Abstract
Smoking is an established risk factor for wound complications. There is limited data on the impact of smoking on artificial urinary sphincter (AUS) outcomes. Thus, the aim of this study was to assess AUS device survival outcomes based on smoking status. From 1985 to 2014, 1,270 patients underwent AUS placement with 728 having smoking status available for review. Smoking status was categorized as never, prior, and active smokers. Kaplan−Meier analysis was performed to evaluate differences in survival, including overall device and erosion/infection−free survival. Hazard regression analysis was utilized to determine the association between smoking and device outcomes. Of the 728 patients in the study, 401 had a history of smoking with 41 active smokers and 360 never smokers at the time of AUS implant. When compared with nonsmokers, past smokers had a higher rate of hypertension and prior transient ischemic attack. Clinical comorbidities were similar between nonsmokers and active smokers. On univariate analysis, patient age, history of transient ischemic attack, diabetes, and coronary artery disease were significantly associated with infection/erosion rate, but prior or active smoking statuses were not. Likewise, when comparing smokers (past or active) with lifelong nonsmokers, there was no significant difference in 1- and 5-year overall device survival. There was no evidence for adverse AUS outcomes in current or past smokers compared with nonsmokers. Given the established risk of perioperative complications secondary to smoking, the recommendation should still be to counsel patients to quit prior to undergoing AUS placement. External validation of these findings is needed.
Keywords: male incontinence, prostate cancer, men’s health interventions
Introduction
The artificial urinary sphincter (AUS), originally introduced in 1972, is considered the preferred surgical treatment for moderate-to-severe stress urinary incontinence (Scott, Bradley, & Timm, 1974), with the majority of patients undergoing AUS placement after having a radical prostatectomy or prostate surgery for benign pathology as the cause of their stress urinary incontinence (Elliott & Barrett, 1998; Lai, Hsu, Teh, Butler, & Boone, 2007; Linder, de Cogain, & Elliott, 2014; Martins & Boyd, 1995). While the AUS is a commonly used treatment in this setting, predictors of adverse device outcomes have been limited with minimal reporting of the impact of patients’ smoking status on the risk of device infection, urethral erosion, or urethral atrophy (Kim et al., 2008; Wang, McGuire, He, Faerber, & Latini, 2012).
Tobacco use is a well-established risk factor for perioperative wound and cardiopulmonary complications documented across a spectrum of specialties and a variety of surgical procedures (Myles et al., 2002; Sørensen, 2012; Sørensen, Hørby, Friis, Pilsgaard, & Jørgensen, 2002). In fact, smoking has a significant negative bearing on all phases of wound healing with the oxidative stress induced by smoking as the primary mechanism. The oxidative stress reduces tissue perfusion, impairs inflammatory cell function, and inhibits cell repair which can lead to delayed or abnormal wound healing (Sørensen, 2012).
Given the established perioperative risks of smoking, it is hypothesized that current smokers or patients with a smoking history may have worse outcomes following AUS placement. The aim of the current study was to assess the impact of smoking on AUS device survival outcomes between current or prior smokers and never smokers.
Material and Method
After obtaining institutional review board approval, 1,270 patients were identified who underwent American Medical Systems 800 AUS surgery at the authors’ institution from 1985 to 2014. Retrospective review identified 728 patients who had smoking status available for study with 401 having a history of smoking, including 41 being active smokers at the time of AUS implantation. Patients were excluded from analysis if they underwent AUS placement secondary to neurogenic bladder, were younger than 18 years, were female, or declined research consent. All devices implanted were American Medical Systems 800 (American Medical Systems, Inc., Minnetonka, Minnesota, USA). The procedure used for placement of the AUS device has been described previously in great detail (Linder, Rivera, Ziegelmann & Elliott, 2015).
Individual charts were reviewed to evaluate pertinent clinical and surgical comorbidities, in particular, smoking status prior to and at the time of AUS placement. Charts were also reviewed to obtain details of the implanted device and device outcomes including reoperations (i.e., explantation for urethral erosion or device infection, revision for device malfunction, urethral atrophy, tubing, or pump complications). The retrospective nature of this study precluded a standardized follow-up protocol in all patients. All patients were evaluated 6 weeks postoperatively for device activation and instruction on device usage. Following this, all participating patients are followed via office evaluation on an as-needed basis as determined by their continence or other device concerns and by mailed patient questionnaires. Furthermore, as part of the Mayo Clinic AUS Registry, which includes AUS patients from 1983 to the present, follow-up correspondence is periodically sent to patients who have undergone AUS placement. Details regarding device survival were obtained from last office examination, any available subsequent operative reports, or written or telephone correspondence.
Statistical analysis was performed using the SAS software package (SAS Institute, Inc., Cary, North Carolina). Continuous features were summarized with medians and interquartile ranges (IQRs); categorical features were summarized with frequency counts and percentages. Device survival was estimated as time from AUS implantation to subsequent repeat surgery (including explantation or device revision for any reason) using the Kaplan–Meier method. Regarding two different AUS-related failures, infection/urethral erosion and urethral atrophy, survival analysis based on competing risks was utilized. All statistical tests were two-sided, with p < .05 considered statistically significant.
Results
From 1985 to 2014, a total of 1,270 male patients underwent AUS surgery at the Mayo Clinic in Rochester, Minnesota. Of these, 728 had smoking status available for review and were included in the study. Notably, 401 (55%) patients had a history of smoking, with 41 (5.6%) being active smokers at the time of implantation. Patients who were current smokers were younger than nonsmokers (median age 66.9 vs. 71.5, p = .02) but had similar body mass index (median 28.2 vs. 28.3, p =.9) and rates of hypertension (51% vs. 60%, p = .3), transient ischemic attack (TIA; 0% vs. 3.4%, p = .2), diabetes mellitus (14.6% vs. 16.5%, p = .8), and coronary artery disease (CAD; 29.3% vs. 24.2%, p = .5). Those who had a history of smoking were of similar age to never smokers (median age 71.0 vs. 71.5, p = .9) but were more likely to have hypertension (68.6% vs. 60%, p = .02) and TIA (7.6% vs. 3.4%, p = .02). They had similar rates of diabetes mellitus (17.9% vs. 16.5%, p = .6) and CAD (28.9% vs. 24.2%, p = .2). All groups had similar rates of history of radiation therapy (current smokers 35%, past smokers 36.8%, nonsmokers 38.3%, p = .7 for both) or androgen deprivation therapy (current smokers 17.9%, p = .5; past smokers 17.4%, p = .2; nonsmokers 13.9%; Table 1).
Table 1.
Clinical and Demographic Information of Patient Undergoing AUS Placement.
| Never smokers (n = 327) | Past smokers (n = 360) | Current smokers (n = 41) |
p
|
||
|---|---|---|---|---|---|
| Past smokers | Current smokers | ||||
| Patient age, year, median (IQR) | 71 (66, 76) | 71 (66, 75) | 68 (64, 72) | .9 | .02 |
| BMI, median (IQR) | 28 (26, 32) | 29 (26, 32) | 28 (27, 30) | .3 | .9 |
| Diabetes, n (%) | 54 (17) | 64 (18) | 6 (15) | .6 | .8 |
| Hypertension, n (%) | 195 (60) | 245 (69) | 21 (51) | .02 | .3 |
| Stroke/TIA, n (%) | 11 (3.4) | 27 (8) | 0 (0) | .02 | .2 |
| Coronary artery disease, n (%) | 79 (24) | 103 (29) | 12 (29) | .2 | .5 |
| Radiation therapy, n (%) | 125 (38) | 132 (37) | 14 (35) | .7 | .7 |
| Androgen deprivation, n (%) | 44 (14) | 61 (17) | 7 (18) | .2 | .5 |
| RRP, n (%) | 240 (81) | 264 (79) | 29 (78) | .4 | .7 |
| Robotic RP, n (%) | 31 (15) | 34 (13) | 3 (12) | .6 | .7 |
Note. AUS = artificial urinary sphincter; BMI = body mass index; IQR = interquartile ranges; TIA = transient ischemic attack; RRP = radical retropubic prostatectomy; RP = radical prostatectomy. bolded values are significant
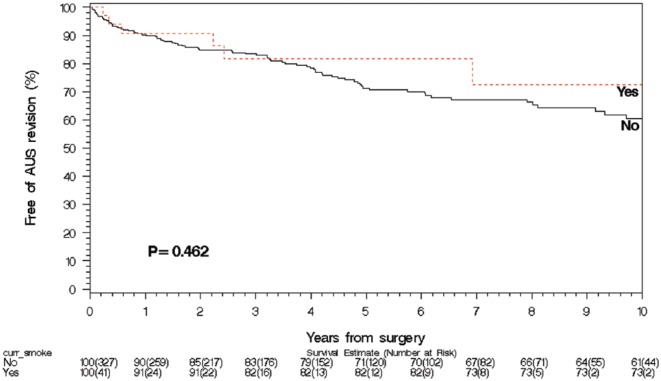
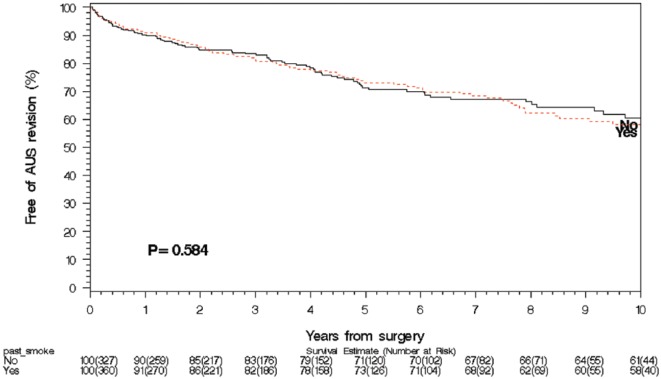
The median follow-up for the never smoker group was 4.0 years (IQR 0.6, 4.9), for the past smoker group was 3.4 years (IQR 1.1, 7.3), and for the current smoker group was 2.2 years (IQR 0.3, 6.9). On univariate hazard regression analysis, neither current smoking nor past smoking history was associated with increased rates of any AUS failure (current smoker hazard ratio [HR] 0.75, p = .5; past smoker HR 1.1, p = .6), infection/urethral erosion (current smoker HR 0.95, p = .9; past smoker HR 1.1, p = .8), mechanical failure (current smoker HR 0.8, p = .7; past smoker HR 1.2, p = .4), or urethral atrophy (current smoker HR 0.85, p = .8; past smoker HR 0.8, p = .5). On univariate analysis, patient age, HR 1.06, 95% confidence interval (CI) [1.02, 1.11], p = .005; history of TIA, HR 2.49, 95% CI [1.14, 5.44], p = .02; diabetes mellitus, HR 1.84, 95% CI [1.07, 3.19], p = .03; and CAD, HR 2.48, 95% CI [1.53, 1.01], p = .002) were significantly associated with infection/erosion rate (Table 2). On multivariable competing risk analysis for infection/erosion in those with a history of smoking, age, HR 1.06, 95% CI [1.02, 1.10], p = .004; CAD, HR 1.78, 95% CI [1.04, 3.03], p = .4; and diabetes mellitus, HR 1.77, 95% CI [1.01, 3.09], p = .05, had ongoing significance (Table 2); whereas, in current smokers, only age had ongoing significance, HR 1.06, 95% CI [1.02, 1.11], p = .008). Survival analysis (Figures 1 and 2) demonstrated no significant difference in overall device survival among those with current or past smoking history versus never smokers, with 1- and 5-year overall device survival rates of never smokers, 90% and 71%, respectively, current smokers, 91% and 82%, respectively, and past smokers, 91% and 73% (p = .46 for current smokers and p = .58 for past smokers).
Table 2.
Univariate and Multivariate Analyses of Factors Associated With Infection/Erosion After AUS Placement.
| Variable | Univariate |
Multivariate |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Past smoker |
Current smoker |
||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.1 | [1.0, 1.1] | .005 | 1.1 | [1.0, 1.1] | .004 | 1.1 | [1.0, 1.1] | .008 |
| BMI | 0.9 | [0.8, 1.0] | .06 | ||||||
| HTN | 1.5 | [0.9, 2.6] | .1 | ||||||
| TIA | 2.5 | [1.1, 5.4] | .02 | 1.4 | [0.6, 3.4] | .4 | |||
| Diabetes | 1.8 | [1.1, 3.2] | .03 | 1.8 | [1.0, 3.1] | .05 | |||
| CAD | 2.5 | [1.5, 4.0] | .0002 | 1.8 | [1.0, 3.0] | .04 | 2.2 | [1.1, 4.4] | .2 |
| Radiation | 1.4 | [0.8, 2.2] | .2 | ||||||
| Current smoker | 0.9 | [0.3, 3.2] | .9 | 0.9 | [0.3, 3.1] | .9 | |||
| Past Smoker | 1.1 | [0.7, 1.8] | .8 | 1.0 | [0.6, 1.6] | .9 | |||
Note. AUS = artificial urinary sphincter; HR = hazard ratio; CI = confidence interval; BMI = body mass index; HTN = hypertension; TIA = transient ischemic attack; CAD = coronary artery disease. bolded values are significant
Figure 1.
Smoking and AUS survival: Current smokers at the time of AUS implant.
Note. AUS = artificial urinary sphincter.
Figure 2.
Smoking and AUS survival: Past smokers at the time of AUS implant.
Note. AUS = artificial urinary sphincter.
Discussion
An increased risk of wound complications from smoking is firmly established and documented in the literature (Myles et al., 2002; Sørensen, 2012; Sørensen et al., 2002) with smoking having a negative bearing on all phases of wound healing. The oxidative stress induced by smoking reduces tissue perfusion, impairs inflammatory cell function, and inhibits cell repair which can lead to delayed or abnormal wound healing (Sørensen, 2012). Given the negative effects on wound healing, it would be expected that active smokers or even those with a smoking history would have worse outcomes. In a large cohort of patients, it was identified that smoking status, whether current or past, was not associated with increased risk to the AUS with similar device survival compared with nonsmokers. This is consistent with prior studies. In two separate reviews of the long-term outcomes of AUS placement, neither reported smoking status to be significantly associated with postoperative complications (Kim et al., 2008; Wang et al., 2012). Smoking was also not a risk factor identified to be significantly associated with poor outcomes in a retrospective review of penile prostheses surgery (Lotan, Roehrborn, McConnell, & Hendin, 2003).
Because of the known link between smoking and perioperative complications, many groups have studied whether discontinuing smoking before operative intervention is beneficial. Several randomized controlled trials and meta-analyses have identified that smoking cessation, even a few weeks prior to planned surgery, is associated with a reduction in postoperative complications (Lindström et al., 2008; Mills et al., 2011; Møller, Villebro, Pedersen, & Tønnesen, 2002; Sørensen, 2012). In a meta-analysis of 6 randomized trials and 15 observational studies examining this topic, the authors identified a relative risk reduction of 41% for prevention of postoperative complications and that each week of smoking cessation increased the magnitude of effect by 19%. The authors concluded that longer periods of smoking cessation were more beneficial in reducing perioperative complications (Mills et al., 2011). In a systematic review on the topic, the authors identified that while former smokers have more healing complications than lifelong nonsmokers, they had fewer complications than current smokers. Perioperative smoking cessation was associated with fewer surgical site infections but not with other healing complications. This suggests that discontinuing smoking preoperatively may have beneficial effects (Sørensen, 2012).
In general, given the current findings regarding overall device survival, smoking status alone should not preclude a patient from AUS implantation. With the established link between smoking, especially current smoking, and wound and cardiopulmonary complications, patients should be encouraged to discontinue smoking prior to AUS placement as it may reduce the risk of surgical complications and improve patient recovery.
Limitations of this study include its nonrandomized, retrospective design, which makes it more difficult to collect all relevant patient clinical data, such as smoking status and specifics regarding the duration of smoking. Given that this study was performed at a tertiary referral center, many patients travel long distances for treatment and may seek follow-up care closer to home. This potentially limits the follow-up data. To help remedy this, the Mayo Clinic AUS Registry periodically contacts patients by mailed survey to obtain follow-up. Finally, while this study presents a large cohort of patients, active smokers represent only 5.6% of all procedures (n = 41). As such, further external validation of the current findings is needed.
Conclusion
This study does not identify an increased risk of infection or erosion or decreased device survival in current smokers or patients with a history of smoking. Despite this, given the established risk of wound infection and respiratory complications from anesthesia secondary to smoking, patients should be counseled to quit smoking prior to undergoing AUS placement.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Elliott D., Barrett D. (1998). Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: A review of 323 cases. Journal of Urology, 159, 1206-1208. [PubMed] [Google Scholar]
- Kim S., Sarmast Z., Daignault S., Faerber G., McGuire E., Latini J. (2008). Long-term durability and functional outcomes among patients with artificial urinary sphincters: A 10-year retrospective review from the University of Michigan. Journal of Urology, 179, 1912-1916. [DOI] [PubMed] [Google Scholar]
- Lai H., Hsu E., Teh B., Butler E., Boone T. (2007). 13 Years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. Journal of Urology, 177, 1021-1025. [DOI] [PubMed] [Google Scholar]
- Linder B., de Cogain M., Elliott D. S. (2014). Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. Journal of Urology, 191, 734-738. [DOI] [PubMed] [Google Scholar]
- Linder B., Rivera M., Ziegelmann M., Elliott D. (2015). Long-term outcomes following artificial urinary sphincter placement: An analysis of 1082 cases at Mayo Clinic. Urology, 86, 602-607. [DOI] [PubMed] [Google Scholar]
- Lindström D., Sadr Azodi O., Wladis A., Tønnesen H., Linder S., Nåsell H., . . . Adami J. (2008). Effects of a perioperative smoking cessation intervention on postoperative complications: A randomized trial. Annals of Surgery, 248, 739-745. [DOI] [PubMed] [Google Scholar]
- Lotan Y., Roehrborn C., McConnell J., Hendin B. N. (2003). Factors influencing the outcomes of penile prosthesis surgery at a teaching institution. Urology, 62, 918-921. [DOI] [PubMed] [Google Scholar]
- Martins F., Boyd S. (1995). Artificial urinary sphincter in patients following major pelvic surgery and/or radiotherapy: Are they less favorable candidates? Journal of Urology, 153, 1188-1193. [PubMed] [Google Scholar]
- Mills E., Eyawo O., Lockhart I., Kelly S., Wu P., Ebbert J. (2011). Smoking cessation reduces postoperative complications: A systematic review and meta-analysis. American Journal of Medicine, 124, 144-154. [DOI] [PubMed] [Google Scholar]
- Møller A., Villebro N., Pedersen T., Tønnesen H. (2002). Effect of preoperative smoking intervention on postoperative complications: A randomised clinical trial. Lancet, 359, 114-117. [DOI] [PubMed] [Google Scholar]
- Myles P., Iacono G., Hunt J., Fletcher H., Morris J., McIlroy D., Fritschi L. (2002). Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: Smokers versus nonsmokers. Anesthesiology, 97, 842-847. [DOI] [PubMed] [Google Scholar]
- Scott F., Bradley W., Timm G. (1974). Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. Journal of Urology, 112, 75-80. [DOI] [PubMed] [Google Scholar]
- Sørensen L. (2012). Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: A systematic review and meta-analysis. Archives of Surgery, 147, 373-383. [DOI] [PubMed] [Google Scholar]
- Sørensen L., Hørby J., Friis E., Pilsgaard B., Jørgensen T. (2002). Smoking as a risk factor for wound healing and infection in breast cancer surgery. European Journal of Surgical Oncology, 28, 815-820. [DOI] [PubMed] [Google Scholar]
- Wang R., McGuire E., He C., Faerber G., Latini J. (2012). Long-term outcomes after primary failures of artificial urinary sphincter implantation. Urology, 79, 922-928. [DOI] [PubMed] [Google Scholar]