-1840. [DOI] [PubMed] [Google Scholar]
- 20. Spitzer P. Essay. Hospital clowns—modern-day court jesters at work. Lancet. 2006;368:S34-S35. [Google Scholar]
- 21. Barkmann C, Siem AK, Wessolowski N, Schulte-Markwort M. Clowning as a supportive measure in paediatrics—a survey of clowns, parents and nursing staff. BMC Pediatr. 2013;13:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vagnoli L, Caprilli S, Robiglio A, Messeri A. Clown doctors as a treatment for preoperative anxiety in children: a randomized, prospective study. Pediatrics. 2005;116:e563-e567. [DOI] [PubMed] [Google Scholar]
- 23. Sridharan K, Sivaramakrishnan G. Therapeutic clowns in pediatrics: a systematic review and meta-analysis of randomized controlled trials. Eur J Pediatr. 2016;175:1353-1360. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Yang Y, Lau WY, Garg S, Lao J. The effectiveness of pre-operative clown intervention on psychological distress: a systematic review and meta-analysis. J Paediatr Child Health. 2016;53:237-245. [DOI] [PubMed] [Google Scholar]
- 25. Bennett MP, Lengacher C. Humor and laughter may influence health IV. Humor and immune function. Evid Based Complement Alternat Med. 2009;6:159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stuber M, Hilber SD, Mintzer LL, Castaneda M, Glover D, Zeltzer L. Laughter, humor and pain perception in children: a pilot study. Evid Based Complement Alternat Med. 2009;6:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saliba FG, Adiwardana NS, Uehara EU, et al. Salivary cortisol levels: the importance of clown doctors to reduce stress. Pediatr Rep. 2016;8:6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sánchez JC, Echeverri LF, Londoño MJ, et al. Effects of a humor therapy program on stress levels in pediatric inpatients. Hosp Pediatr. 2017;7:46-53. [DOI] [PubMed] [Google Scholar]
- 29. Caserta MT, O’Connor TG, Wyman PA, et al. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain Behav Immun. 2008;22:933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gordis L. Epidemiology. 4th ed. Philadelphia, PA: WB Saunders; 2008. [Google Scholar]
- 31. Stalder T, Kirschbaum C, Kudielka BM, et al. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414-432. [DOI] [PubMed] [Google Scholar]
- 32. Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392-401. [DOI] [PubMed] [Google Scholar]
- 33. Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486-496. [DOI] [PubMed] [Google Scholar]
- 34. Lipschitz DL, Kuhn R, Kinney AY, Donaldson GW, Nakamura Y. Reduction in salivary α-amylase levels following a mind-body intervention in cancer survivors—an exploratory study. Psychoneuroendocrinology. 2013;38:1521-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Lima RAG, Azevedo EF, Nascimento LC, Rocha SM. The art of clown theater in care for hospitalized children [in Portuguese]. Rev Esc Enferm USP. 2009;43:186-193. [DOI] [PubMed] [Google Scholar]
- 36. Lucarelli MDM, Lipp MEN. Validação do Inventário de Sintomas de Stress Infantil—ISS-I. Psicol Reflex Crit. 1999;12:71-88. [Google Scholar]
- 37. Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090-2106. [DOI] [PubMed] [Google Scholar]
- 38. Nascimento LC, Nunes MD, Rocha EL, et al. Validity and reliability of the PedsQL™ Multidimensional Fatigue Scale for Brazilian children with cancer. J Pediatr Oncol Nurs. 2015;32:57-64. [DOI] [PubMed] [Google Scholar]
- 39. Hinds PS, Hockenberry-Eaton M, Gilger E, et al. Comparing patient, parent, and staff descriptions of fatigue in pediatric oncology patients. Cancer Nurs. 2000;22:277-289. [DOI] [PubMed] [Google Scholar]
- 40. Chang PC, Yeh CH. Agreement between child self-report and parent proxy-report to evaluate quality of life in children with cancer. Psychooncology. 2005;14:125-134. [DOI] [PubMed] [Google Scholar]
- 41. Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163-171. [DOI] [PubMed] [Google Scholar]
- 42. Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: an indicator of sympathetic activity? Ann N Y Acad Sci. 2004;1032:258-263. [DOI] [PubMed] [Google Scholar]
- 43. Rohleder N, Wolf JM, Maldonado EF, Kirschbaum C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 2006;43:645-652. [DOI] [PubMed] [Google Scholar]
- 44. Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time dependent change. Psychoneuroendocrinology. 2003;28:916-931. [DOI] [PubMed] [Google Scholar]
- 45. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 46. Pinquart M, Skolaude D, Zaplinski K, Maier RF. Do clown visits improve psychological and sense of physical well-being of hospitalized pediatric patients? A randomized-controlled trial. Klin Padiatr. 2011;223:74-78. [DOI] [PubMed] [Google Scholar]
- 47. Kingsnorth S, Blain S, McKeever P. Physiological and emotional responses of disabled children to therapeutic clowns: a pilot study. Evid Based Complement Alternat Med. 2011;2011:732394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jett PL, Samuels MH, McDaniel PA, et al. Variability of plasma cortisol levels in extremely low birth weight infants. J Clin Endocrinol Metab. 1997;82:2921-2925. [DOI] [PubMed] [Google Scholar]
- 49. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374-381. [DOI] [PubMed] [Google Scholar]
- 50. Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265-278. [DOI] [PubMed] [Google Scholar]
- 51. Nunes MD, Bomfim EO, Olson K, et al. Interventions minimizing fatigue in children/adolescents with cancer: an integrative review. J Child Health Care. 2018;22:186-204. [DOI] [PubMed] [Google Scholar]
- 52. Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2-18. [DOI] [PubMed] [Google Scholar]
- 53. Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dmitrieva OS, Shilovskiy IP, Khaitov MR, Grivennikov SI. Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochemistry (Mosc). 2016;81:80-90. [DOI] [PubMed] [Google Scholar]
- 55. Blundell S, Ray KK, Buckland M, White PD. Chronic fatigue syndrome and circulating cytokines: a systematic review. Brain Behav Immun. 2015;50:186-195. [DOI] [PubMed] [Google Scholar]
- 56. de Omena Bomfim E, Anatrielo E, Nunes MDR, et al. Correlations between functional Interleukin-1 and changes in fatigue and quality of life in children and adolescents with cancer. J Clin Oncol. 2015;33(29 suppl):95. [Google Scholar]
- 57. Saligan LN, Olson Filler K, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23:2461-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901-912. [DOI] [PubMed] [Google Scholar]
- 59. Vallance K, Yang J, Li J, Crabtree VM, Hinds PS, Mandrell BN. Disturbed sleep in pediatric patients with leukemia: the potential role of interleukin-6 (-174GC) and tumor necrosis factor (-308GA) polymorphism. Oncol Nurs Forum. 2011;38:E365-E372. [DOI] [PubMed] [Google Scholar]
- 60. Bien E, Krawczyk M, Izycka-Swieszewska E, et al. Serum IL-10 and IL-12 levels reflect the response to chemotherapy but are influenced by G-CSF therapy and sepsis in children with soft tissue sarcomas. Postepy Hig Med Dosw (Online). 2013;67:517-528. [DOI] [PubMed] [Google Scholar]
- 61. Ahlberg K, Ekman T, Gaston-Johansson F. Levels of fatigue compared to levels of cytokines and hemoglobin during pelvic radiotherapy: a pilot study. Biol Res Nurs. 2004;5:203-210. [DOI] [PubMed] [Google Scholar]
- 62. Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788-793. [DOI] [PubMed] [Google Scholar]
- 63. Rich TA. Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. J Support Oncol. 2007;5:167-174. [PubMed] [Google Scholar]
- 64. Wang J, Shi Q, Yuan TX, et al. Matrix metalloproteinase 9 (MMP-9) in osteosarcoma: review and meta-analysis. Clin Chim Acta. 2014;433:225-231. [DOI] [PubMed] [Google Scholar]
- 65. Li H, Zhang K, Liu LH, et al. A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma. Tumour Biol. 2014;35:5487-5491. [DOI] [PubMed] [Google Scholar]
- 66. Lopes-Júnior LC, Silveira DSC, Vulczak A, et al. Emerging cytokine networks in osteosarcoma. Can Cell Microenviron. 2017;4:e1510. doi: 10.14800/ccm.1510. [Google Scholar]
- 67. Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466-475. [DOI] [PubMed] [Google Scholar]
- 69. Brulé D, Gillmeister B, Lee M, et al. A feasibility pilot trial of individualized homeopathic treatment of fatigue in children receiving chemotherapy. Integr Cancer Ther. 2016;15:495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cohen J. The earth is round (p < .05). Am Psychol. 1994;49:997-1003. [Google Scholar]
- 71. Chow SL. Significance test or effect size? Psychol Bull. 1988;103:105-110. [Google Scholar]
- 72. Snyder P, Lawson S. Evaluating results using corrected and uncorrected effect size estimates. J Exp Educ. 1993;61:334-349. [Google Scholar]
- 73. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34:917-928. [DOI] [PubMed] [Google Scholar]
- 74. Berben L, Sereika SM, Engberg S. Effect size estimation: methods and examples. Int J Nurs Stud. 2012;49:1039-1047. [DOI] [PubMed] [Google Scholar]
- 75. Wilkinson L; Task Force on Statistical Inference; American Psychological Association; Science Directorate. Statistical methods in psychology journals: guidelines and explanations. Am Psychol. 1999;54:594-604. [Google Scholar]
- 76. Ward J, Swanson B, Fogg L, Rodgers C. Pilot study of parent psychophysiologic outcomes in pediatric hematopoietic stem cell transplantation. Cancer Nurs. 2017;40:E48-E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lopes-Júnior LC, by Omena Bomfim E, Nascimento LC, Pereira-da-Silva G, from Lima RA. Theory of unpleasant symptoms: support for the management of symptoms in children and adolescents with cancer. Rev Gaucha Enferm. 2015; 36 (3): 109-12. doi: 10.1590 / 1983-1447.2015.03.51465. [DOI] [PubMed] [Google Scholar]