Abstract
A number of performance-enhancing drugs (PEDs) are used illicitly to improve muscle strength by the bodybuilders. The misuse of these drugs is associated with serious adverse effects to different organs. A previously healthy 22-year-old male bodybuilder after taking stanozolol, clenbuterol, and triiodothyronine for 10 days presented to the hospital with symptoms of icteric sclera, progressive dyspnea, intermittent cough, and bloody sputum. He was diagnosed with dilated cardiomyopathy and acute hepatic injury. Rapidly progressive dilated cardiomyopathy and acute hepatic injury among bodybuilders in such a short period of time have not been reported. People using these drugs must monitor liver and cardiac functions regularly, and they should discontinue using PEDs after diagnosis of liver or cardiac abnormalities. Physicians should always consider the possibility of the PED abuse in the context of a young athlete suffering cardiomyopathy or hepatic injury.
Keywords: cardiomyopathy, hepatic injury, heart failure, stanozolol, clenbuterol, triiodothyronine
Several performance-enhancing drugs (PEDs) are currently used to improve activity performances in humans. Anabolic steroids are among the most frequently used PEDs. Other drugs include human growth hormone, insulin-like growth factor 1, insulin, clenbuterol, amphetamine, and thyroid hormones (THs). Some competitive bodybuilders also use diuretics (e.g., thiazides and furosemide) to reduce the body weight and to improve muscle definition onstage. These drugs are associated with a number of side effects and a wide variety of cardiovascular, hepatic, metabolic, renal, endocrine, neurological, psychiatric, as well as musculoskeletal disorders (Pope et al., 2014; Richard & Kirk, 2017). Several cases with cardiomyopathy or liver disorders have been reported to be associated with anabolic steroids, growth hormones, or thyroxine (T4) consumption (Bispo et al., 2009; Mark, Watkins, & Dargie, 2005). Ascertaining the side effects of PEDs is important for diagnosis and drug administration.
Case Report
A 22-year-old man was presented to the hospital with symptoms of progressive dyspnea, paroxysmal nocturnal dyspnea, intermittent cough, and bloody sputum. No previous history of any systemic diseases was reported by the patient. There was no personal history of tobacco use and recreational drug abuse. The patient admitted to have self-administered stanozolol, clenbuterol, and triiodothyronine (T3) to increase his muscle mass and his strength for 10 days, and discontinued after symptoms of chest discomfort and exertional dyspnea 12 days before his admission to the hospital. During the first 7 days, he consumed stanozolol 10 mg/day and clenbuterol 40 μg/day, and then doubled the dose during the next 3 days. The dose of T3 was 25 μg/day for the reported 10 days of administration. On admission, vital signs showed body temperature 36.5 °C, heart rate 102 bpm, blood pressure 118/78 mmHg, and respiratory rate 20 breaths/min. Physical examination revealed icteric sclera and mild pitting edema of the bilateral lower limbs. Cardiovascular examination showed muffled heart sounds, gallop rhythm, and grade 2/6 systolic ejection murmur at mitral auscultatory area. The relevant lab reports are listed in Table 1.
Table 1.
Lab Reports.
| Parameters | Result | Reference range |
|---|---|---|
| White blood cell | 14.82 × 109/L ↑ | 3.5–9.5 × 109/L |
| Hemoglobin | 143 g/L * | 130–175 g/L |
| Platelet count | 106 × 109/L ↓ | 125–350 × 109/L |
| Creatine kinase | 7.20 ng/mL ↑ | 0–4.3 ng/mL |
| Myoglobin | >500 ng/mL ↑ | 0–107 ng/mL |
| D-dimer | 2,640 ng/mL ↑ | 100–600 ng/mL |
| Brain natriuretic peptide | 4,330 pg/mL ↑ | 0–100 pg/mL |
| Aspartate transaminase | 2,576.3 U/L ↑ | 15–40 U/L0 |
| Alanine aminotransferase | 4,892.7 U/L ↑ | 9–50 U/L |
| Total bilirubin | 160.7 μmol/L ↑ | 6.8–30 μmol/L |
| Direct bilirubin | 61.3 μmol/L ↑ | 0–8.6 μmol/L |
| Alkaline phosphatase | 125.7 U/L ↑ | 45–125 U/L |
| γ-Glutamyl transpeptidase | 59.5 U/L * | 10–60 U/L |
| Blood urea nitrogen | 15.59 mmol/L ↑ | 3.2–7.0 mmol/L |
| Creatinine | 126.9 μmol/L ↑ | 44–115 μmol/L |
| Serum potassium | 5.83 mmol/L ↑ | 3.5–5.3 mmol/L |
| Serum sodium | 127.6 mmol/L ↓ | 137–147 mmol/L |
| Serum chlorine | 87.9 mmol/L ↓ | 99–110 mmol/L |
| Serum calcium | 1.91 mmol/L ↓ | 2.1–2.6 mmol/L |
| Prothrombin time | 44.4 s ↑ | 9–13 s |
| International normalized ratio | 3.85 ↑ | 0.8–1.2 |
| Fibrinogen | 0.70 g/L ↓ | 2.0–4.0 g/L |
| Free T3 | 2.12 pmol/L ↓ | 3.1–6.8 pmol/L |
| Free T4 | 14.06 pmol/L * | 12–22 pmol/L |
| Thyroid-stimulating hormone | 2.51 μIU/mL * | 0.27–4.2 μIU/mL |
Note. ↑ = increased; ↓ = decreased; * = normal.
The electrocardiogram showed sinus tachycardia and left ventricular hypertrophy (Figure 1). The transthoracic echocardiography (Figure 2) revealed ejection fraction 20%; left atrium (36 mm); right ventricle (30 mm); left ventricle (62 mm); diffused hypokinetic ventricular wall motion, severe tricuspid regurgitation, moderate mitral regurgitation, pulmonary artery systolic pressure (45 mmHg), and mild pericardial effusion. The pulmonary CT (Figure 3) showed bilateral pneumonia, pleural effusion, and pericardial effusion. The abdominal ultrasonography (Figure 4) showed cholestasis and intestinal fluid. During admission period, patient was managed with symptomatic and supportive medical therapy. His blood biochemistry reports including cardiac markers and the liver function test showed gradual improvement and he was discharged after 12 days of hospitalization.
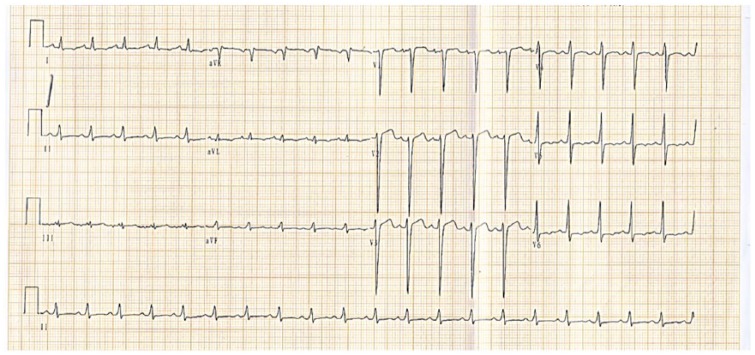
Figure 1.
ECG showing sinus tachycardia (heart rate 128 bpm) and left ventricular hypertrophy (RV5 + SV1 > 4.0 mV).
Figure 2.
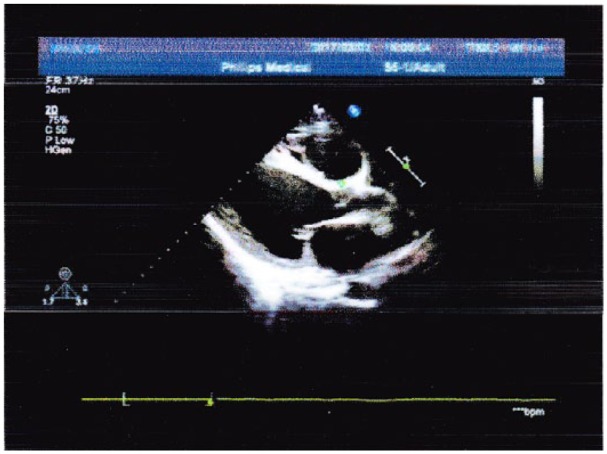
Transthoracic echocardiography reveals ejection fraction (EF) 20%; left atrium (36 mm); right ventricle (30 mm); left ventricle (62 mm); diffused hypokinetic ventricular wall motion, severe tricuspid regurgitation, moderate mitral regurgitation, pulmonary artery systolic pressure (45 mmHg), and mild pericardial effusion.
Figure 3.
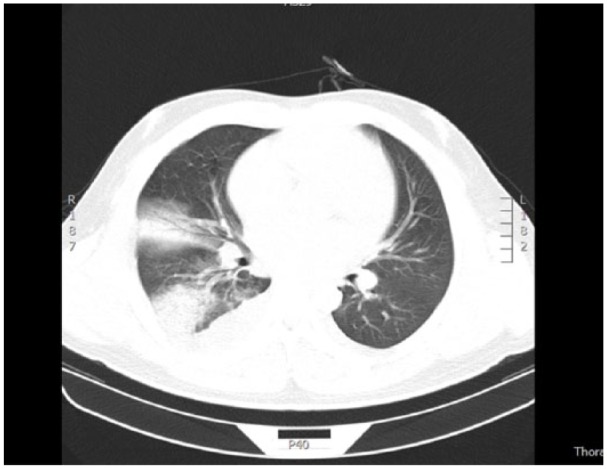
Pulmonary CT reveals bilateral pneumonia (inflammatory changes present in middle lobe of right lung, superior lobe of left lung, and inferior lobe of both lungs), bilateral pleural effusion, and pericardial effusion.
Figure 4.
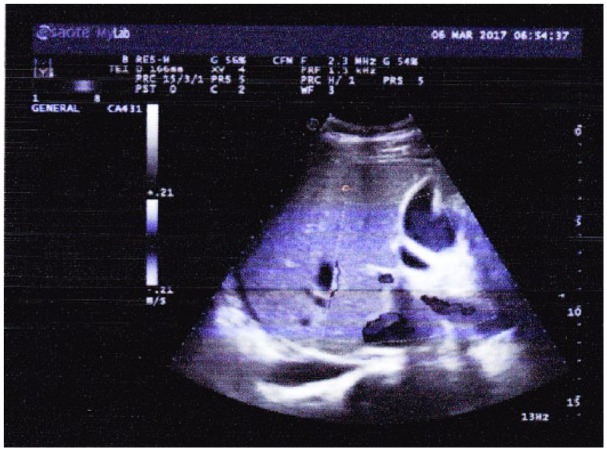
Abdominal ultrasonography shows cholestasis (accumulation of punctiform sediments in gall bladder) and intestinal fluid (fluid density echoes present in intestinal space, depth 21 mm).
Discussion
There is widespread misperception that only athletes are the PED users. A vast majority of PEDs are used by nonathlete weightlifters, power lifters, boxers, kickboxers, or bodybuilders. Recently, several cases of PEDs misuse by bodybuilders have been reported. The PED users often combine multiple drugs. In this case, the patient combined stanozolol, clenbuterol, and T3. The stanozolol is a 17-α-alkylation androgenic steroid which is derived from dihydrotestosterone. Therapeutically, stanozolol is used to treat muscle wasting, anemia, hereditary angioedema, and hypogonadal status (Sloane, Lee, & Sheffer, 2007; Thevis & Schanzer, 2010). The 17-α-alkylated steroids are notorious for their hepatotoxicity. They can cause abnormal liver function, liver hyperplasia, cholestasis, adenomas, and liver failure (Blue & Lombardo, 1999; Soe, Soe, & Gluud, 1992). A previously reported evidence states that steroids disturb canalicular excretion of conjugated bile and sinusoidal uptake of bile resulting in elevated level of bilirubin (Ishak & Zimmerman, 1987). The treatment with stanozolol has been reported to increase the level of lipid peroxidation and reactive oxygen species (ROS) in liver of rats. The overproduction of ROS causes oxidative stress, damages polyunsaturated fatty acids of the cell membranes by altering their initial chemical conformation (Dornelles et al., 2017). In humans, severe hepatotoxicity and cholestasis because of the stanozolol abuse have been reported (Ampuero et al., 2014; Segal, Cooper, & Bolognia, 2000). Few cases of severe dilated cardiomyopathy were reported in young patients associated with anabolic steroid abuse (Ferrera, Putnam, & Verdile, 1997; Nieminen et al., 1996). A study report pointed that the heart failure, caused by anabolic steroids, might be the unrecognized cause of liver injury (Bispo et al., 2009). The thrombus is mostly present in ventricles or venous system of patients with a long history of anabolic steroid use (Sveinsson & Herrman, 2013), which is probably due to increased platelet aggregation, elevated thromboxane A2, or decreased prostacyclin level (Ferenchick, 1990).
The drug clenbuterol, a selective β2-adrenergic receptor agonist, was used therapeutically in the management of the reversible airway obstructions such as bronchial asthma and chronic obstructive pulmonary disease. It is particularly well known for its ability to increase muscle mass and decrease fat mass (MacLennan & Edwards, 1989). The effect of β-agonist on fat tissue is due to the fact that the body fat reduction is related to the stimulation of lipolysis, suppression of lipogenesis, enhancement of fatty acid turnover and oxidation, and the decreased preadipocyte recruitment (Prezelj, Obreza, & Pecar, 2003). Thus, clenbuterol and other β-agonist drugs have been widely used in horse racing in hippodromes, and in athletics. However, clenbuterol treatment has been reported to induce cardiac hypertrophy and increase collagen infiltration around the blood vessels and in the left ventricle wall (Bhavsar et al., 2010; Duncan, Williams, & Lynch, 2000). Clenbuterol administration was reported to increase serum cardiac isoenzyme CK-MB in mice, which may be because of the myocardial damage (Cubria, Reguera, Balana-Fouce, Ordonez, & Ordonez, 1998). Chronic administration of clenbuterol was also reported to induce cellular hypertrophy, elevate oxidative carbohydrate utilization, and increase sarcoplasmic reticulum Ca2+ content in adult rat cardiac muscle (Soppa et al., 2005). It was reported that the atrial and ventricular size, the stroke volume, the cardiac output, and the heart rate were significantly increased following a maximal exercise test in horses that received clenbuterol (Sleeper, Kearns, & McKeever, 2002). The aortic root diameter was also increased significantly, which may increase the risk of the rupture of the aortic root and can lead in this case to the death of the horse.
The TH facilitates oxidative phosphorylation and glycolysis of the skeletal muscles. It is an important regulator of cardiac function and cardiovascular hemodynamics. It is involved in the key regulatory processes of cardiac contractility and electrophysiological function. Relaxation and contraction properties of the myocardium are mediated by TH via the regulation of the expression of calcium ion coupling protein and myosin heavy chain, respectively. T3 is commonly considered the biologically active form of TH. It is mostly generated by the T4 in peripheral tissues by type I and type II deiodinase enzymes (Jabbar et al., 2017). The synthesis and secretion of TH are strictly regulated by the negative feedback system, that is, the hypothalamic/pituitary/thyroid axis. Taking ectogenic T3 disturbs the normal regulatory mechanism of TH that causes factitious hyperthyroidism status. T3 has shown to act on the cardiac myocyte by regulating myocyte genes, such as α-myosin heavy chain and sarcoendoplasmic reticulum Ca2+-ATPase (Davis, Goglia, & Leonard, 2016; Hiroi et al., 2006). The high TH concentration causes the rapid use of oxygen, increased production of metabolic end products, and the relaxation of arterial smooth muscle fibers, resulting in peripheral vasodilatation and further increase in heart rate (Danzi & Klein, 2004; Martinez, 2016). In addition, TH has shown to stimulate both the synthesis and the release of brain natriuretic peptide (Ozmen, Ozmen, Parildar, Mutaf, & Bayindir, 2007).
Conclusion
The nonmedical use of PEDs has become a global health problem. Self-administration of these drugs to enhance physique and self-esteem by bodybuilders may lead to serious health consequences. They should be considered as people at risk of developing cardiomyopathy and hepatic injury. Bodybuilders should be suggested to avoid taking PEDs. The possibility of PED abuse should always be considered in the context of a young bodybuilder suffering cardiomyopathy or hepatic injury. People using these drugs must monitor liver and cardiac function regularly and should discontinue using PEDs after diagnosis of liver or cardiac abnormalities.
Acknowledgments
We especially thank our patient for allowing us to share their information to all readers of medical society.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grant from the Jilin Province Science and Technology Project (grant no. 20170520012JH) and the National Natural Science Foundation (grant no. 81400279).
Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying figures.
References
- Ampuero J., Garcia E. S., Lorenzo M. M., Calle R., Ferrero P., Gomez M. R. (2014). Stanozolol-induced bland cholestasis. Gastroenterologia Hepatologia, 37, 71–72. [DOI] [PubMed] [Google Scholar]
- Bhavsar P. K., Brand N. J., Felkin L. E., Luther P. K., Cullen M. E., Yacoub M. H., Barton P. J. (2010). Clenbuterol induces cardiac myocyte hypertrophy via paracrine signalling and fibroblast-derived IGF-1. Journal of Cardiovascular Translational Research, 3, 688–695. [DOI] [PubMed] [Google Scholar]
- Bispo M., Valente A., Maldonado R., Palma R., Glória H., Nóbrega J., Alexandrino P. (2009). Anabolic steroid-induced cardiomyopathy underlying acute liver failure in a young bodybuilder. World Journal of Gastroenterology, 15(23), 2920–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue J. G., Lombardo J. A. (1999). Steroids and steroid-like compounds. Clinics in Sports Medicine, 18, 667–689. [DOI] [PubMed] [Google Scholar]
- Cubria J. C., Reguera R., Balana-Fouce R., Ordonez C., Ordonez D. (1998). Polyamine-mediated heart hypertrophy induced by clenbuterol in the mouse. The Journal of Pharmacy and Pharmacology, 50, 91–96. [DOI] [PubMed] [Google Scholar]
- Danzi S., Klein I. (2004). Thyroid hormone and the cardiovascular system. Minerva Endocrinologica, 29, 139–150. [PubMed] [Google Scholar]
- Davis P. J., Goglia F., Leonard J. L. (2016). Nongenomic actions of thyroid hormone. Nature Reviews Endocrinology, 12, 111–121. [DOI] [PubMed] [Google Scholar]
- Dornelles G. L., Bueno A., de Oliveira J. S., da Silva A. S., Franca R. T., da Silva C. B., … de Andrade D. M. (2017). Biochemical and oxidative stress markers in the liver and kidneys of rats submitted to different protocols of anabolic steroids. Molecular and Cellular Biochemistry, 425,181–189. [DOI] [PubMed] [Google Scholar]
- Duncan N. D., Williams D. A., Lynch G. S. (2000). Deleterious effects of chronic clenbuterol treatment on endurance and sprint exercise performance in rats. Clinical Science, 98, 339–347. [PubMed] [Google Scholar]
- Ferenchick G. S. (1990). Are androgenic steroids thrombogenic? The New England Journal of Medicine, 322, 476. [DOI] [PubMed] [Google Scholar]
- Ferrera P. C., Putnam D. L., Verdile V. P. (1997). Anabolic steroid use as the possible precipitant of dilated cardiomyopathy. Cardiology, 88, 218–220. [DOI] [PubMed] [Google Scholar]
- Hiroi Y., Kim H. H., Ying H., Furuya F., Huang Z., Simoncini T., … Liao J. K. (2006). Rapid nongenomic actions of thyroid hormone. Proceedings of the National Academy of Sciences of the United States of America, 103, 14104–14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak K. G., Zimmerman H. J. (1987). Hepatotoxic effects of the anabolic/androgenic steroids. Seminars in Liver Disease, 7, 230–236. [DOI] [PubMed] [Google Scholar]
- Jabbar A., Pingitore A., Pearce S. H., Zaman A., Iervasi G., Razvi S. (2017). Thyroid hormones and cardiovascular disease. Nature Reviews Cardiology, 14, 39–55. [DOI] [PubMed] [Google Scholar]
- MacLennan P. A., Edwards R. H. (1989). Effects of clenbuterol and propranolol on muscle mass: Evidence that clenbuterol stimulates muscle beta-adrenoceptors to induce hypertrophy. Biochemistry Journal, 264(2), 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark P. B., Watkins S., Dargie H. J. (2005). Cardiomyopathy induced by performance enhancing drugs in a competitive bodybuilder. Heart, 91, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F. (2016). Thyroid hormones and heart failure. Heart Failure Reviews, 21, 361–364. [DOI] [PubMed] [Google Scholar]
- Nieminen M. S., Ramo M. P., Viitasalo M., Heikkila P., Karjalainen J., Mantysaari M., Heikkila J. (1996). Serious cardiovascular side effects of large doses of anabolic steroids in weight lifters. European Heart Journal, 17, 1576–1583. [DOI] [PubMed] [Google Scholar]
- Ozmen B., Ozmen D., Parildar Z., Mutaf I., Bayindir O. (2007). Serum N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) levels in hyperthyroidism and hypothyroidism. Endocrine Research, 32, 1–8. [DOI] [PubMed] [Google Scholar]
- Pope H. G., Wood R. I., Rogol A., Nyberg F., Bowers L., Bhasin S. (2014). Adverse health consequences of performance-enhancing drugs: An endocrine society scientific statement. Endocrine Reviews, 35(3), 341–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezelj A., Obreza A., Pecar S. (2003). Abuse of clenbuterol and its detection. Current Medicinal Chemistry, 10, 281–290. [DOI] [PubMed] [Google Scholar]
- Richard J. A., Kirk J. B. (2017). The public health consequences of performance enhancing substances: Who bears responsibility? JAMA, 318(20), 1983–1984. [DOI] [PubMed] [Google Scholar]
- Segal S., Cooper J., Bolognia J. (2000). Treatment of lipodermatosclerosis with oxandrolone in a patient with stanozolol-induced hepatotoxicity. Journal of American Academy of Dermatology, 43, 558–559. [DOI] [PubMed] [Google Scholar]
- Sleeper M. M., Kearns C. F., McKeever K. H. (2002). Chronic clenbuterol administration negatively alters cardiac function. Medicine and Science in Sports and Exercise, 34, 643–650. [DOI] [PubMed] [Google Scholar]
- Sloane D. E., Lee C. W., Sheffer A. L. (2007). Hereditary angioedema: Safety of long-term stanozolol therapy. The Journal of Allergy and Clinical Immunology, 120, 654–658. [DOI] [PubMed] [Google Scholar]
- Soe K. L., Soe M., Gluud C. (1992). Liver pathology associated with the use of anabolic-androgenic steroids. Liver, 12, 73–79. [DOI] [PubMed] [Google Scholar]
- Soppa G. K., Smolenski R. T., Latif N., Yuen A. H., Malik A., Karbowska J., … Yacoub M. H. (2005). Effects of chronic administration of clenbuterol on function and metabolism of adult rat cardiac muscle. American Journal of Physiology-Heart and Circulatory Physiology, 288, H1468–H1476. [DOI] [PubMed] [Google Scholar]
- Sveinsson O., Herrman L. (2013). Cortical venous thrombosis following exogenous androgen use for bodybuilding. BMJ Case Reports, 2013, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevis M., Schanzer W. (2010). Synthetic anabolic agents: Steroids and nonsteroidal selective androgen receptor modulators. In Thieme D., Hemmerbach P. (Eds.), Handbook of experimental pharmacology (Vol. 195, pp. 99–126). Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]