Abstract
The high frequency of treatment-related side effects for men with localized prostate cancer creates uncertainty for treatment outcomes. This study assessed the comparative effectiveness of treatment-related side effects associated with conservative management and cryotherapy in patients with localized prostate cancer. A retrospective longitudinal cohort study was conducted, using the linked data of the Surveillance, Epidemiology, and End Results and Medicare, which included patients diagnosed from 2000 through year 2013, and their Medicare claims information from 2000 through 2014. To compare the differences in baseline characteristics and treatment-related side effects between the study cohorts, χ2 tests were conducted. Multivariate logistic regression was used to assess the association between treatment selection and side effects. There were 7,998 and 3,051 patients in the conservative management and cryotherapy cohort, respectively. The likelihood of erectile dysfunction, lower urinary tract obstruction, urinary fistula, urinary incontinence, and hydronephrosis was reported to be significantly lower (53%, 35%, 69%, 65%, and 36%, respectively) in the conservative management cohort. Conservative management had a lower likelihood of treatment-related side effects compared to cryotherapy. However, further research is needed to compare other significant long-term outcomes such as costs associated with these treatment choices and quality of life.
Keywords: prostate cancer, conservative management, cryotherapy, treatment side effects, surveillance, epidemiology, end results
Prostate cancer is the most prevalent cancer and a second leading cause of death in American men (Siegel, Miller, & Jemal, 2018). Men aged 65 years or older are more often diagnosed with prostate cancer (Siegel et al., 2018). It has been reported that nearly four out of five cases are diagnosed with localized prostate cancer (early-stage prostate cancer; Patton, 2014). Several treatment options are available for localized prostate cancer (Drachenberg, 2000; Hargraves & Hadley, 2003; Shore, 2014; Venderbos & Roobol, 2011). The impacts of these treatment options on survival and patients’ quality of life may vary due to different side effects (Klotz et al., 2010; Rodriguez et al., 2014). Minimally invasive treatment options with fewer side effects than surgery and radiation, such as conservative management and cryotherapy, may be preferable to patients with localized prostate cancer (Roberts et al., 2011).
Conservative management is an observational treatment strategy, in which no treatment is given to patients until cancer progresses to advanced or metastatic stage (Drachenberg, 2000). Conservative management is commonly used for localized prostate cancer (Venderbos & Roobol, 2011). Patients on conservative management were reported to have higher survival rates than active treatment strategies (Venderbos & Roobol, 2011). Further, patients on conservative management are often followed by repeated biopsies, digital rectal examinations (DREs), and prostate-specific antigen (PSA) testing (Venderbos & Roobol, 2011). However, research suggests that there is an increased risk of erectile dysfunction among patients who undergo repeated prostate biopsies (Fujita, Landis, McNeil, & Pavlovich, 2009; Kirby & Fitzpatrick, 2012). In addition, biopsies are associated with bleeding, infections, pain, and a low risk of urinary track symptoms (Loeb et al., 2013).
Cryotherapy involves the destruction of cancer cells through rapid freezing and thawing and injection of very cold gases, while avoiding lethal freezing to the surrounding healthy tissues (Hargraves & Hadley, 2003; Shore, 2014). This procedure is usually performed on an outpatient basis and typically only requires one treatment (Roberts et al., 2011). The frequency of side effects associated with cryotherapy is lower than that in other treatments. Futher, less commonly reported side effects of cryotherapy are bleeding, urinary and bowel fistulas, lower urinary tract obstruction, hydronephrosis, and incontinence (Aus, Pileblad, & Hugosson, 2002; Moore et al., 2013; Pirtskhalaishvili, Hrebinko, & Nelson, 2001; Williams et al., 2012). Compared with other treatment modalities such as surgery and radical prostatectomy, the long-term outcomes of cryotherapy are not known because cryotherapy is a relatively newer technique (Babaian et al., 2008; Shore, 2014).
Previous research examining the clinical outcomes associated with conservative management has focused on shorter follow-up periods, which may not capture important events. The Agency for Healthcare Research and Quality (AHRQ) conducted a review on the comparative effectiveness of treatments used in localized prostate cancer (Wilt et al., 2008) and the authors concluded that there were uncertainties about the comparative effectiveness and harm of these treatments due to inadequate evidence on long-term outcomes (Wilt et al., 2008). Insufficient evidence and uncertainties associated with conservative management and cryotherapy highlight the need for further evaluation of these two treatments. Thus, this study aimed to compare conservative management and cryotherapy based on treatment-related side effects in patients with localized prostate cancer. Further, this study hypothesized that men on conservative management may experience lower rates of treatment-related side effects and higher rates of survival compared to men on cryotherapy.
Methods
Study Design
This retrospective longitudinal cohort study used the linked data of Surveillance, Epidemiology, and End Results (SEER) and Medicare and included patients diagnosed from 2000 through year 2013, and their claims information available from 2000 through 2014. The linkage of the SEER and Medicare data is the combined effort of the National Cancer Institute, the SEER registries, and the Center for Medicare and Medicaid Services (Warren, Klabunde, Schrag, Bach, & Riley, 2002). The SEER program captures clinical, demographic, and survival information for approximately 28% of the U.S. population and is 98% complete for case ascertainment (Warren et al., 2002). The Medicare program covers approximately 97% of persons aged 65 years and older (Warren et al., 2002). This study was approved by the University of Georgia’s Institutional Review Board (IRB Study ID # 00003555).
Study Cohorts
Patients with localized prostate cancer were identified as those with stage I or stage II cancer diagnosis (Mathers et al., 2011; Mohan & Schellhammer, 2011). Patients in the conservative management group were identified as those who did not receive any immediate treatment within the first 6 months of diagnosis of localized prostate cancer (Lu-Yao et al., 2008; Schymura et al., 2010). Patients in the cryotherapy cohort were identified using either the International Classification of Diseases, Ninth Revision (ICD-9), Procedural Code (60.62), Healthcare Common Procedure Coding System (HCPCS) codes (G0160 or G0161), or Current Procedural Terminology (CPT) code (55873). Patients were excluded from the study if they were (a) first diagnosed at autopsy or by death certificate, (b) had other types of cancer, (c) enrolled in a health maintenance organization, (d) at other stages of cancer, or (e) below 66 years of age (restricting the cohort to patients aged 66 years allowed at least 12 months of Medicare claims data).
Outcomes
The following seven treatment-related side effects were identified using the relevant diagnostic and procedural codes (Table 1) in these patients: erectile dysfunction, lower urinary tract obstruction, bowel fistula, urinary fistula, urinary incontinence, bleeding, and hydronephrosis (Aus et al., 2002; Moore et al., 2013; Pirtskhalaishvili et al., 2001; Roberts et al., 2011; Williams et al., 2012). Cancer-specific survival was measured as the time from prostate cancer diagnosis until death as a result of prostate cancer.
Table 1.
ICD-9 and CPT/HCPCS Codes for Treatment-Related Side Effects.
| Side effects | ICD-9 diagnosis codes | ICD-9 procedure codes | CPT/HCPCS codes |
|---|---|---|---|
| Erectile dysfunction | 607.84 | 64.94, 64.95, 64.96, 64.97 | 54400, 54401, 54402, 54405, 54407, 54408, 54409, 54410, 54411, 54415, 54416, 54417, C1007, C1813, C2622, C3500, C8514, C8516, C8534, L7900, 54231, 54235, J0270, J0275, J2440, J2760 |
| Lower urinary tract obstruction | 596.0, 598.x, 599.6, 788.2x | 57.85, 57.91, 57.92, 58.0, 58.1, 58.3x, 58.44, 58.46, 58.47, 58.5, 58.6, 58.99, 60.95, 60.2x | 52275, 52276, 52281,52510, 53010, 53400, 53405,53410, 53415, 53420, 53425, 53600, 53601, 53605, 53620, 53621, 52601, 52612, 52614, 52620, 52630, 53850, 53852, 2282, 52283 |
| Bowel fistula | 569.41, 569.81 | 48.73, 48.93, 46.1x, 48.31, 48.32, 48.33 | 45800, 45805, 45820, 45825, 45562, 45563 |
| Urinary fistula | 596.1, 596.2, 599.1 | 57.83, 57.84, 58.43 | 44660, 44661, 53520 |
| Urinary incontinence | 788.3x, 599.82 | 59.72, 58.93, 59.3, 59.4, 59.5, 59.6, 59.71, 59.79 | 51715, 53445, 53447, 53440, 51840, 51841, 53442, 53443 |
| Bowel bleeding | 569.41, 569.81 | ||
| Hydronephrosis | 591 |
Note. CPT = Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System; ICD-9 = International Classification of Diseases, Ninth Revision.
Covariates
Demographic information included patients’ age (66–69 years, 70–74 years, 75–79 years, and 80 and above), race (Caucasians, African American, and others), marital status (married, unmarried/single, and unknown), year of diagnosis (2000–2004, 2005–2009, and 2010–2013), geographic location (Northeast, South, Central, West), and urban residency (yes/no). Education (e.g., the proportion of population with less than a high school degree) was obtained from the census tract file of the SEER. The education variable was categorized into quartiles. The categories for the proportion of population with less than a high school degree were 0% to 7.13% (representing high educational level), 7.14% to 11.91% (medium), 11.92% to 20.46% (lower), 20.47% to 100% (lowest), and unknown (Srokowski et al., 2008). The Charlson Comorbidity Index (CCI) was derived from the Medicare claims using a validated algorithm (Deyo, Cherkin, & Ciol, 1992; Klabunde, Potosky, Legler, & Warren, 2000) and was categorized as 0, 1, and 2 and above.
Cancer-related information including tumor stage and grade were also extracted. Tumor grades were determined by Gleason score and was classified as well differentiated (Gleason scores of 2–4), moderately differentiated (Gleason scores of 5–7), and poorly differentiated (Gleason score of 8 and above; Li, Djenaba, Soman, Rim, & Master, 2012; Wallis et al., 2016). Tumor stage was classified as T1 and T2.
Statistical Analysis
Descriptive statistics were performed to characterize the conservative management and cryotherapy cohorts. To compare the differences in baseline characteristics and treatment-related side effects between the study cohorts, χ2 tests were conducted. logistic regression was utilized to assess the association between each side effect and the treatment selection. The Kaplan-Meier survival method was used to test for the crude differences between the two cohorts using a log-rank test. A multivariate cox proportional hazard model was used to estimate the cancer-specific survival rates. All analyses were performed using SAS statistical software (version 9.4, SAS Institute, Cary, NC).
Results
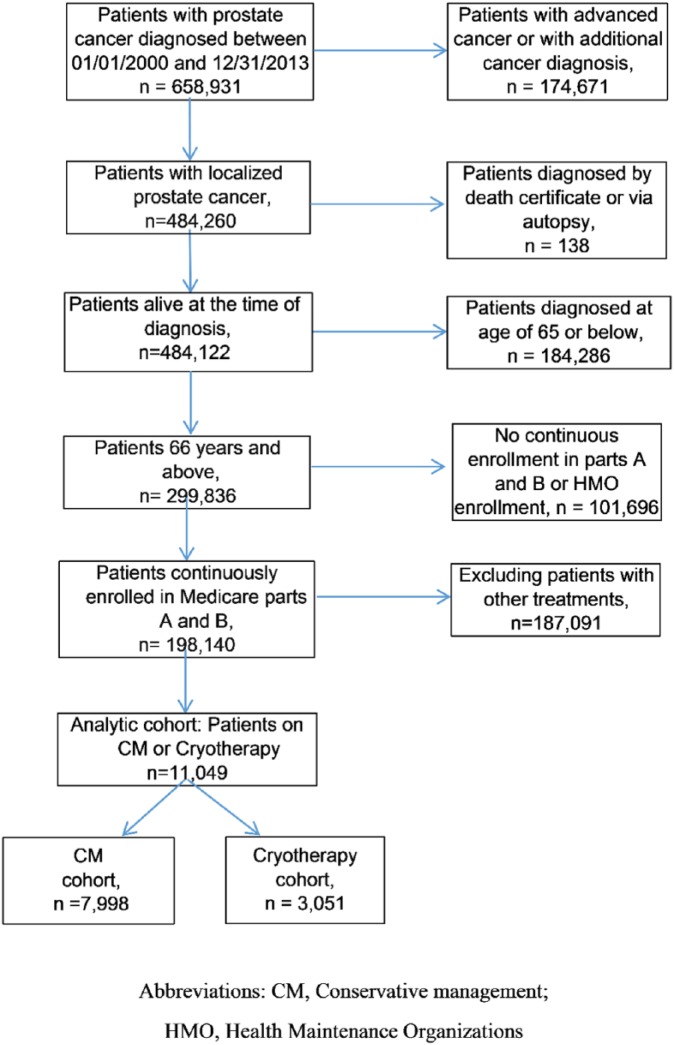
Figure 1 shows the derivation of the final analytical cohort based on the inclusion and exclusion criteria. There were 7,998 patients in the conservative management cohort and 3,051 patients in the cryotherapy cohort. Table 2 describes the baseline characteristics of the study cohorts. Overall, the conservative management and cryotherapy cohorts were identified to be statistically different in terms of age, race, education, geographic location, marital status, urban residency, year of diagnosis, tumor grade, and stage (p < .05). Compared to the cryotherapy cohort, patients in the conservative management cohort were more likely to be aged 66 to 69 years, while patients in the cryotherapy cohorts were more likely to be aged between 70 and 74 years. A majority of patients in the conservative management and cryotherapy cohort were Caucasians, married, had a comorbidity score of 0, resided in urban areas, and were at T2 stage. Similarly, compared to the cryotherapy cohort, patients in the conservative management cohort were less likely to belong to the West, were more likely to have more than high school education, were less likely to be diagnosed between 2005 and 2009, and were more likely to have moderately differentiated tumors.
Figure 1.
Derivation of the final analytical cohort.
Table 2.
Baseline Characteristics of the Study Cohorts.
| Baseline characteristics | Conservative management (n = 7,998) | Cryotherapy (n = 3,051) |
p value |
|---|---|---|---|
| Age group, n (%) | |||
| 66–69 | 2,903 (36.30%) | 762 (24.98%) | <.0001 |
| 70–74 | 2,661 (33.27%) | 1,104 (36.18%) | |
| 75–79 | 1,599 (19.99%) | 815 (26.71%) | |
| 80+ | 835 (10.44%) | 370 (12.13%) | |
| Race, n (%) | |||
| Caucasians | 6,191 (77.41%) | 2,453 (80.40%) | .0029 |
| African Americans | 1,136 (14.20%) | 372 (12.19%) | |
| Others | 671 (8.39%) | 226 (7.41%) | |
| Tumor grade, n (%) | |||
| Well differentiated | 123 (1.54%) | 18 (0.59%) | <.0001 |
| Moderately differentiated | 4,510 (56.39%) | 1,317 (43.17%) | |
| Poorly differentiated | 3,140 (39.26%) | 1,584 (51.92%) | |
| Unknown | 225 (2.81%) | 132 (4.33%) | |
| Tumor stage, n (%) | |||
| T1 | 3,414 (42.69%) | 1,425 (46.71%) | .0001 |
| T2 | 4,584 (57.31%) | 1,626 (53.29%) | |
| Charlson Comorbidity Index, n (%) | |||
| 0 | 5,992 (74.92%) | 2,290 (75.06%) | .3600 |
| 1 | 1,833 (22.92%) | 708 (23.21%) | |
| 2+ | 173 (2.16%) | 53 (1.74%) | |
| Marital status, n (%) | |||
| Married | 5,214 (65.19%) | 2,073 (67.94%) | .0019 |
| Unmarried/single | 1,758 (21.98%) | 577 (18.91%) | |
| Unknown/missing | 1,026 (12.83%) | 401 (13.14%) | |
| Education, n (%) | |||
| First quartile (highest) | 2,715 (33.95%) | 816 (26.75%) | <.0001 |
| Second quartile | 1,526 (19.08%) | 599 (19.63%) | |
| Third quartile | 1,372 (17.15%) | 558 (18.29%) | |
| Fourth quartile (lowest) | 1,986 (24.83%) | 942 (30.88%) | |
| Unknown | 399 (4.99%) | 136 (4.46%) | |
| Geographic location, n (%) | |||
| Northeast | 2,074 (25.93%) | 277 (9.08%) | <.0001 |
| South | 905 (11.32%) | 540 (17.70%) | |
| Central | 1,320 (16.50%) | 592 (19.40%) | |
| West | 3,699 (46.25%) | 1,642 (53.82%) | |
| Urban residency, n (%) | |||
| Yes | 7,415 (92.71%) | 2,625 (86.04%) | <.0001 |
| No | 583 (7.29%) | 426 (13.96%) | |
| Year of diagnosis, n (%) | |||
| 2000–2004 | 2,597 (32.47%) | 713 (23.37%) | <.0001 |
| 2005–2009 | 3,328 (41.61%) | 1,639 (53.72%) | |
| 2010–2013 | 2,073 (25.92%) | 699 (22.91%) | |
Treatment-Related Side Effects
The multivariate logistic regression, which identifies the crude rates of treatment-related side effects in both the cohorts, is presented in Table 3. Overall, patients in the cryotherapy cohort experienced significantly higher rates of any side effects compared to patients in the conservative management cohort (29.29% vs. 42.61%, p < .0001). Compared to the conservative management cohort, patients in the cryotherapy cohort experienced significantly higher rates of erectile dysfunction (12.02% vs. 20.65%, p < .0001), lower urinary tract obstruction (9.06% vs. 13.63%, p < .0001), urinary fistula (<0.14% vs. 0.39%, p < .05), and hydronephrosis (1.74% vs. 2.79%, p < .05). There were no statistically significant differences observed in the rates of urinary incontinence, bleeding, and bowel fistula between the two cohorts.
Table 3.
Frequency of Side Effects in the Conservative Management and Cryotherapy Cohort.
| Side effects | Conservative management (n = 7,998) |
Cryotherapy (n = 3,051) | p value |
|---|---|---|---|
| Overall side effects, n (%) | 2,343 (29.29%) | 1,300 (42.61%) | <.0001 |
| Side effects by categories | |||
| Erectile dysfunction, n (%) | 961 (12.02%) | 630 (20.65%) | <.0001 |
| Lower urinary tract obstruction, n (%) | 725 (9.06%) | 416 (13.63%) | <.0001 |
| Urinary fistula, n (%) | <11 (<0.14%) | 12 (0.39%) | .0047 |
| Hydronephrosis, n (%) | 139 (1.74%) | 85 (2.79%) | .0005 |
| Urinary incontinence, n (%) | <11 (<0.14%) | <11 (0.36%) | .1573 |
| Bleeding, n (%) | 547 (6.84%) | 187 (6.13%) | .1803 |
| Bowel fistula, n (%) | 43 (0.54%) | 13 (0.43%) | .4604 |
Note. Cell size less than 11 are not shown in accordance with data use agreement of Surveillance, Epidemiology, and End Results (SEER) and Medicare.
After controlling for all the covariates, it was identified that patients in the conservative management cohort were nearly 50% less likely to experience any side effects compared to patients in the cryotherapy cohort (Table 4). Compared to patients in the cryotherapy cohort, patients in the conservative management cohort were 53% less likely to experience erectile dysfunction (odds ratio [OR]: 0.47; 95% confidence interval [CI] [0.41, 0.53]). Similarly, patients in the conservative management had lower odds of experiencing lower urinary tract obstruction (OR: 0.65; 95% CI [0.57, 0.75]), urinary fistula (OR: 0.31; 95% CI [0.13, 0.74]), hydronephrosis (OR: 0.64; 95% CI [0.48, 0.86]), and urinary incontinence (OR: 0.35; 95% CI [0.13, 0.93]).
Table 4.
Likelihood of Treatment-Related Side Effects in Patients Receiving Conservative Management Versus Cryotherapy.
| Side effects (conservative management vs. cryotherapy) | Adjusted odds ratio (95% CI) | p value |
|---|---|---|
| Overall side effects, n (%) | 0.54 [0.49, 0.59] | <.0001 |
| Side effects by categories | ||
| Erectile dysfunction, n (%) | 0.47 [0.41, 0.53] | <.0001 |
| Lower urinary tract obstruction, n (%) | 0.65 [0.57, 0.75] | <.0001 |
| Urinary fistula, n (%) | 0.31 [0.13, 0.74] | .0085 |
| Hydronephrosis, n (%) | 0.64 [0.48, 0.86] | <.0001 |
| Urinary incontinence, n (%) | 0.35 [0.13, 0.93] | .0344 |
| Bleeding, n (%) | 1.18 [0.98, 1.41] | .0763 |
| Bowel fistula, n (%) | 1.42 [0.75, 2.70] | .2865 |
Note. Odds ratio adjusted for age, race, tumor grade, tumor stage, Charlson Comorbidity Index, geographic location, urban density, year of diagnosis, marital status, and education. CI = confidence interval.
Cancer-Specific Survival
Overall, cancer-specific survival rates in the conservative management and cryotherapy cohort were reported to be 95.79% and 93.59%, respectively, at the end of the study period. Figure 2 shows the cancer-specific survival curves across the treatment groups. Survival rates were found to be significantly different between the patients receiving conservative management and cryotherapy (log-rank p value < .05). Results from the multivariate cox model are presented in Table 5. Patients in the conservative management cohort had significantly 12% lower hazard of dying as compared to patients in the cryotherapy cohort (hazard ratio [HR]: 0.78, 95% CI [0.56, 0.98]), after controlling for all the covariates.
Figure 2.
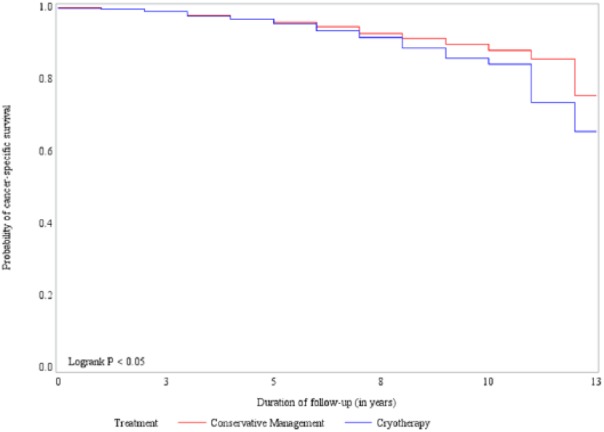
Cancer-specific survival across the treatment groups.
Table 5.
Multivariate Cox Proportional Hazards Model of Cancer-Specific Survival.
| Cancer-specific survival | ||
|---|---|---|
| Variable | Hazard ratio (95% CI) | p value |
| Treatment group | ||
| Conservative management | 0.78 [0.56, 0.98] | .031 |
| Cryotherapy | 1 | |
| Age | ||
| 66–69 | 1 | |
| 70–74 | 1.44 [1.12, 1.86] | .005 |
| 75–79 | 1.47 [1.12, 1.93] | .006 |
| 80–84 | 2.70 [2.10, 3.61] | <.0001 |
| Race | ||
| Caucasians | 1 | |
| African Americans | 1.30 [1.01, 1.68] | .044 |
| Others | 1.20 [0.86, 1.66] | .283 |
| Tumor grade | ||
| Unknown | 0.58 [0.36, 0.94] | .026 |
| Well differentiated | 0.40 [0.16, 0.97] | .042 |
| Moderately differentiated | 0.38 [0.31, 0.46] | <.0001 |
| Poorly differentiated | 1 | |
| Tumor stage | ||
| T1 | 0.88 [0.71, 1.10] | .229 |
| T2 | 1 | |
| Charlson Comorbidity Index | ||
| 0 | 1 | |
| 1 | 1.82 [1.65, 1.92] | .0002 |
| 2+ | 1.87 [1.68, 1.98] | .026 |
| Marital status | ||
| Married | 1 | |
| Unmarried | 1.08 [0.82, 1.43] | .576 |
| Unknown | 1.24 [1.01, 1.54] | .050 |
| Education | ||
| First quartile (highest) | 1.16 [0.92, 1.47] | .216 |
| Second quartile | 1.14 [0.87, 1.49] | .344 |
| Third quartile | 1.18 [0.92, 1.53] | .197 |
| Fourth quartile (lowest) | 1 | |
| Geographic location | ||
| West | 1 | |
| South | 1.28 [0.97, 1.70] | .079 |
| Central | 1.25 [0.97, 1.59] | .080 |
| Northeast | 0.93 [0.72, 1.19] | .549 |
| Urban residency | ||
| Yes | 1 | |
| No | 1.05 [0.75, 1.48] | .762 |
| Year of diagnosis | ||
| 2000–2004 | 1 | |
| 2005–2009 | 1.43 [1.13, 1.82] | .003 |
| 2010–2013 | 5.94 [3.45, 10.21] | <.0001 |
Note. Cancer-specific survival hazard ratios are mutually adjusted for treatment group, age, race, tumor grade, tumor stage, Charlson Comorbidity Index, geographic location, urban density, year of diagnosis, marital status, and education. CI = confidence interval.
Demographic characteristics such as age, race, tumor grade, comorbidities, and year of diagnosis were found to be significantly associated with cancer-specific survival. Patients in the age group of 70 to 74 years (HR: 1.44; 95% CI [1.12, 1.86]), 75 to 79 years (HR: 1.47; 95% CI [1.12, 1.93]), and 80+ years (HR: 2.70; 95% CI [2.10, 3.61]) had significantly higher hazard of dying as compared to those in the age group of 66 to 69 years. African American patients had 30% higher hazard of dying as compared to Caucasian patients (HR: 1.30; 95% CI [1.01, 1.68]). Compared to patients with poorly differentiated tumors, patients with well-differentiated (HR: 0.40; 95% CI [0.16, 0.97]) and moderately differentiated tumors (HR: 0.38; 95% CI [0.31, 0.46]) had significantly lower hazard of dying. The hazard of dying in patients with a comorbidity score of 1 (HR: 1.82; 95% CI [1.65, 1.92]) and 2 and above (HR: 1.87; 95% CI [1.68, 1.98]) was significantly higher as compared to those with no comorbidities. The hazard of dying was also found to be significantly higher in patients diagnosed between 2005 and 2009 (HR: 1.43; 95% CI [1.13, 1.82]) and 2010 and 2013 (HR: 5.94; 95% CI [3.45, 10.21]), as compared to those diagnosed between 2000 and 2004.
Discussion
This study addresses a significant gap in the literature by providing evidence about the side effects and long-term outcomes associated with two treatment choices for localized prostate cancer. The study findings suggest lower rates of side effects and higher rates of survival in patients who choose conservative management over cryotherapy. Further, the crude rates of erectile dysfunction, lower urinary tract obstruction, urinary fistula, and hydronephrosis were significantly lower in patients who chose conservative management as compared to those who chose cryotherapy. After controlling for all the covariates, patients in the conservative management cohort had significantly lower odds of erectile dysfunction, lower urinary tract obstruction, urinary fistula, hydronephrosis, and urinary incontinence.
The lower rates of side effects associated with conservative management reported in this study are similar to previous research that compared conservative management approaches with other active treatments (Acar et al., 2014; Wilt et al., 2012). Wilt et al. compared radical prostatectomy with conservative management and reported that radical prostatectomy was associated with a significant increase in urinary incontinence and erectile dysfunction (Acar et al., 2014; Wilt et al., 2012). Acar et al. conducted a study that compared brachytherapy, robot-assisted laparoscopic prostatectomy, and conservative management and identified that rates of erectile dysfunction were lower with conservative management (Acar et al., 2014). Side effects associated with cryotherapy are also reported to be low, with lower urinary tract obstruction and erectile dysfunction being the most common side effects (Roberts et al., 2011). In a cost-effectiveness analysis of cryotherapy and brachytherapy, rates of erectile dysfunction and urinary complications were reported to be higher with cryotherapy as compared to brachytherapy (Williams et al., 2012). However, the rates of bowel complications were reported to be lower in the cryotherapy cohort (Williams et al., 2012).
Although nonsignificant, the current study also showed that the rates of bowel fistula and bleeding were slightly higher in the conservative management cohort compared to the cryotherapy cohort. Conservative management involves periodic monitoring, which involves procedures such as biopsy (Brandes et al., 2016). Evidence suggests that biopsies in prostate cancer are associated with various complications such as bleeding and fistulas (Loeb et al., 2013; Nomura et al., 2005). The higher rates of bleeding and bowel fistula in the conservative management cohort in the current study may be associated with frequent biopsies. These treatment-related side effects could significantly impact patients’ quality of life, and therefore it is important to take them into consideration when making treatment choices.
The 13-year survival rates associated with conservative management and cryotherapy were found to be 95.79% and 93.59%, respectively. Although the rates of survival are comparable to those in the literature, there is inconclusive evidence on the treatment option with higher survival rates. A prospective, single-arm study conducted by Klotz et al. on a conservative management cohort found the 10-year cancer-specific survival rate to be 97.2% with conservative management (Klotz et al., 2010). Similarly, a prospective study conducted by Rodriguez et al. on a cryotherapy cohort in Spain found cancer-specific survival rates of 98.1% (Rodriguez et al., 2014). However, this study lacked a comparison group. A recent review conducted on cryotherapy concluded comparable survival benefits of cryotherapy over other treatments such as radiation therapy (Gao et al., 2016). However, due to the limitations in available evidence in literature, assessment of comparative benefits and harm of these treatments has been difficult. Overall, choosing conservative management provides slightly higher survival benefit over cryotherapy.
Additionally, factors such as increasing age, patients being African American, and having poorly differentiated tumors and higher comorbidities were identified to be associated with a significantly higher likelihood of dying. to the available evidence in the literature, the current study identified considerable factors that could influence survival (Jani & Hellman, 2003; Wilt et al., 2008). Significant racial disparities exist in survival rates among patients with prostate cancer. African American patients are approximately twice as likely as Caucasian patients are to die from prostate cancer (Mordukhovich et al., 2011). The literature also suggests that patients with well-differentiated tumors have minimal risk of dying from prostate cancer, while men with poorly differentiated tumors have a high risk of dying from their disease within 10 years of diagnosis (Wilt et al., 2008). When choosing treatments, these factors play an important role and therefore should be carefully considered.
These outcomes are extremely relevant for the treatment decision–making process. Evidence on the long-term outcomes generated from this study could be helpful and convincing in preferring conservative management over cryotherapy in elderly patients with localized prostate cancers, thus avoiding the associated risks and their impact on survival. A study examining literature on patient preferences indicated that patients with prostate cancer are not well-informed (Aning, Wassersug, & Goldenberg, 2012). In this era of shared decision-making, it is increasingly important for health-care providers to provide better quality of life with minimal posttreatment decision regrets to patients. Therefore, health-care providers should actively engage in translating the available evidence to practice not only through communication with their patients but also by practicing evidence-based decision-making.
This study has several limitations that warrant mentioning. First, the study cohorts were restricted to only Medicare beneficiaries who were aged 66 years and above. Therefore, study findings may not be generalizable to other patient populations such as Medicaid, Health Maintenance Organizations, other insurance plans, or to those younger than 66 years of age. There might be a possibility of selection bias due to nonrandomized nature of the study. Further, the conservative management cohort was defined as patients who did not undergo definitive therapy within the first 6 months of diagnosis for localized prostate cancer. Second, a distinction was not made between patients who deferred treatment as part of an active surveillance protocol and those who declined treatment due to significant comorbidities or advanced age. Furthermore, whether the patients who underwent cryotherapy were initially on surveillance protocols was not included in the study cohort definition of conservative management. These are potential sources of cohort contamination.
Third, an examination of the patient characteristics shows that the intervention group had a higher percentage of patients with poorly differentiated prostate cancer (39.26% vs. 51.92%). This may be a result of selection bias, with higher risk patients electing for treatment over observation. Fourth, increased risk of death was identified to be associated with increasingly risky disease parameters among all the study subjects; however, the current study did not compare survival rates of the high-risk patients from the two cohorts. Fifth, the study results may also be due to chance, as several other confounders were not included in the model that could reduce the likelihood of side effects, such as physical activity, healthy diet, normal weight, and diabetes medication. Finally, the current study did not account for other factors that may influence treatment choices such as physician or patient preferences or self-management strategies, which may have influenced the study results.
Despite these limitations, findings from this comparative effectiveness research can assist policy makers, health-care providers, and patients in making informed treatment decisions to optimize care. Conservative management seems to be a better treatment option over cryotherapy in terms of treatment-related side effects and survival. Better quality of care can be provided to patients suffering from prostate cancer with careful consideration of these outcomes. Further research is needed to compare other significant long-term outcomes such as costs associated with these treatment choices and quality of life.
Footnotes
Authors’ Contributions: Surbhi Shah, Henry N. Young, and Ewan K. Cobran carried out the study, participated in the design of the study, performed quantitative analysis, and drafted the article. All authors read and approved the final version of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Institutional Research Grant # 14-193-01 from the American Cancer Society.
Ethics Statement: This study was approved by the University of Georgia’s Institutional Review Board.
References
- Acar C., Schoffelmeer C. C., Tillier C., de Blok W., van Muilekom E., van der Poel H. G. (2014). Quality of life in patients with low-risk prostate cancer: A comparative retrospective study: Brachytherapy versus robot-assisted laparoscopic prostatectomy versus active surveillance. Journal of Endourology, 28, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aning J. J., Wassersug R. J., Goldenberg S. L. (2012). Patient preference and the impact of decision-making aids on prostate cancer treatment choices and post-intervention regret. Current Oncology, 19, S37–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aus G., Pileblad E., Hugosson J. (2002). Cryosurgical ablation of the prostate: 5-year follow-up of a prospective study. European Urology, 42, 133–138. [DOI] [PubMed] [Google Scholar]
- Babaian R. J., Donnelly B., Bahn D., Baust J. G., Dineen M., Ellis D, … Thrasher J. B. (2008). Best practice statement on cryosurgery for the treatment of localized prostate cancer. Journal of Urology, 180, 1993–2004. [DOI] [PubMed] [Google Scholar]
- Brandes A., Koerber F., Schwarzkopf L., Hunger M., Rogowski W. H., Waidelich R. (2016). Costs of conservative management of early-stage prostate cancer compared to radical prostatectomy–a claims data analysis. BMC Health Services Research, 16, 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo R. A., Cherkin D. C., Ciol M. A. (1992). Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology, 45, 613–619. [DOI] [PubMed] [Google Scholar]
- Drachenberg D. E. (2000). Treatment of prostate cancer: Watchful waiting, radical prostatectomy, and cryoablation. Seminars in Surgical Oncology, 18, 37–44. [DOI] [PubMed] [Google Scholar]
- Fujita K., Landis P., McNeil B. K., Pavlovich C. P. (2009). Serial prostate biopsies are associated with an increased risk of erectile dysfunction in men with prostate cancer on active surveillance. Journal of Urology, 182, 2664–2669. [DOI] [PubMed] [Google Scholar]
- Gao L., Yang L., Qian S., Tang Z., Qin F., Wei Q., … Yuan J. (2016). Cryosurgery would be an effective option for clinically localized prostate cancer: A meta-analysis and systematic review. Scientific Reports, 6, 27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargraves J. L., Hadley J. (2003). The contribution of insurance coverage and community resources to reducing racial/ethnic disparities in access to care. Health Services Research, 38, 809–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani A. B., Hellman S. (2003). Early prostate cancer: Clinical decision-making. The Lancet, 361, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Kirby R., Fitzpatrick J. M. (2012). Optimising repeat prostate biopsy decisions and procedures. BJU International, 109, 1750–1754. [DOI] [PubMed] [Google Scholar]
- Klabunde C. N., Potosky A. L., Legler J. M., Warren J. L. (2000). Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology, 53, 1258–1267. [DOI] [PubMed] [Google Scholar]
- Klotz L., Zhang L., Lam A., Nam R., Mamedov A., Loblaw A. (2010). Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 28, 126–131. [DOI] [PubMed] [Google Scholar]
- Li J., Djenaba J. A., Soman A., Rim S. H., Master V. A. (2012). Recent trends in prostate cancer incidence by age, cancer stage, and grade, the United States, 2001–2007. Prostate Cancer, 2012, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb S., Vellekoop A., Ahmed H. U., Catto J., Emberton M., Nam R., … Lotan Y. (2013). Systematic review of complications of prostate biopsy. European Urology, 64, 876–892. [DOI] [PubMed] [Google Scholar]
- Lu-Yao G. L., Albertsen P. C., Moore D. F., Shih W., Lin Y., DiPaola R. S., Yao S. L. (2008). Survival following primary androgen deprivation therapy among men with localized prostate cancer. Journal of the American Medical Association, 300, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers M. J., Roth S., Klinkhammer-Schalke M., Gerken M., Hofstaedter F., Wilm S., Klotz T. (2011). Patients with localised prostate cancer (t1 - t2) show improved overall long-term survival compared to the normal population. Journal of Cancer, 2, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R., Schellhammer P. F. (2011). Treatment options for localized prostate cancer. American Family Physician, 84, 413–420. [PubMed] [Google Scholar]
- Moore A. D., Hamilton J. B., Knafl G. J., Godley P. A., Carpenter W. R., Bensen J. T., … Mishel M. (2013). The influence of mistrust, racism, religious participation, and access to care on patient satisfaction for African American men: The North Carolina-Louisiana prostate cancer project. Journal of the National Medical Association, 105, 59–68. [DOI] [PubMed] [Google Scholar]
- Mordukhovich I., Reiter P. L., Backes D. M., Family L., McCullough L. E., O’Brien K. M., … Olshan A. F. (2011). A review of African American-White differences in risk factors for cancer: Prostate cancer. Cancer Causes & Control, 22, 341–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Mimata H., Hata S., Yamasaki M., Tanaka E., Noguchi T., … Nomura Y. (2005). Recto-peritoneal fistula following transperineal prostate biopsy. International Journal of Urology: Official Journal of the Japanese Urological Association, 12, 322–324. [DOI] [PubMed] [Google Scholar]
- Patton M. Q. (2014). Qualitative research & evaluation methods: Integrating theory and practice (4th ed., p. 832). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Pirtskhalaishvili G., Hrebinko R. L., Nelson J. B. (2001). The treatment of prostate cancer: An overview of current options. Cancer Practice, 9, 295–306. [DOI] [PubMed] [Google Scholar]
- Roberts C. B., Jang T. L., Shao Y.-H., Kabadi S., Moore D. F., Lu-Yao G. L. (2011). Treatment profile and complications associated with cryotherapy for localized prostate cancer: A population-based study. Prostate Cancer and Prostatic Diseases, 14, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S. A., Funez A. F., Bravo C. B., Rodriguez R. R.-P., Mayayo E. S., Palacios V. H., Revilla F. J. B. (2014). Cryotherapy for primary treatment of prostate cancer: Intermediate term results of a prospective study from a single institution. Prostate Cancer, 2014, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymura M. J., Kahn A. R., German R. R., Hsieh M.-C., Cress R. D., Finch J. L., … Stuckart E. (2010). Factors associated with initial treatment and survival for clinically localized prostate cancer: Results from the CDC-NPCR Patterns of Care Study (PoC1). BMC Cancer, 10, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore N. (2014). Management of early-stage prostate cancer. American Journal of Managed Care, 20, S260–S272. [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D, Jemal A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68, 7–30. [DOI] [PubMed] [Google Scholar]
- Srokowski T. P., Fang S., Duan Z., Buchholz T. A., Hortobagyi G. N., Goodwin J. S., Giordano S. H. (2008). Completion of adjuvant radiation therapy among women with breast cancer. Cancer, 113, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venderbos L. D., Roobol M. J. (2011). PSA-based prostate cancer screening: The role of active surveillance and informed and shared decision making. Asian Journal of Andrology, 13, 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C. J. D., Mahar A. L., Satkunasivam R., Herschorn S., Kodama R. T., Lee Y., … Nam R. K. (2016). Cardiovascular and skeletal-related events following localized prostate cancer treatment: Role of surgery, radiotherapy, and androgen deprivation. Urology, 97, 145–152. [DOI] [PubMed] [Google Scholar]
- Warren J. L., Klabunde C. N., Schrag D., Bach P. B., Riley G. F. (2002). Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Medical Care, 40, Iv-3-18. [DOI] [PubMed] [Google Scholar]
- Williams S. B. Lei Y. Nguyen P. L. Gu X. Lipsitz S. R. Yu H.-Y. … Hu, J. C (2012). Comparative effectiveness of cryotherapy vs brachytherapy for localised prostate cancer. BJU International, 110, E92–E98. [DOI] [PubMed] [Google Scholar]
- Wilt T. J., Brawer M. K., Jones K. M., Barry M. J., Aronson W. J., Fox S., … Wheeler T. (2012). Radical prostatectomy versus observation for localized prostate cancer. New England Journal of Medicine, 367, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt T. J., Shamliyan T., Taylor B., MacDonald R., Tacklind J., Rutks I., … Kane R. L. (2008). AHRQ comparative effectiveness reviews. In Comparative effectiveness of therapies for clinically localized prostate cancer. Rockville, MD: Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]