Abstract
Methadone is largely recognized as an effective treatment for opiate-dependent patients; however, it causes reduced brain dopaminergic action resulting in significant sexual dysfunction. Bupropion is a dopamine reuptake inhibitor which can potentially improve erectile function among male patients on methadone (MMT). This is a phase II, randomized, double-blind, parallel-group, placebo-controlled trial, involving 80 MMT male patients (73.4%) with mean age of 42.83 years ±9.68. These MMT male patients were randomly assigned into two groups to receive bupropion and placebo, respectively. The primary efficacy outcome measure was the difference between the two groups in end-point mean improvement scores using the measurement of Clinical Global Impression Scale adapted for Sexual Function (CGI-SF) at baseline (week 0) and at weeks 2, 4, and 6. Malay version of the sexual desire inventory-2 (SDI-2-BM) and Malay version of International Index of Erectile Function 15 (Mal-IIEF-15) domain scores were evaluated as secondary parameters. Improvement of the end-point mean from baseline were seen across the scores of SDI-2-BM (mean difference = 11.77 ± 2.90, 95% confidence interval (CI) [3.89, 19.54], p < .001) and Mal-IIEF-15 (mean difference = 8.37 ± 2.71, 95% CI [15.75, 0.99], p = .02), and the total plasma testosterone level (mean difference = 4.03, 95% CI [0.90, 7.15], p = .01). A categorical improvement of “much/very much improved” (CGI-SF score = 2) was reported by 58.3% (n = 21/36) of bupropion SR-assigned versus 27.7% (n = 10/36) placebo-assigned patient. Bupropion was well tolerated with no serious adverse events reported other than insomnia (17.7%). Six weeks of bupropion SR treatment reported significant improvement in key aspects of sexual function among male opiate-dependent patients on methadone maintenance treatment with emergent sexual dysfunction.
Keywords: sexual dysfunction, sexuality, erectile dysfunction, sexuality, male reproductive health, sexuality, bupropion, methadone
Sexual dysfunctions, such as reduced libido or arousal and impairments in erectile, ejaculatory, and orgasmic functions, are commonly reported in men among both the mentally ill as well as the healthy populations. The prevalence of sexual dysfunction among healthy men ranges from 20% to 35%, and in male populations with psychiatric illness, 50% to 65%. (Grube & Weigand-Tomiuk, 2002; Laumann, Paik, & Rosen, 1999; Macdonald et al., 2003; Oksuz & Malhan, 2005; Rosen, 2000).
Methadone maintenance treatment (MMT) is a comprehensive treatment program that involves the long-term prescribing of methadone as a substitution therapy for opiate dependence. Despite the effectiveness of the methadone maintenance therapy (Mattick, Breen, Kimber, & Davoli, 2009), the meta-analytical pooled prevalence for sexual dysfunction among methadone users was 52% (95% confidence interval (CI) [0.39, 0.65]), whereby the hypoactive sexual desire and low libido were the most prevalent sexual dysfunctions (Yee, Loh, & Ng, 2014). Previous studies reported that sexual dysfunction decreased quality of patient’s sexual life (Brown, Balousek, Mundt, & Fleming, 2005) and damaged intimate relationships, while at the same time increased the risk of dropout from methadone maintenance therapy prematurely (Hallinan et al., 2008). Management for methadone-induced sexual dysfunction remains a challenge for the physicians because by reducing or stopping of methadone in this group of patients may not be always possible. Some of the ex-opiate-dependent patients relapsed after their methadone dosage became too low. Moreover, some of them used other illicit drugs, especially stimulants, to boost up their sex function (La Pera et al., 2008). Hence, the physicians need other strategies to manage sexual dysfunction in this group of patients.
Methadone is a slow-and-long acting opiate agonist that causes the stimulation of mu (µ) opiate receptors in various areas of the brain. There are few hypotheses that try to explain the effect of methadone on sexual dysfunction. One of the oldest hypotheses revealed that methadone alters the function of tubero-infundibular and hypothalamus-pituitary-gonadal (HPG) axis through dopaminergic pathway by chronic stimulation of the μ opiate receptors. Hence, the reduced neurotransmitter dopamine secretion causes disinhibition of prolactin production, which subsequently exerts a negative effect on the gonadotrophin releasing hormone (GnRH). This action lowers the level of sex hormones, particularly testosterone, which then causes low sexual desire and interests (Balon & Segraves, 2005). However, in a recent meta-analysis (Yee, Loh, Hashim, et al., 2014), the prolactin level was reported to be not statistically significantly associated with sexual dysfunction in the methadone group. Therefore, this hypothesis could not explain the sexual dysfunction in this group of patients.
Previous studies have demonstrated that opioids, both endogenous and exogenous, led to a decline in luteinizing hormone (LH), follicle-stimulating hormone (FSH) from the pituitary gland, and testosterone or estradiol (E2) from the gonads (Daniell, 2002; Hallinan et al., 2009; Vuong, Van Uum, O’Dell, Lutfy, & Friedman, 2010). de la Rosa and Hennessey (1996) suggested that opioids decreased testosterone level in men, either by altering the release of normal pulsatility of GnRH or affecting the response of the anterior pituitary to GnRH. Previous studies also reported that opioids may also have direct effects on the pituitary gland and the testes which resulted in low serum testosterone concentrations and caused symptoms of testosterone deficiency (De Maddalena, Bellini, Berra, Meriggiola, & Aloisi, 2012; Vuong et al., 2010). The clinical manifestations of testosterone deficiency included significant decreases in sexual desire, erectile dysfunction, delayed ejaculation, depressive mood and irritability (Buvat, Maggi, Guay, & Torres, 2013).
Another well-known hypothesis was the interplay effects of excitation and inhibition sexual systems in the brain. Brain dopamine in the hypothalamic and mesolimbic systems appears to form the core of sexual excitatory system. In contrast, brain opiate, endocannabinoid, and serotonin systems are activated during periods of sexual inhibition, and blunt the ability of excitatory systems (Pfaus, 2009). Animal studies (Coolen, Fitzgerald, Yu, & Lehman, 2004; van Furth, van Emst, & van Ree, 1995; Vuong et al., 2010) reported that opioids have significant suppressive effects on sexual behavior, which appear to be modulated primarily via activation of μ- and δ-receptors in the medial preoptic area and the ventromedial hypothalamus. A recent imaging study identified that the methadone maintenance patients had reduced dopamine receptor 2 (D2) in various parts of their brains, with consequences of reduced brain dopaminergic function (Gradin, Baldacchino, Balfour, Matthews, & Steele, 2013). Some researchers ( Salehi et al., 2015; Tatari, Shakeri, Farnia, Heidari, & Rezaei, 2013) proposed to use dopamine reuptake inhibitor such as bupropion to treat sexual dysfunction in this group of patients. There was an open-labeled, quasiexperimental study done by Tatari et al. in 2013 (Tatari et al., 2013), whereby 100 mg of bupropion was administered for 6 weeks on 67 methadone maintenance men who had erectile dysfunction. In this study, bupropion significantly improved the erectile function in this group of patients (International Index of Erectile Function score improved from 12.79 ± 1.37 to 15.94 ± 2.14). The sample size of that study was small and had many methodological limitations.
The main objective of this research was to determine the therapeutic effect of bupropion hydrochloride sustained-release in the treatment of methadone emergent sexual dysfunction in men.
Materials and Methods
Study Design and Population
This phase II, randomized, double-blind, parallel-group, placebo-controlled trial was conducted from December 2015 to December 2017 in University Malaya Medical Center (UMMC) and University Malaya Center of Addiction Science Studies (UMCAS). This study was approved by the Medical Ethics Committee of University Malaya Medical Center (UMMC, Kuala Lumpur, Malaysia, NO: 20154-1212) and National Medical Research Register (NMRR, No: NMRR-15-303-24854). This study was conducted according to the Declaration of Helsinki, and registered at the ClinicalTrials.gov with the following number: NCT02593396.
All the male subjects who attended the methadone maintenance clinic were approached. Screening data were reviewed to determine subject eligibility. Subjects who met all inclusion criteria and none of the exclusion criteria were recruited into the study. The inclusion criteria included: (a) they were aged from 18 to 60 years; (b) had a diagnosis of opiate dependence based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria; (c) they were taking a stable dose of methadone for ≥6 months; (d) they were experiencing constant sexual dysfunction for ≥4 weeks receiving MMT; (e) they were in a stable sexual relationship for ≥6 months. The exclusion criteria were: (a) the subjects exhibited severe behavior disturbances or psychotic symptoms; (b) obvious organic illnesses which caused the sexual dysfunction (such as patients with diabetes, heart disease, or vascular disease); (c) those with history of sexual dysfunction before methadone therapy; (d) those who were receiving antiviral treatment for viral hepatitis or HIV; (e) those who were receiving androgen replacement treatment, phosphodiesterase type 5 inhibitors (PDE5i), or any traditional sexual enhancement remedies; (f) those with a history of an eating disorder (e.g., anorexia, bulimia) or seizures (e.g., epilepsy); (g) those who fulfilled other axis 1 diagnoses (e.g., Bipolar disorder) base on DSM-5; (h) or those who were using other psychotropic medications other than methadone; (i) QTc prolongation >450 ms in the electrocardiogram; (j) those who refused to participate.
All patients gave their written informed consent before study procedures began. At the baseline, the patients were assessed by using the Opiate Treatment Index (OTI), Malay Version of the Golombok-Rust Inventory of Marital State (Mal-GRIMS), the Malay version of the self-rated Montgomery-Asberg Depression Rating Scale (MADRS-BM), Malay version of the Fagerstrom Test for Nicotine Dependence (FTND-M), Malay version of International Index of Erectile Function 15 (Mal-IIEF-15), the Malay version of the sexual desire inventory-2 (SDI-2-BM), and Clinical Global Impression Scale adapted for Sexual Function (CGI-SF). Besides, all patients received a physical examination, including body mass index (BMI), blood pressure, 12-lead electrocardiogram, rapid urine drug test for opiate (non-methadone), marijuana, methamphetamine, and ecstasy. Blood samples were obtained from all study participants and assayed for prolactin and total testosterone in a single laboratory.
After a diagnostic screening and baseline visit, eligible patients were randomly and double-blindly assigned to either receiving the Bupropion Hydrochloride Sustained-Release (Bupropion SR) 150 mg twice daily (n = 40) or a placebo (Calcium Lactate) 300 mg twice daily (n = 40), in a 1:1 ratio using a computer-generated table of random numbers using the Randomization.com program. Investigators and patients were both blinded to the allocation of the study drug throughout the study period. A non-blind physician had access to the study randomization list so that he could provide appropriate follow-up care to patients who dropped out of the study.
All study medications were repacked in sealed opaque packets and dispensed by the research assistant who was not involved in the assessment of the study. All study medications were packaged in sets of 11 (1 tablet per day for 3 days, followed by 2 tablets per day for 4 days) for the first week. Subsequently, all study medications were packaged in sets of 14 (2 tablets per day for 7 days). All study medications were identical both in their color and shape, and they were kept in sealed opaque packets before inserted into sealed envelopes.
All patients were assessed biweekly by a consultant psychiatrist (A Yee) using CGI-SF, SDI-2-BM, Mal-IIEF-15, MADRS-BM, and FTND-M. Additionally, any treatment-related emergent side effects were all recorded and documented. All the data were analyzed by a statistician who was also blinded to the allocation of the study drugs.
Efficacy Criteria
The primary efficacy outcome measure was the difference between the two groups in end-point mean improvement scores using the measurement of CGI-SF at baseline (week 0) and at weeks 2, 4, and 6. SDI-2-BM and Mal-domain scores were evaluated as secondary parameters.
Main Outcome Measurements
CGI-SF
This is a clinician-rated severity and improvement scale adapted from the CGI Scale (Guy, 1976). This scale assesses the changes of the sexual function from baseline to weeks 2, 4, and 6 (final or last visit) with anchored scores from 1 (normal/very much improved) to 7 (most extreme sexual dysfunction/very much worse). Lower scores indicate better sexual functioning.
SDI-2-BM
This is a self-assessment of sexual desire in cognitive terms. This scale contains 14 items which yield two domain scores: dyadic sexual desire (DSD) and solitary sexual desire (SSD) (Spector, Carey, & Steinberg, 1996). DSD is defined as individuals’ desire for intimacy with another person and SSD is defined as individuals’ desire in engaging sexual behavior by oneself. For all the questionnaire items, respondents rate their sexual desire on an 8-point Likert scale except for items 1, 2, and 10, which are only rated on a 7-point Likert scale. DSD consists of 8 items and SSD consists of 3 items. All items are summed up to dictate the total sexual desire. Higher scores reflect higher sexual desire. The total possible sores for DSD range from 0 to 62, and for SSD, range from 0 to 23 (Spector et al., 1996). This instrument was validated in Malay version (SDI-2-BM) with good internal consistency (DSD, Cronbach’s α = 0.93 and SSD, Cronbach’s α = 0.88) (Yee et al., in press).
Mal-IIEF-15
IIEF-15 is a 15-item, multidimensional self-reporting instrument for the evaluation of five distinct domains of the male sexual function for the past 4 weeks (Rosen, Cappelleri, Gendrano, & Correspondence, 2002). The five domains include erectile function (items 1, 2, 3, 4, 5, and 15), orgasmic function (items 9 and 10), sexual desire (items 11 and 12), intercourse satisfaction (items 6, 7, and 8), and overall satisfaction (items 13 and 14). Each item has a score ranging from 0 to 5 with higher score reflecting a better sexual function. Mal-IIEF-15 has been validated among Malaysian male population with Cronbach’s α value of 0.74 and intra-class correlation coefficient is of 0.59 (Quek, Low, Razack, Chua, & Loh, 2002).
Other Measurements
OTI
OTI is a multidimensional, structured interview for the evaluation of methadone treatment. It assesses five independent outcome domains including drug use, HIV risk-taking behavior, social functioning, criminality, and health status. The drug use domain is examined by calculating Q score for each drug class. A Q score is a score which is the adding the number of use episodes and divided by the total of the two intervals between use. The higher the value of “Q” indicated the heavier the drug usage. In other domains, all the questions are ranged from 0 (good) to 5 (worse) Likert scale. The total score for each domain was derived by adding up the scores for each question. Higher scores reflect greater degree of dysfunction in that domain (Darke, Ward, Hall, Heather, & Wodak, 1991).
Malay Version of the Golombok-Rust Inventory of Marital State
The Golombok Rust Inventory of Marital State (GRIMS) is a new short (28 items) questionnaire for the assessment of the quality of a relationship (Rust, Bennun, Crowe, & Golombok, 1986). Each item is answered on a four-point scale from strongly agree, agree, disagree, and strongly disagree. A total score is computed (range 0–84), with a high score indicating problematic marital relationship. In this study, this instrument was used to detect the marital satisfaction in this group of participants. GRIMS has been validated in Malay with excellent internal consistency (Cronbach’s α = 0.43 to 1.00), good test–retest correlation coefficient and intra-class correlation coefficient (ICC = 0.51 and above), high sensitivity and specificity (Quek, Low, Razack, Loh, & Chua, 2001).
MADRS-BM
MADRS-S is a 9-item self-report measure of depression developed by Svanborg and Asberg (Svanborg & Åsberg, 1994). Participants rate items on a 4-point Likert scale ranging from 0 (no depressive symptoms) to 3 (worst depressive symptoms). Possible score ranges from 0 to 27, with higher scores indicating greater symptom severity. The MADRS-BM was validated and exhibited good internal consistency with Cronbach’s α of 0.78 among Malaysians (Yee, Yassim, Loh, Ng, & Tan, 2015).
FTND-M
FTND-M is a self-report questionnaire in Malay language to assess the nicotine dependence among the smokers. FTND-M consists of 6 items and has similar internal consistency with the original FTND (Anne Yee, Ng, & Rusdi, 2011). The total score ranges from 0 to 10 with higher score indicating more severe nicotine dependence.
Data Analysis
A sample size of 66 patients (33 per group) was expected to detect a significant difference in sexual dysfunction with 90% power for a type I error rate of α = 0.05 between bupropion and placebo. Assuming 20% attrition, 80 patients were entered. Statistical significance was set at p < .05.
The χ2 or the Fisher’s exact test for categorical variables were used to test group differences (bupropion SR and placebo) at baseline. For continuous variable, Mann–Whitney U test (nonparametric distribution) and t test (parametric distribution) were used to test the baseline differences between bupropion SR and placebo.
Intention-to-treat (ITT) analyses were used for all efficacy variables and included all patients who had been randomized, took at least 1 dose of study trial medication and had at least one efficacy assessment after the baseline visit. The safety population included all patients who took at least one dose of study medication. A case-wise interpolation was used to replace missing values. The changes in sexual functioning measured by CGI-SF, SDI-2-BM, and Mal-IIEF-15 from baseline to each patient’s own end-point were the dependent measures of efficacy. A two-way repeated measures analysis of covariance was used to determine study group (bupropion SR and placebo) in the change from baseline to end-point for the measures of efficacy and sexual functioning (time × group interaction). Pairwise comparisons using Bonferroni adjustment correction of all sexual-functioning variables were performed between bupropion SR and placebo group across the time.
The safety population consisted of all patients who consumed at least 1 dose of study trial medication and after baseline measurement. Adverse events were compared between groups using frequency counts. SPSS v. 23 (IBM Corp, Armonk, NY) used for data analysis.
Results
This study screened 109 and included 80 (73.4%) male patients aged 25–60 (mean age 42.83 years ±9.68) who met all inclusion criteria and none of the exclusion criteria. A total of 80 men were randomly assigned to receive bupropion SR (n = 40) or placebo (n = 40). They had been taking 70.44 ± 29.01 mg methadone for 46.09 ± 29.5 months. There were no statistically significant differences between baseline demographics in the assigned treatment groups (Table 1).
Table 1.
Baseline Sociodemographic, Treatment Characteristics, and Sexual Function of All Male Participants.
| Variables | Bupropion (n = 40) | Placebo (n = 40) | df | χ2, z, t | p value |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 42.30 ± 9.49 | 43.50 ± 10.09 | 78 | 0.55 | .62 |
| Daily dose, mg, mean ± SD | 71.67 ± 28.57 | 69.25 ± 29.75 | 78 | −0.37 | .71 |
| Duration of MMT, months, mean ± SD | 50.03 ± 32.46 | 42.25 ± 26.15 | 78 | −1.17 | .24 |
| BMI, mean ± SD | 25.83 ± 5.91 | 24.47 ± 4.74 | 78 | −1.13 | .26 |
| SBP, mean ± SD | 130.43±15.89 | 135.43±18.79 | 78 | 1.29 | .20 |
| DBP, mean ± SD | 80.25 ± 10.79 | 81.63 ± 9.39 | 78 | 0.61 | .54 |
| QTc, ms, mean ± SD | 428.60 ± 24.51 | 426.90 ± 26.90 | 78 | −0.29 | .77 |
| Prolactin, μIU/ml, mean ± SD |
181.69 ± 118.47 | 194.96 ± 122.98 | 78 | 0.42 | .68 |
| Total testosterone (nmol/L) (ng/dl) |
8.72 ± 5.98 | 10.56 ± 5.98 | 78 | 1.37 | .18 |
| Ethnic group, n (%) | |||||
| Malay | 34 (85.0) | 34 (85.0) | 3 | 2.50 | .54 |
| Chinese | 3 (7.5) | 5 (12.5) | |||
| Indian | 1 (2.5) | 1 (2.5) | |||
| Others | 2 (5.0) | 0 (0) | |||
| Religion, n (%) | |||||
| Islam | 36 (90.0) | 34 (85.0) | 3 | 1.20 | .64 |
| Christianity | 0 (0) | 1 (2.5) | |||
| Buddhism | 3 (7.5) | 4 (10.0) | |||
| Hinduism | 1 (2.5) | 1 (2.5) | |||
| Education level, n (%) | |||||
| No formal education | 0 | 1 (2.5) | 3 | 1.11 | .77 |
| Primary | 3 (7.5) | 3 (7.5) | |||
| Secondary | 32 (80.0) | 32 (80.0) | |||
| Tertiary | 5 (12.5) | 4 (10.0) | |||
| Employment, n (%) | 36 (90.0) | 35 (87.5) | 1 | 0.13 | 1.00 |
| Family history of drug use, n (%) | 6 (15.4) | 7 (17.9) | 1 | 0.09 | 1.00 |
| HBV, n (%) | 1 (2.5) | 1 (2.5) | 1 | 0 | 1.00 |
| HCV, n (%) | 12 (30.0) | 12 (30.0) | 1 | 0.24 | .81 |
| Total FTND scorea, mean ± SD |
2.95 ± 2.19 | 4.70 ± 5.63 | 72 | −1.69 | .09 |
| OTI, mean ± SD | |||||
| Q scores of drugs usea | |||||
| Tobaccoa | 11.79 ± 9.04 | 9.63 ± 6.96 | 78 | −0.88 | .38 |
| Alcohola | 0.05 ± 0.316 | 0 | 78 | −1.00 | .32 |
| Marijuanaa | 0 | 0.0005 ± 0.003 | 78 | −1.00 | .32 |
| Amphetaminesa | 0.002 ± 0.009 | 0.0009 ± 0.005 | 78 | −0.47 | .64 |
| Heroina | 0.0008 ± 0.005 | 0.03 ± 0.16 | 78 | −1.03 | .30 |
| HIV risk-takinga | 5.85 ± 2.23 | 4.87 ± 2.93 | 78 | −1.57 | .12 |
| Criminality | 0 | 0 | − | ||
| Social functioninga | 8.45 ± 4.81 | 8.20 ± 4.75 | 78 | −0.04 | .97 |
| Healtha | 1.43 ± 1.08 | 1.15 ± 0.74 | 78 | −0.69 | .49 |
| Total Mal-GRIMS score, mean ± SD |
46.63 ± 5.25 | 47.65 ± 5.40 | 78 | 0.75 | .46 |
| Total MADRS-BM scorea, mean ± SD | 2.68 ± 3.59 | 3.53 ± 4.44 | 78 | 0.92 | .36 |
| Mal-IIEF-15 domain, mean ± SD | |||||
| Erectile function | 17.15 ± 9.28 | 18.15 ± 7.73 | 78 | 0.52 | .69 |
| Orgasmic functiona | 6.17 ± 3.21 | 5.44 ± 3.29 | 78 | −1.00 | .32 |
| Sexual desire | 5.38 ± 1.78 | 5.74 ± 1.57 | 78 | 0.98 | .33 |
| Intercourse satisfaction | 6.75 ± 4.01 | 6.59 ± 3.87 | 78 | −0.18 | .86 |
| Overall satisfaction | 6.35 ± 2.67 | 6.51 ± 2.37 | 78 | 0.29 | .78 |
| Total | 41.80 ± 19.13 | 42.44 ± 16.43 | 78 | 0.16 | .88 |
| SDI-2-BM, mean ± SD | |||||
| DSD | 27.48 ± 9.76 | 29.62 ± 10.24 | 78 | 0.95 | .35 |
| SSD | 6.20 ± 4.27 | 4.54 ± 4.57 | 78 | −1.67 | .10 |
| Total | 42.58 ± 14.97 | 42.87 ± 15.83 | 78 | 0.09 | .93 |
| CGI-S score | 5.35 ± 0.48 | 5.25 ± 0.54 | 78 | −0.87 | .57 |
Note. aBased on Mann–Whitney test. MMT = methadone maintenance treatment; BMI = body mass index; HBV = hepatitis B; HCV = hepatitis C; OTI = Opioid Treatment Index; Mal-GRIMS = Malay Version of the Golombok-Rust Inventory of Marital State; MADRS-BM = Malay version of self-rated Montgomery-Asberg Depression Rating Scale; Mal-IIEF-15 = Malay version of the International Index of Erectile Function 15; SDI-2-BM = Malay version of the sexual desire inventory-2; DSD = dyadic sexual desire; SSD = solitary sexual desire; CGI = clinical global impression-severity; df = degrees of freedom; SD = standard deviation; t = t test; χ2 = chi-square test; z = z test; nmol/L = nanomoles per liter; ng/dl = nanograms per deciliter; μIU/ml = macro international units per milliliter.
p < .05. **p < .01.
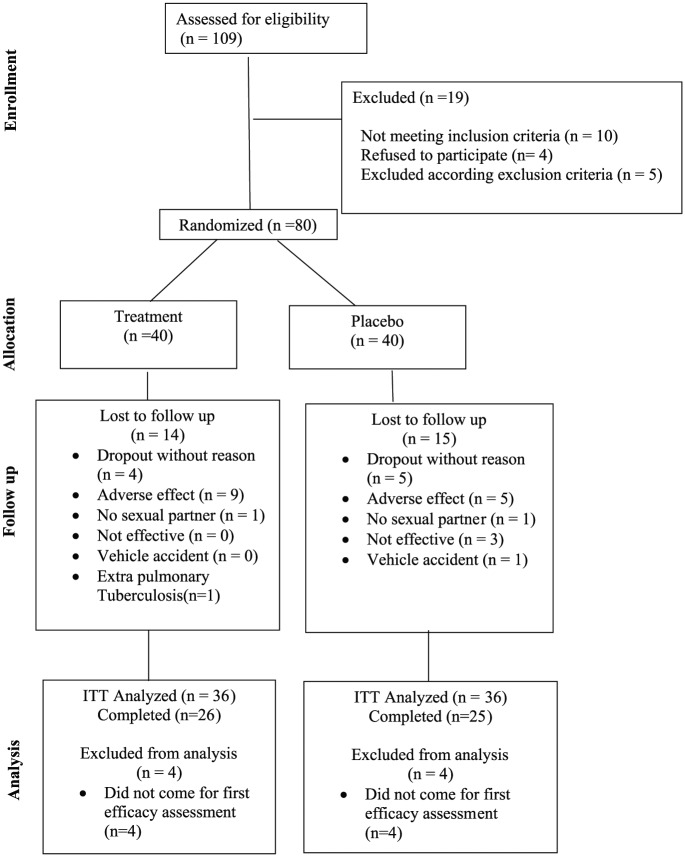
Systematic review for any protocol deviations in patient enrollment was undertaken before unblinding. The 72 randomized men constituted the ITT analysis. Fifty-one men (63.8%) completed the study: with 65% (26 of 40) in the bupropion SR and 62.5% (25 of 40) in the placebo group completed the 6-week study. Three men in the placebo group and none in bupropion SR group discontinued prematurely for lack of efficacy (Figure 1).
Figure 1.
CONSORT flow diagram for evaluation of bupropion efficacy on sexual dysfunction among male patients on methadone maintenance therapy: A double-blind placebo-controlled trial.
Efficacy Measures
CGI-SF
The difference from baseline to end-point in the mean ± SD change in the Clinical Global Impression scale sexual function improvement by intent-to-treat analyses was 5.33 ± 0.48 to 2.64 ± 1.61 with a difference of 2.57 (95% CI [1.87, 3.28]) for the bupropion SR group versus 5.25 ± 0.60 to 3.75 ± 1.59 with a difference of 1.62 (95% CI [0.91, 2.33]) for the placebo group, which showed a significant difference of 0.83 (95% CI [−1.5, −1.30], p = .02) between groups (Figure 2; Table 2). A categorical improvement of “much/very much improved” (CGI-SF score ≤2) was reported by 58.3% (n = 21/36) of bupropion SR-assigned versus 27.7% (n = 10/36) placebo-assigned patient.
Figure 2.
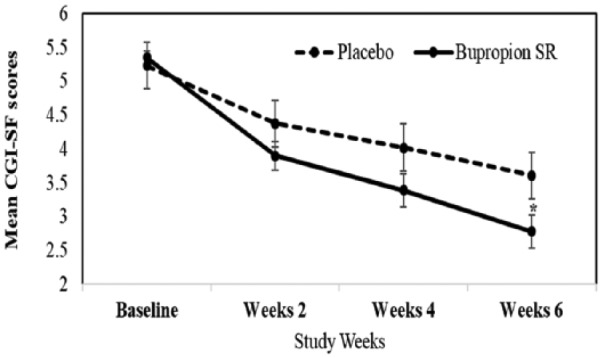
Mean Clinical Global Impression Scale adapted for Sexual Function scores (CGI-SF) in methadone-emergent sexual dysfunction patients receiving sustained-release bupropion (bupropion SR) or placebo. *p < .05.
Table 2.
Summary of All the Sexual Functioning Outcomes
| Bupropion (n = 36) |
Placebo (n = 36) |
Mean EOT differenceaFor bupropion vs. placebo |
95% CI for differencea |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
EOT |
Baseline |
EOT |
|||||
| Outcome measure | Mean ± SD | Mean ± SD | Mean ± SE | Lower bound | Upper bound | p value | ||
| CGI-SF | 5.33 ± 0.48 | 2.64 ± 1.61 | 5.25 ± 0.60 | 3.75 ± 1.59 | −0.83 ± 0.35* | −1.5 | −0.13 | .02 |
| SDI-2-BM | ||||||||
| DSD | 26.19 ± 8.59 | 36.08 ± 10.17 | 27.67 ± 8.44 | 29.99 ± 13.44 | 5.83 ± 2.78* | 0.28 | 11.37 | .04 |
| SSD | 6.22 ± 4.34 | 7.45 ± 4.97 | 5.06 ± 4.29 | 6.87 ± 5.97 | 0.58 ± 1.34 | −2.09 | 3.24 | .67 |
| Total | 41.14 ± 14.00 | 53.53 ± 15.88 | 41.06 ± 14.12 | 47.36 ± 21.09 | 5.69 ± 4.41 | −3.10 | 14.49 | .20 |
| Mal-IIEF-15 domain | ||||||||
| Erectile function | 18.14 ± 8.74 | 22.56 ± 7.69 | 17.44 ± 8.07 | 18.06 ± 9.43 | 4.51 ± 2.02* | 0.47 | 8.56 | .03 |
| Orgasmic function | 6.50 ± 3.01 | 7.30 ± 2.92 | 5.17 ± 3.31 | 5.99 ± 3.75 | 1.30 ± 0.81 | −0.33 | 2.94 | .12 |
| Sexual desire | 5.00 ± 1.41 | 6.77 ± 1.91 | 5.25 ± 1.34 | 6.04 ± 1.97 | 0.70 ± 0.44 | −0.18 | 1.59 | .12 |
| Intercourse satisfaction | 7.06 ± 3.89 | 8.78 ± 3.77 | 6.33 ± 4.01 | 6.46 ± 4.18 | 2.36 ± 0.97* | 0.44 | 4.29 | .02 |
| Overall satisfaction | 6.50 ± 2.71 | 7.10 ± 2.29 | 6.64 ± 2.11 | 7.14 ± 2.41 | 0.05 ± 0.52 | −0.99 | 1.09 | .92 |
| Total | 43.19 ± 18.23 | 52.53 ± 16.14 | 40.83 ± 16.73 | 43.81 ± 19.86 | 8.69 ± 4.24* | 0.24 | 17.15 | .04 |
| Plasma TT (nmol/L) | 7.28 ± 4.50 | 12.04 ± 8.46 | 8.07 ± 2.79 | 10.18 ± 6.25 | 0.75 ± 2.12 | −3.51 | 5.01 | .73 |
| (ng/dl) | 209.79 ± 129.68 | 346.97 ± 243.80 | 232.56 ± 80.40 | 293.37 ± 180.12 | ||||
| Plasma prolactin (μIU/ml) |
182.68 ± 91.49 | 171.15 ± 90.97 | 174.79 ± 99.22 | 170.05 ± 87.95 | 3.18 ± 26.64 | −50.34 | 56.72 | .91 |
Note. aAdjustment for multiple comparisons: Bonferroni with covariate daily methadone dosage and duration of the methadone usage. EOT = end of treatment; CGI-SF = Clinical Global Impression Scale adapted for Sexual Function; Mal-IIEF-15 = Malay version of the International Index of Erectile Function 15; SDI-2-BM = Malay version of the sexual desire inventory-2; DSD = dyadic sexual desire; SSD = solitary sexual desire; TT = total testosterone; SD = standard deviation; SE = standard error. nmol/L = nanomoles per liter; ng/dl = nanograms per deciliter; μIU/ml = macro international units per milliliter.
p < .05. **p < .01.
SDI-2-BM
At end-point, total SDI-2-BM scores improved from baseline in bupropion SR group (mean difference = 11.77 ± 2.90, 95% CI [3.89, 19.54], p < .001) when compared to those in placebo group (Table 2). DSD scores improved from baseline for patients receiving bupropion SR (mean difference = 9.41 ± 1.82, 95% CI [4.46, 14.35], p < .001) compared with those receiving placebo (mean difference = 2.81 ± 0.36, 95% CI [−2.14, 7.76], p = .76) (Table 3 and Figure 3). SSD scores between bupropion SR and control group were statistically not significant.
Table 3.
Pairwise Comparison Within Group Over Time.
| Outcome measure | Group | (I) Time | (J) Time | Mean differencea (I−J) | SE | p value | 95% CI for differencea | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| CGI-SF | ||||||||
| Placebo | Baseline | Week 2 | 0.86* | 0.22 | .01 | 0.27 | 1.45 | |
| Baseline | Week 4 | 1.21** | 0.25 | <.01 | 0.54 | 1.88 | ||
| Baseline | Week 6 | 1.62** | 0.26 | <.01 | 0.91 | 2.33 | ||
| Bupropion | Baseline | Week 2 | 1.45*** | 0.22 | <.001 | 1.52 | 2.57 | |
| Baseline | Week 4 | 1.96*** | 0.25 | <.001 | 1.58 | 2.74 | ||
| Baseline | Week 6 | 2.57*** | 0.36 | <.001 | 1.87 | 3.28 | ||
| SDI-2-BM | ||||||||
| DSD | Placebo | Baseline | Week 2 | −3.51 | 1.49 | .13 | −7.56 | 0.54 |
| Baseline | Week 4 | −3.25 | 1.69 | .34 | −7.85 | 1.35 | ||
| Baseline | Week 6 | −2.81 | 1.82 | .76 | −7.76 | 2.14 | ||
| Bupropion | Baseline | Week 2 | −7.77*** | 1.49 | <.001 | −11.81 | −3.72 | |
| Baseline | Week 4 | −8.62*** | 1.69 | <.001 | −13.22 | −4.02 | ||
| Baseline | Week 6 | −9.41*** | 1.82 | <.001 | −14.35 | −4.46 | ||
| SSD | Placebo | Baseline | Week 2 | −1.73 | 0.92 | .38 | −4.22 | 0.77 |
| Baseline | Week 4 | −1.26 | 0.81 | .73 | −3.46 | 0.93 | ||
| Baseline | Week 6 | −1.86 | 0.83 | .17 | −4.11 | 0.40 | ||
| Bupropion | Baseline | Week 2 | −1.48 | 0.92 | .68 | −3.97 | 1.02 | |
| Baseline | Week 4 | −1.51 | 0.81 | .40 | −3.70 | 0.69 | ||
| Baseline | Week 6 | −1.19 | 0.83 | .93 | −3.44 | 1.06 | ||
| Total | Placebo | Baseline | Week 2 | −6.39 | 2.66 | .12 | −13.63 | 0.85 |
| Baseline | Week 4 | −5.82 | 2.81 | .25 | −13.45 | 1.81 | ||
| Baseline | Week 6 | −6.84 | 2.90 | .13 | −14.72 | 1.04 | ||
| Bupropion | Baseline | Week 2 | −11.30*** | 2.66 | <.001 | −18.54 | −4.06 | |
| Baseline | Week 4 | −11.56*** | 2.81 | <.001 | −19.19 | −3.94 | ||
| Baseline | Week 6 | −11.77*** | 2.90 | <.001 | −19.65 | −3.89 | ||
| Mal-IIEF-15 domain | ||||||||
| Erectile function | Placebo | Baseline | Week 2 | −0.87 | 1.45 | 1.00 | −4.80 | 3.07 |
| Baseline | Week 4 | −1.17 | 1.40 | 1.00 | −4.96 | 2.63 | ||
| Baseline | Week 6 | −1.24 | 1.38 | 1.00 | −5.00 | 2.51 | ||
| Bupropion | Baseline | Week 2 | −1.50 | 1.45 | 1.00 | −5.44 | 2.43 | |
| Baseline | Week 4 | −2.67 | 1.40 | .36 | −6.47 | 1.12 | ||
| Baseline | Week 6 | −3.93* | 1.38 | .04* | −7.68 | −0.17 | ||
| Orgasmic function | Placebo | Baseline | Week 2 | −0.92 | 0.53 | .53 | −2.38 | 0.53 |
| Baseline | Week 4 | −1.40 | 0.56 | .09 | −2.92 | 0.13 | ||
| Baseline | Week 6 | −1.08 | 0.62 | .51 | −2.76 | 0.60 | ||
| Bupropion | Baseline | Week 2 | 0.35 | 0.53 | 1.00 | −1.10 | 1.80 | |
| Baseline | Week 4 | −0.46 | 0.56 | 1.00 | −1.99 | 1.06 | ||
| Baseline | Week 6 | −0.63 | 0.62 | 1.00 | −2.31 | 1.05 | ||
| Sexual desire | Placebo | Baseline | Week 2 | −0.20 | 0.27 | 1.00 | −0.93 | 0.53 |
| Baseline | Week 4 | −0.49 | 0.29 | .60 | −1.29 | 0.31 | ||
| Baseline | Week 6 | −0.88 | 0.31 | .05 | −1.72 | −0.06 | ||
| Bupropion | Baseline | Week 2 | −0.86*** | 0.27 | <.001 | −1.61 | −0.16 | |
| Baseline | Week 4 | −1.31*** | 0.29 | <.001 | −2.11 | −0.52 | ||
| Baseline | Week 6 | −1.68*** | 0.31 | <.001 | −2.51 | −0.85 | ||
| Intercourse | Placebo | Baseline | Week 2 | 0.11 | 0.63 | 1.00 | −1.61 | 1.83 |
| Satisfaction | Baseline | Week 4 | −0.56 | 0.65 | 1.00 | −2.34 | 1.22 | |
| Baseline | Week 6 | −0.33 | 0.66 | 1.00 | −2.12 | 1.46 | ||
| Bupropion | Baseline | Week 2 | −0.37 | 0.63 | 1.00 | −2.08 | 1.35 | |
| Baseline | Week 4 | −0.72 | 0.65 | 1.00 | −2.49 | 1.06 | ||
| Baseline | Week 6 | −1.63 | 0.66 | .09 | −3.42 | 0.16 | ||
| Overall satisfaction | Placebo | Baseline | Week 2 | −0.03 | 0.40 | 1.00 | −1.10 | 1.04 |
| Baseline | Week 4 | −0.09 | 0.37 | 1.00 | −1.09 | 0.92 | ||
| Baseline | Week 6 | −0.45 | 0.37 | 1.00 | −1.46 | 0.56 | ||
| Bupropion | Baseline | Week 2 | −0.57 | 0.40 | .91 | −1.65 | 0.50 | |
| Baseline | Week 4 | −0.19 | 0.37 | 1.00 | −1.20 | 0.81 | ||
| Baseline | Week 6 | −0.54 | 0.37 | .91 | −1.55 | 0.47 | ||
| Total | Placebo | Baseline | Week 2 | −2.66 | 2.86 | 1.00 | −10.43 | 5.11 |
| Baseline | Week 4 | −3.89 | 2.65 | .88 | −11.10 | 3.31 | ||
| Baseline | Week 6 | −4.19 | 2.71 | .76 | −11.57 | 3.18 | ||
| Bupropion | Baseline | Week 2 | −3.01 | 2.86 | 1.00 | −10.78 | 4.76 | |
| Baseline | Week 4 | −5.32 | 2.65 | .29 | −12.52 | 1.88 | ||
| Baseline | Week 6 | −8.37* | 2.71 | .02 | −15.75 | −1.00 | ||
| Plasma TT (nmol/L) | Placebo | Baseline | Week 6 | −3.32 | 1.62 | .05 | −6.57 | −0.07 |
| Bupropion | Baseline | Week 6 | −4.03* | 1.56 | .01 | −7.15 | −0.90 | |
| Plasma prolactin (μIU/ml) | Placebo | Baseline | Week 6 | 3.94 | 18.61 | .83 | −33.45 | 41.33 |
| Bupropion | Baseline | Week 6 | 13.70 | 17.88 | .45 | −22.23 | 49.63 | |
Note. aAdjustment for multiple comparisons: Bonferroni with covariate daily methadone dosage and duration of the methadone usage. CGI-SF = Clinical Global Impression Scale adapted for Sexual Function; Mal-IIEF-15 = Malay version of the International Index of Erectile Function 15; SDI-2-BM = Malay version of the sexual desire inventory-2; DSD = dyadic sexual desire; SSD = solitary sexual desire; TT = total testosterone; SD, standard deviation; SE, standard error. nmol/L = nanomoles per liter; μIU/ml = macro international units per milliliter.
p < .05. **p < .01. ***p < .001.
Figure 3.
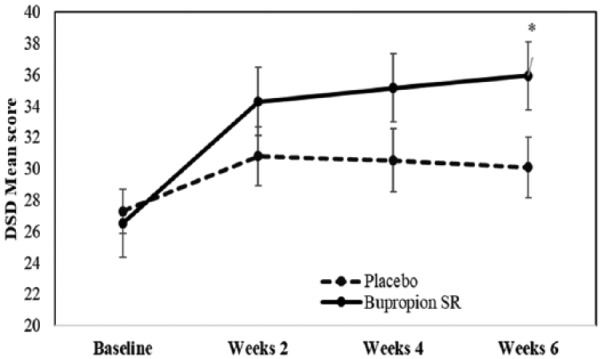
Mean dyadic sexual desire (DSD) in methadone-emergent sexual dysfunction patients receiving sustained-release bupropion (bupropion SR) or placebo. *p < .05.
Mal-IIEF-15
At end-point, the total Mal-IIEF-15 mean scores improved from baseline for patients receiving bupropion SR (mean difference = 8.37 ± 2.71. 95% CI [15.75, 0.99], p = .02) compared with those receiving placebo (mean difference = 4.19 ± 2.71, 95% CI [10.43, 5.12], p = .76) (Table 2; Figure 4). Patients receiving bupropion SR showed significant improvements from baseline to end-point on IIEF domains of erectile function (mean difference = 3.93, p = .04) and sexual desire (mean difference = 1.68, p < .001) but not in patients receiving placebo (Table 3).
Figure 4.
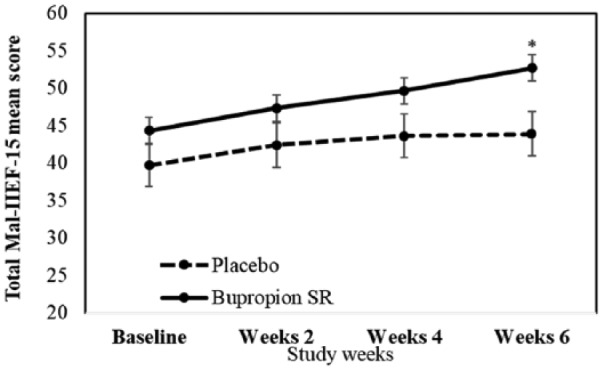
Total Malay Version of International Index of Erectile Function mean scores (Mal-IIEF-15) in methadone-emergent sexual dysfunction patients receiving sustained-release bupropion (bupropion SR) or placebo. *p < .05.
Plasma Testosterone and Prolactin Levels
At end-point, the total plasma testosterone improved from baseline in bupropion SR group (mean difference = 4.03, 95% CI [0.90, 7.15], p = .01) when compared to those in the placebo group (mean difference = 3.32, 95% CI [0.07, 6.57], p = .05). There were no statistically significant differences in plasma testosterone levels between the two groups.
There was no improvement noted in plasma prolactin levels from baseline to end-point in bupropion SR or placebo group. There were also no statistically significant differences in prolactin levels between the two groups.
Adverse Events
The most common adverse event was insomnia, reported by 17.7% (n = 6) of men taking bupropion SR and 2.8% (n = 1) taking placebo (p = .11). Less frequent were skin rash, 8.3% (n = 3) versus 0% (p = .24); inability to concentrate, 5.6% (n = 2) versus 0% (p = .49); constipation, 5.6% (n = 2) versus 5.6% (n = 2) (p = .61); and headache, 2.8% (n = 1) versus 2.8% (n = 1) (p = .49), respectively. More common adverse events in the placebo group than in the bupropion SR group were ineffectiveness 8.3% (n = 3) versus 0% (p = .24), nausea 5.6% (n = 2) versus 0% (p = .49) and loss of appetites, 2.8% (n = 1) versus 0% (p = 1.00), respectively. Nine patients treated with bupropion SR and 8 patients treated with placebo discontinued the study prematurely because of adverse events, mainly insomnia (Table 4). No patient in any group had clinically significant changes in systolic and diastolic blood pressure, and BMI. No serious adverse events related to trial medication were reported.
Table 4.
Number of Patients Prematurely Discontinued From the Study Because of Adverse Events.
| Adverse events, n (%) | Bupropion (n = 36) | Placebo (n = 36) | p value |
|---|---|---|---|
| Insomnia | 4 (11.1) | 0 | .24 |
| Skin itchiness | 2 (5.6) | 0 | .49 |
| Not effective | 0 | 3 (8.3) | .24 |
| Cannot concentrate | 2 (5.6) | 0 | .49 |
| Constipation | 1 (2.8) | 0 | 1 |
| Nausea | 0 | 1 (2.8) | 1 |
| Headache | 0 | 1 (2.8) | 1 |
| Loss of appetites | 0 | 1 (2.8) | 1 |
| Chest discomfort | 0 | 2 (5.6) | .49 |
Note. *p < .05. **p < .01. ***p < .001.
Discussion
To our knowledge, this is the first randomized trial to demonstrate a significant reduction in adverse sexual effects, measured by CGI-SF, SDI-2-BM, and Mal-IIEF-15 that compared bupropion SR with placebo among men with methadone emergent sexual dysfunction, specifically low sexual desire while continuing stable-dose methadone treatment. A categorical improvement of “much/very much improved” in CGI-SF score was reported by 58.3% of bupropion SR-assigned versus 27.7% (n = 10/36) placebo-assigned patients.
DSD scores improved from baseline for patients receiving bupropion SR with mean difference of 9.41 ± 1.82 when compared with those receiving placebo. Besides, patients receiving bupropion SR showed significant improvements from baseline to end-point on IIEF domains of erectile function (mean difference = 3.93, p = .04) and sexual desire (mean difference = 1.68, p <.001) but not in those receiving placebo. In addition, the total plasma testosterone also improved from baseline in bupropion SR group (mean difference = 4.03, 95% CI [7.15, 0.90], p < .01).
Hypoactive sexual desire is one of the major adverse effects for ex-heroin-dependent patients who are receiving methadone (Yee, Loh, & Ng, 2014). This could be one of the reasons MMT patients failed to comply with MMT. In current study, the patients who received bupropion SR had shown statistically significant improvements from baseline to end of trial point on their DSD scores (<.001) and IIEF sexual desire domain scores (<.001). This finding is similar to the previous studies (Salehi et al., 2015; Tatari et al., 2013). This may indicate that bupropion SR, by blocking the reuptake of norepinephrine and dopamine, is able to alleviate the loss of sexual desire as a result of long-term methadone usage. The sexual desire pathway is an interplay between the sexual excitatory and inhibitory mechanisms in the brain. Dopamine is one of the main neurotransmitters which plays a major role in enhancing the sexual excitatory mechanism, while the endogenous opiate and methadone blunting it. Bupropion SR probably exerts effect on this dopamine-mediated enhancement of sexual excitatory mechanisms in the sexual desire pathway (Pfaus, 2009).
In this study, patients receiving bupropion SR showed significant improvements on IIEF domains of erectile function from baseline (mean = 18.14 ± 8.74) to end-point (22.56 ± 7.69) but not in patients receiving placebo. In one local study, the prevalence of erectile dysfunction among MMT patients was up to 67% and the patients with depression were four times more likely to have erectile dysfunction than patients without depression (Teoh, Yee, Danaee, Ng, & Sulaiman, 2016). Given that bupropion SR is an effective amino-ketone antidepressant, along with the potential for improved erectile function, bupropion SR may serve as an attractive choice of treatment for MMT patients who have depression and also for those whose sexual dysfunction is a concern.
This study also identified that the total plasma testosterone had statistically significantly improved from baseline (mean = 7.98 ± 4.57) to end-point (mean = 12.04 ± 8.46) in bupropion SR group but not in placebo group (p = .01). In contrast, this observation was not seen in the plasma prolactin. This has further proved that the effect of methadone on sexual function is possible through direct blocking of the dopamine-mediated release of gonadotrophin-releasing hormone rather than prolactin-releasing hormone at the hypothalamic-pituitary-gonadal axis, resulting in decreased serum testosterone concentrations and symptoms of testosterone deficiency. Perhaps the addition of bupropion SR to ongoing MMT can ameliorate MMT-induced sexual dysfunction through the enhancement of activity of dopamine-mediate gonadotrophin releasing hormone, which in turn increases the level of plasma testosterone. Up to date, research on exploring the relationship between bupropion and testosterone levels remained scarce. In animal study (Bulmuş et al., 2015), serum testosterone levels were observed significantly higher in male rats receiving bupropion (47.74 ± 2.33 ng/ml) compared with control rats (39.69 ± 2.27 ng/ml, p < .05). Clayton et al. (2004) conducted a placebo-controlled, double-blind comparison of bupropion SR versus placebo in 42 patients with selective serotonin reuptake inhibitor (SSRI)-induced sexual dysfunction. Frequency of sexual activity was significantly correlated to total testosterone level at baseline (r = .36, p = .027) and at week 4 (r = .41, p = .025) in this study. However, this study only consisted of 5 male patients, hence cautious interpretation must be carried out. Testosterone replacement therapy is one of the potential therapeutic options for sexual dysfunction where there is testosterone deficiency (Buvat et al., 2013). Previous studies have demonstrated that testosterone replacement therapy improved sexual desire in men who have opioid-induced androgen deficiency (Basaria et al., 2015; Blick et al., 2012). However, the benefit of combination of testosterone replacement therapy and bupropion SR to treat this condition remained uncertain. Furthermore, in current study, the total testosterone level also improved at placebo group (mean difference from baseline to weeks 6 is −3.32) and there were no statistically significant differences in plasma testosterone levels between the bupropion SR and placebo group at the end-point. Further research is needed to determine the effect of bupropion SR on plasma testosterone before any conclusion could be made.
Both bupropion and methadone are extensively metabolized by cytochrome P4502B6 (CYP2B6) in the human liver (Hedrich, Hassan, & Wang, 2016). Although bupropion has been reported to be a CYP2B6 inhibitor, CYP2B6 is highly polymorphic. Previous study identified CYP2B6*4, a variant of CYP2B6, to be an apparent rapid metabolizer phenotype. CYP2B6*4 increases methadone metabolism but decreases methadone concentrations, and demonstrates a particular susceptibility to CYP2B6 inhibitory drug interactions (Kharasch, Regina, Blood, & Friedel, 2015). Besides, bupropion has been demonstrated to be an effective inhibitor of CYP2D6 which is also involved in methadone metabolism, leading to clinically significant higher methadone concentrations (Hedrich et al., 2016). This indicates that individuals who concurrently take bupropion and methadone will not need high dose of methadone which may in turn experience less side effect such as sexual dysfunction from methadone. Due to large inter-individual variability in methadone’s pharmacokinetics and its pharmacodynamics, the effect of methadone–bupropion interaction on sexual function and plasma testosterone remained unclear. More research is needed to determine the effect of methadone-bupropion interaction in sexual functioning.
Bupropion SR was well tolerated in this study. The most frequently reported adverse event in the present study was insomnia, which occurred with similar frequency in previous studies (Aubin, 2002; Croft et al., 1999).
Current study was not without a few limitations. The sample size was relatively small. Additionally, this study relied on patient self-report on their sexual dysfunction, which could be subjective, potentially inaccurate due to recall bias. Besides, this study might not be generalizable to all MMT men who had not met the criteria of this study. Limitation of this trial to a 6-week duration was also a consideration and it was unknown whether bupropion SR would have been beneficial in the long run.
Conclusion
Despite the limitations, our results supported the effectiveness of a 6-week adjunctive bupropion SR treatment. Bupropion SR treatment had significantly improved the key aspects of sexual function in men with MMT-emergent sexual dysfunction in this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Huai Seng Loh  https://orcid.org/0000-0003-0904-0503
https://orcid.org/0000-0003-0904-0503
References
- Anne Yee H. A., Ng C. G., Rusdi A. R. (2011). Validation of the Malay version of Fagerstrom Test for Nicotine Dependence (FTND-M) among a group of male staffs in a university hospital. Malaysian Journal of Psychiatry, 20(1). [Google Scholar]
- Aubin H.-J. (2002). Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs, 62(2), 45–52. [DOI] [PubMed] [Google Scholar]
- Balon R., Segraves R. T. (2005). Handbook of sexual dysfunction. New York, NY: Taylor & Francis. [Google Scholar]
- Basaria S., Travison T. G., Alford D., Knapp P. E., Teeter K., Cahalan C., … Mensing G. (2015). Effects of testosterone replacement in men with opioid-induced androgen deficiency: A randomized controlled trial. Pain, 156(2), 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blick G., Khera M., Bhattacharya R. K., Nguyen D., Kushner H., Miner M. M. (2012). Testosterone replacement therapy outcomes among opioid users: The Testim Registry in the United States (TRiUS). Pain Medicine, 13(5), 688–698. [DOI] [PubMed] [Google Scholar]
- Brown R., Balousek S., Mundt M., Fleming M. (2005). Methadone maintenance and male sexual dysfunction. Journal of Addictive Diseases, 24(2), 91–106. [DOI] [PubMed] [Google Scholar]
- Bulmuş Ö., Yardımcı A., Ülker N., Özdemir G., Özcan M., Canpolat S., Keleştimur H. (2015). Effects of paroxetine, bupropion and agomelatine on reproduction hormones and sperm parameters in male rats. Acta Physiologica, 215, 102–103. [Google Scholar]
- Buvat J., Maggi M., Guay A., Torres L. O. (2013). Testosterone deficiency in men: Systematic review and standard operating procedures for diagnosis and treatment. The Journal of Sexual Medicine, 10(1), 245–284. [DOI] [PubMed] [Google Scholar]
- Clayton A. H., Warnock J. K., Kornstein S. G., Pinkerton R., Sheldon-Keller A., McGarvey E. L. (2004). A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. The Journal of Clinical Psychiatry, 65(1), 62–67. [DOI] [PubMed] [Google Scholar]
- Coolen L., Fitzgerald M., Yu L., Lehman M. (2004). Activation of μ opioid receptors in the medial preoptic area following copulation in male rats. Neuroscience, 124(1), 11–21. [DOI] [PubMed] [Google Scholar]
- Croft H., Settle E., Houser T., Batey S. R., Donahue R. M., Ascher J. A. (1999). A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clinical Therapeutics, 21(4), 643–658. [DOI] [PubMed] [Google Scholar]
- Daniell H. W. (2002). Narcotic-induced hypogonadism during therapy for heroin addiction. Journal of Addictive Diseases, 21(4), 47–53. [DOI] [PubMed] [Google Scholar]
- Darke S., Ward J., Hall W., Heather N., Wodak A. (1991). The Opiate Treatment Index (OTI) researchers’ manual: NDARC. Sydney: National Drug and Alcohol Research Centre. [Google Scholar]
- de la Rosa R. E., Hennessey J. V. (1996). Hypogonadism and methadone: Hypothalamic hypogonadism after long-term use of high-dose methadone. Endocrine Practice, 2(1), 4–7. [DOI] [PubMed] [Google Scholar]
- De Maddalena C., Bellini M., Berra M., Meriggiola M. C., Aloisi A. M. (2012). Opioid-induced hypogonadism: Why and how to treat it. Pain Physician, 15(3), ES111–ES118. [PubMed] [Google Scholar]
- Gradin V. B., Baldacchino A., Balfour D., Matthews K., Steele J. D. (2013). Abnormal brain activity during a reward and loss task in opiate-dependent patients receiving methadone maintenance therapy. Neuropsychopharmacology, 39(4), 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M., Weigand-Tomiuk H. (2002). Sexual dysfunctions in a group of psychiatric in-patients. Psychiatrische Praxis, 29(4), 194. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976). ECDEU assessment manual for psychopharmacology (pp. 534–537). Washington, DC: US Department of Health, and Welfare. [Google Scholar]
- Hallinan R., Byrne A., Agho K., McMahon C., Tynan P., Attia J. (2008). Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. The Journal of Sexual Medicine, 5(3), 684–692. [DOI] [PubMed] [Google Scholar]
- Hallinan R., Byrne A., Agho K., McMahon C. G., Tynan P., Attia J. (2009). Hypogonadism in men receiving methadone and buprenorphine maintenance treatment. International Journal of Andrology, 32(2), 131–139. [DOI] [PubMed] [Google Scholar]
- Hedrich W. D., Hassan H. E., Wang H. (2016). Insights into CYP2B6-mediated drug–drug interactions. Acta Pharmaceutica Sinica B, 6(5), 413–425. doi: 10.1016/j.apsb.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch E. D., Regina K. J., Blood J., Friedel C. (2015). Methadone pharmacogenetics: CYP2B6 polymorphisms determine plasma concentrations, clearance and metabolism. Anesthesiology, 123(5), 1142–1153. doi: 10.1097/aln.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Pera G., Carderi A., Marianantoni Z., Peris F., Lentini M., Taggi F. (2008). Sexual dysfunction prior to first drug use among former drug addicts and its possible causal meaning on drug addiction: Preliminary results. The Journal of Sexual Medicine, 5(1), 164. [DOI] [PubMed] [Google Scholar]
- Laumann E. O., Paik A., Rosen R. C. (1999). Sexual dysfunction in the United States. JAMA: The Journal of the American Medical Association, 281(6), 537–544. [DOI] [PubMed] [Google Scholar]
- Macdonald S., Halliday J., MacEwan T., Sharkey V., Farrington S., Wall S., McCreadie R. (2003). Nithsdale schizophrenia surveys 24: Sexual dysfunction case—control study. The British Journal of Psychiatry, 182(1), 50–56. [DOI] [PubMed] [Google Scholar]
- Mattick R. P., Breen C., Kimber J., Davoli M. (2009). Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Systematic Reviews, 3, CD002209. [DOI] [PubMed] [Google Scholar]
- Oksuz E., Malhan S. (2005). The prevalence of male sexual dysfunction and potential risk factors in Turkish men: A web-based survey. International Journal of Impotence Research, 17(6), 539–545. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G. (2009). Reviews: Pathways of sexual desire. The Journal of Sexual Medicine, 6(6), 1506–1533. [DOI] [PubMed] [Google Scholar]
- Quek K. F., Low W. Y., Razack A. H., Chua C. B., Loh C. S. (2002). Reliability and validity of the Malay version of the International Index of Erectile Function (IIEF-15) in the Malaysian population. International Journal of Impotence Research, 14, 310–315. doi: 10.1038/sj.ijir.3900902 [DOI] [PubMed] [Google Scholar]
- Quek K. F., Low W. Y., Razack A. H., Loh C. S., Chua C. B. (2001). Measurement properties of the Malay version of the Golombok-Rust Inventory of Marital State (GRIMS) among urological patients. Malaysian Journal of Psychiatry, 9(1), 23–28. [Google Scholar]
- Rosen R., Cappelleri J., Gendrano N., Correspondence N. (2002). The International Index of Erectile Function (IIEF): A state-of-the-science review. International Journal of Impotence Research, 14(4), 226–244. [DOI] [PubMed] [Google Scholar]
- Rosen R. C. (2000). Prevalence and risk factors of sexual dysfunction in men and women. Current Psychiatry Reports, 2(3), 189–195. [DOI] [PubMed] [Google Scholar]
- Rust J., Bennun I., Crowe M., Golombok S. (1986). The Golombok Rust Inventory of Marital State (GRIMS). Sexual and Marital Therapy, 1(1), 55–60. [Google Scholar]
- Salehi M., Barekatain M., Faghani F., Karimian N., Molaeinezhad M., Asadalloahi G. A., Maracy M. R. (2015). Bupropion efficacy on sexual dysfunction among male patients on methadone maintenance therapy: A double-blind placebo-controlled trial. Sexual and Relationship Therapy, 30(3), 364–375. doi: 10.1080/14681994.2015.1016494 [DOI] [Google Scholar]
- Spector I. P., Carey M. P., Steinberg L. (1996). The sexual desire inventory: Development, factor structure, and evidence of reliability. Journal of Sex & Marital Therapy, 22(3), 175–190. [DOI] [PubMed] [Google Scholar]
- Svanborg P., Åsberg M. (1994). A new self-rating scale for depression and anxiety states based on the comprehensive psychopathological rating scale. Acta Psychiatrica Scandinavica, 89(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Tatari F., Shakeri J., Farnia V., Heidari F., Rezaei M. (2013). Bupropion in methadone induced erectile dysfunction. Life Science Journal, 10(9s), 330–332. [Google Scholar]
- Teoh J. B. F., Yee A., Danaee M., Ng C. G., Sulaiman A. H. B. (2016). Erectile dysfunction among patients on methadone maintenance therapy and its association with quality of life. Journal of Addiction Medicine, 11(1), 40–46. [DOI] [PubMed] [Google Scholar]
- van Furth W. R., van Emst M. G., van Ree J. M. (1995). Opioids and sexual behavior of male rats: Involvement of the medial preoptic area. Behavioral Neuroscience, 109(1), 123. [DOI] [PubMed] [Google Scholar]
- Vuong C., Van Uum S. H. M., O’Dell L. E., Lutfy K., Friedman T. C. (2010). The effects of opioids and opioid analogs on animal and human endocrine systems. Endocrine Reviews, 31(1), 98–132. doi: 10.1210/er.2009-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A., Loh H. S., Hashim H. H., Ng C. G. (2014). Clinical factors associated with sexual dysfunction among men in methadone maintenance treatment and buprenorphine maintenance treatment: A meta-analysis study. International Journal of Impotence Research, 26(5), 161–166. [DOI] [PubMed] [Google Scholar]
- Yee A., Loh H. S., Ng C. G. (2014). The prevalence of sexual dysfunction among male patients on methadone and buprenorphine treatments: A meta-analysis study. The Journal of Sexual Medicine, 11(1), 22–32. [DOI] [PubMed] [Google Scholar]
- Yee A., Ong T. K., Kuppusamy S., Yeoh W. S., Sulaiman A. H., Ng C. G., Tan K. A. (in press). Factor structure, reliability, and validity of the Malay version of Sexual Desire Inventory-2 (SDI-2-BM) in a sample of benign prostatic hyperplasia patients and healthy individuals. [Google Scholar]
- Yee A., Yassim A. R. M., Loh H. S., Ng C. G., Tan K.-A. (2015). Psychometric evaluation of the Malay version of the Montgomery-Asberg Depression Rating Scale (MADRS-BM). BMC Psychiatry, 15(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]