Abstract
To evaluate the characteristics of lichen sclerosus (LS) accompanied by urethral squamous cell carcinoma (USCC) and to raise urologists’ awareness about the early management of LS, a retrospective analysis was performed on the clinical features, diagnosis, treatment, and prognosis of 18 male genital LS accompanied by USCC patients who were referred to Shanghai Sixth People’s Hospital between June 2000 and August 2014. All of the patients had a long-term history of LS, urethral strictures, and urethral dilatation. Seven patients are with distal (glanular or penile) USCC, 10 patients with proximal USCC, and one with entire USCC. The most common presentation, except for LS and urethral strictures, was periurethral abscess, followed by extraurethral mass, pelvic pain, urethrocutaneous fistula, hematuria, and bloody urethral discharge. All had primary surgical excision that was adapted to tumor location and extension. All of the USCC were positive for P53 and Ki-67. P16 was positive in four cases of human papillomavirus (HPV)-associated USCC and negative in 14 cases of HPV-independent USCC. Patients with distal USCC had a significant longer survival time than proximal USCC (p < .05). LS should be treated early to prevent the disease progression. LS probably has some associations with USCC. Distal USCC has a relatively better prognosis than proximal USCC.
Keywords: lichen sclerosus, squamous cell carcinoma, male urethra, balanitis xerotica obliterans
Male genital lichen sclerosus (LS), historically referred to as balanitis xerotica obliterans, is a chronic progressive inflammatory skin disease that may affect any cutaneous surface but shows a predilection for the anogenital area (Tausch & Peterson, 2012). The disease is usually limited to the prepuce and glans penis but urethral involvement has been reported in 20% of cases (Potts, Belsante, & Peterson, 2016). One theory in the development and progression of LS suggests that the disease starts with external manifestations involving the skin, and progresses with time to include the urethra, starting distally and spreading more proximally (Liu et al., 2014).
The incidence of LS is 1.4/100,000 individuals, developing most commonly in White males after the third decade of life (Nelson & Peterson, 2011). The exact causes are unknown, but chronic infection, poor hygiene, hormonal and autoimmune factors have been implicated (Zhang, Fu, & Zhang, 2016). The diagnosis is mostly clinical, with secondary phimosis, white xerotic appearance of the glans and foreskin, dysuria, and even urinary retention, and confirmed by biopsy (Fistarol & Itin, 2013). For affected patients, it is essential that dermatologists, histopathologists, urologists, and general practitioners, respectively, have a solid knowledge of the disease (Fistarol & Itin, 2013). When not recognized and treated early, the progression of this disease can cause destructive genitourinary scarring and may result in debilitating symptoms (Liu et al., 2014).
Urethral squamous cell carcinoma (USCC) is considered a rare cancer, accounting for <1% of all malignancies (Gatta et al., 2011). Chronic urethral inflammation/urethritis and urethral strictures have been reported as predisposing factors for USCC (Gakis et al., 2013). LS is a chronic inflammatory disease that may cause urethral strictures and has been regarded as a strong risk factor for penile squamous cell carcinoma (pSCC; Algaba et al., 2002). During the past few years, some cases of LS accompanied by USCC and the potential tumorigenicity of LS implied that LS may also have some associations with USCC. In the present study, the aim is to share some experiences on the diagnosis and treatment of LS accompanied by USCC, to raise urologists’ awareness about the early management of LS, and to alert them to USCC’s aggressive malignancy.
Materials and Methods
After approval by the Shanghai Sixth People’s Hospital ethics committee, 18 male patients who were diagnosed with genital LS accompanied by USCC were included in this retrospective study. All patients who were referred to Shanghai Sixth People’s Hospital between June 2000 and August 2014 were East Asians, with an average age of 58.39 years (from 52 to 69 years) at the time of surgery. They were of Chinese nationality. All cases had biopsy-proven squamous cell carcinoma of the urethra and underwent accurate preoperative evaluation including clinical history; clinical appearance; physical examination; hematological laboratory investigation; chest, abdominal computer tomography (CT), and pelvic magnetic resonance imaging (MRI) for metastases; and multiple biopsies from the foreskin, glans, and urethra under local anesthesia. Patients with secondary urethral tumors arising from surrounding organs were excluded from this study.
LS was defined according to strict, accepted pathologic criteria: an epithelial-stromal lesion characterized by squamous atrophy or hyperplasia, band-like lymphocytes infiltration, hyalinization of the papillar dermis, hyperkeratosis, pigment incontinence, and/or dermal edema (Powell & Wojnarowska, 1999; Pugliese, Morey, & Peterson, 2007). USCC was staged according to the tumor-node-metastasis (TNM) classification system (Gakis et al., 2013). All patients underwent surgical treatment on the basis of clinical and radiological staging and after appropriate consultation. The excised USCC tissues were sent for histological analysis.
All cases received chemoradiotherapy after surgery and had a close follow-up. Chemotherapy consisted of cisplatin and 5-FU, which were started concurrently with DT45-55GY/25F/5W radiation therapy. The field of radiation included the inguinal region for anterior urethral USCC and pelvic LN for those with posterior urethral USCC. Survival time was defined as the time from the date of operation to death.
Statistical Analysis
Statistical analysis was performed using the graphpad 6.0 software program for Windows. The log-rank test by the Kaplan–Meier method was used for comparison of survival time between distal USCC and proximal USCC. The data were presented as the mean ± standard deviation. A p < .05 was considered statistically significant.
Results
Between June 2000 and August 2014, 554 patients with LS, USCC, or LS accompanied by USCC underwent surgical treatment at Shanghai Sixth People’s Hospital. Among them, 524 patients were diagnosed with LS, two with USCC, and 18 with LS accompanied by USCC. The 18 male patients who were included in this study had a long-term history of LS, urethral strictures, and urethral dilatation and received suprapubic cystostomy (SPC) placement at another hospital. The median during of urethral stricture disease prior to USCC diagnosis was 13 years. All cases had no history of trauma, drug abuse, infectious disease, or calculus. Their familial history of USCC or LS was negative. Hematological laboratory investigation revealed that four cases were positive for HPV infection (Table 1).
Table 1.
Demographics of Partial Urethrectomy of 18 Patients
| Case/age | LS position | USCC position | US dur. /year | Physical examination | Stage | Surgical intervention | Sur T /Mo | P 53 |
Ki-67 | P 16 |
HPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/60 | G, U | GU | 6 | US, H | T1N0M0 | PPT | 68 | + | + | − | − |
| 2/58 | F, G, U | GU | 9 | US, H | T1N0M0 | PPT | 64 | + | + | − | − |
| 3/60 | G, U | GU | 10 | US, EM | T2N0M0 | PPT | 54 | + | + | − | − |
| 4/63 | G, U | PU | 13 | US, BUD | T1N0M0 | PPT | 52 | + | + | − | − |
| 5/56 | F, G, U | PU | 12 | US, BUD | T1N0M0 | PPT | 52 | + | + | − | − |
| 6/60 | G, U | PU | 15 | US, EM | T2N0M0 | PPT | 50 | + | + | − | − |
| 7/69 | G, U | PU | 12 | US, EM | T2N0M0 | PPT | 48 | + | + | − | − |
| 8/54 | G, U | BU | 18 | US, EM, PP | T3N0M0 | OSS | 24 | + | + | + | + |
| 9/54 | F, G, U | BU | 14 | US, PA, PP | T2N0M0 | OSS | 22 | + | + | − | − |
| 10/59 | G, U | BU | 16 | US, PA, LN | T2N1M0 | OSS + LND | 20 | + | + | + | + |
| 11/57 | F, G, U | BU | 10 | US, PA, LN, EM | T2N1M0 | OSS + LND | 16 | + | + | − | − |
| 12/56 | G, U | BMU | 14 | US, PA, PP, UF | T3N0M0 | OSS + fistulectomy | 14 | + | + | − | − |
| 13/58 | F, G, U | BMU | 16 | US, PA, PP, EM | T3N0M0 | OSS | 14 | + | + | − | − |
| 14/55 | F, G, U | BMU | 12 | US, PA, EM | T3N0M0 | OSS | 12 | + | + | − | − |
| 15/63 | G, U | BMU | 18 | US, PA, PP, UF, LN | T3N1M0 | OSS + LND + fistulectomy | 11 | + | + | − | − |
| 16/52 | G, U | BMU | 10 | US, PA, EM | T2N0M0 | OSS | 10 | + | + | − | − |
| 17/65 | G, U | BMU | 16 | US, PA, PP, UF | T3N0M0 | OSS + fistulectomy | 10 | + | + | + | + |
| 18/52 | G, U | EU | 18 | US, PA, PP, UF, LN | T4N2M0 | TPU + LND + fistulectomy | 4 | + | + | + | + |
Note. LS = lichen sclerosus; G = glans; U = urethra; F = foreskin; USCC = urethral squamous cell carcinoma; GU = glanular urethra; PU = penile urethra; BU = bulbar urethra; BMU = bulbomembranous urethra; EU = entire urethra; US dur. = duration of urethral stricture prior to USCC diagnosis; US = urethral stricture; H = hematuria; EM = extraurethral mass; BUD = bloody urethral discharge; PP = pelvic pain; PA = periurethral abscess; LN = palpable lymph nodes; UF = urethrocutaneous fistula; PPT = partial phallectomy; OSS = organ sparing surgery; LND = lymph nodes dissection; TPU = total phallectomy and urethrectomy; Sur T = survival time.
Physical examination, chest, abdominal CT, and pelvic MRI demonstrated that 14 cases were negative for metastatic disease (T1-T3N0M0). Three cases demonstrated enlarged firm left or right inguinal node in a single ⩽ 2 cm in greatest dimension; inguinal node biopsies revealed invasive squamous cell carcinoma (T2/T3N1M0). Two palpable 2.5 cm left inguinal nodes with biopsy-proven squamous cell carcinoma were presented in one case of entire USCC, which extended to prostate, anterior rectal wall, left testis, and the base of the penis (T4N2M0).
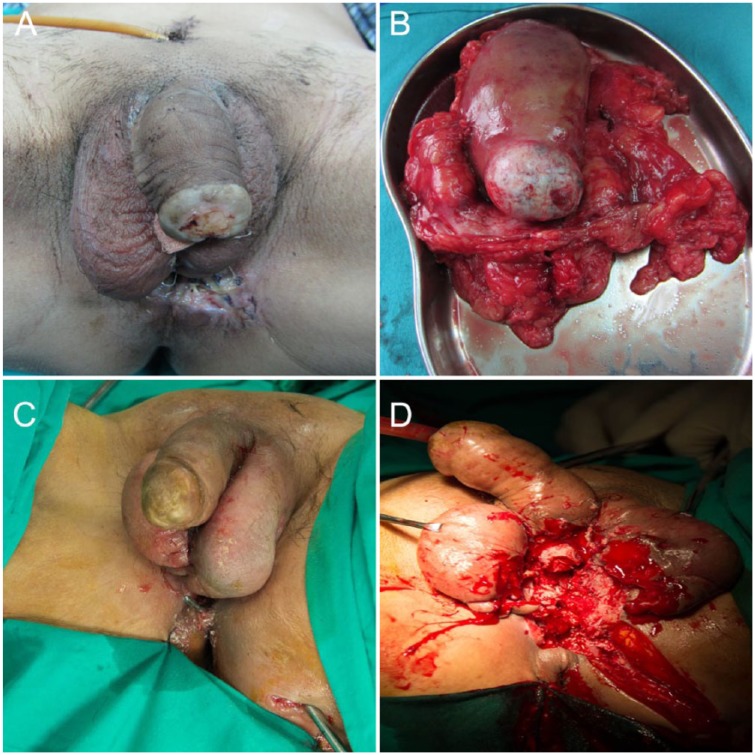
LS occurs in glans and urethra in 12 cases (Figure 1A) and in foreskin, glans, and urethra in six cases (Table 1). The predominant presentation of LS accompanied by USCC, except for LS and urethral strictures, was periurethral abscess (n = 10, 55.56%), followed by extraurethral mass (n = 8, 44.44%), pelvic pain (n = 7, 38.89%), urethrocutaneous fistula (n = 4, 22.22%), hematuria (n = 2, 11.11%), and bloody urethral discharge (n = 2, 11.11%).
Figure 1.
(A) The appearance of lichen sclerosus, with white xerotic plaques on the glans. (B) The excised tumors extended to prostate, anterior rectal wall, left testis, and penis. (C) Urethral sounds revealed urethrocutaneous fistula. (D) Residue-like pus mixed with necrotic tissues draining from the surgical wounds.
Surgical therapy was tailored according to tumor location and clinical stage. Patients with glanular or penile USCC underwent partial phallectomy. And patients with bulbar or bulbomembranous USCC underwent bladder, prostate, and penile-sparing perineal resection, with aggressive surgical excision of the primary lesion with a negative surgical margin (Table 1). Three cases with urethrocutaneous fistula also received fistulectomy (Table 1). During operation, residue-like pus mixed with necrotic tissues drained from the periurethral abscess (Figure 1C and 1D). One case of entire USCC was managed with total phallectomy and urethrectomy (Figure 1B). Four cases with inguinal lymph node involved also received superficial lymphadenectomy (Table 1). The excised tissues were sent for histological analysis.
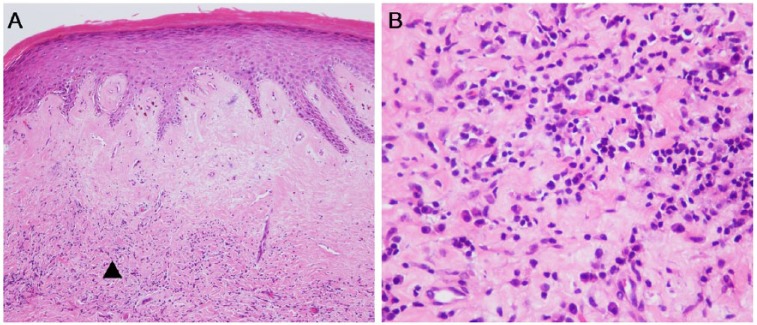
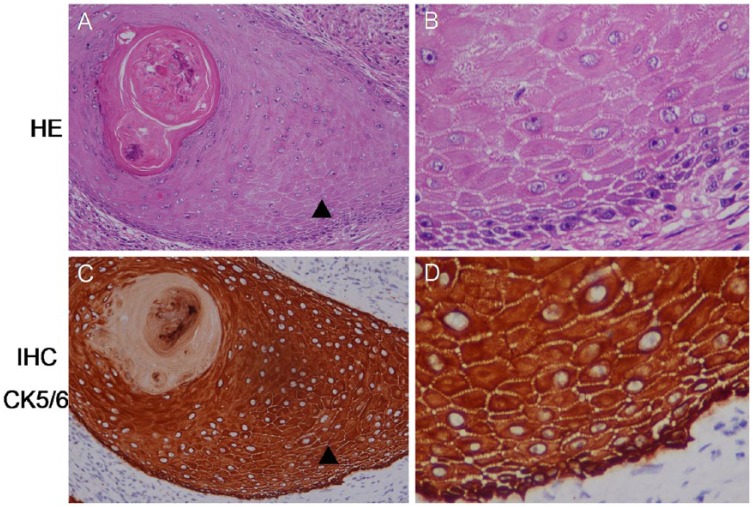
Hematoxylin and eosin (HE) staining of the foreskin, glans, and urethra revealed classical LS, with epidermal atrophy, dermal sclerosis, and underlying band-like lymphocytic infiltration (Figure 2). HE and immunohistochemical (IHC) staining with CK5/6 of the excised mass and necrotic tissues showed well differentiated USCC, with apparent keratin pearl and intercellular bridges in the carcinoma nest (Figure 3). IHC evaluation with P53, Ki-67, and P16 staining showed that all of the USCC were positive for P53 and Ki-67, and P16 were positive in four cases of HPV associated USCC and negative in 14 cases of HPV independent USCC (Table 1).
Figure 2.
(A) Hematoxylin and eosin staining of the urethra demonstrating lichen sclerosus: band-like infiltrate of lymphocytes in the dermis, hyalinization of collagen in the upper dermis, vacuolar degeneration of basal cells, and orthokeratotic hyperkeratosis of the squamous epithelium. (B) The magnifying area of “▲” indicates infiltration of lymphocytes. Original magnifications: ×100 (panel A) and ×400 (panel B).
Figure 3.
(AC) Hematoxylin and eosin and immunohistochemical staining showed well-differentiated urethral squamous cell carcinoma, with keratin pearl in the carcinoma nest. (BD) The magnifying area of “▲” indicates intercellular bridges. Original magnifications: ×200 (panels A and C) and ×400 (panels B and D).
Two patients with glanular USCC survived more than 5 years and the other 16 patients survived no more than 5 years and had a distant metastasis or died after a mean (range) follow-up of 25.81 (4-54) months. Among these 18 patients, eight patients (44.4%) died from lung metastasis, five patients (27.8%) died from liver metastasis, three patients died from bone metastasis (17.7%) and two patients (11.1%) died from brain metastasis. Patients with distal USCC had a significantly longer survival time (p < .05) compared with proximal USCC (Figure 4). The 52-year-old patient with entire USCC (T4N2M0) only survived 4 months after surgery (Table 1).
Figure 4.
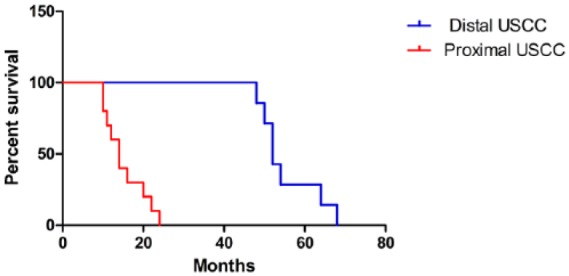
Kaplan–Meier survival curves according to tumor location.
Discussion
LS accompanied by USCC is extremely rare. As such, much of the published literature consists of case reports and limited case series from a single institution making it difficult to systematically analyze the characteristics of LS accompanied by USCC. The Department of Urology of the Shanghai Sixth People’s Hospital is the largest urethral referral center in China, and the large number of patients offered a possibility to retrospectively analyze patient records of LS accompanied by USCC.
When not recognized and treated early, LS may progress and cause debilitating symptoms. Urethral LS is thought to originate at the meatus and/or preputial skin with potential disease progression proximally along the urethra. LS-related urethral strictures have a higher rate of treatment-related morbidity and disease recurrence, which necessitates accurate diagnosis and staging to plan appropriate management (Liu et al., 2014). The diagnosis is mostly clinical initially, with secondary phimosis, white xerotic appearance of glans and foreskin, dysuria, and even urinary retention (Fistarol & Itin, 2013). The diagnosis is confirmed via classical histopathology exhibiting epidermal atrophy or hyperkeratosis, hyalinization of the dermis, and an underlying band-like lymphocytic infiltration. Historically, LS limited to the glans and urethral meatus has acceptable outcomes with minimally invasive approaches such as circumcision and meatotomy; more extensive or panurethral disease warrants more invasive therapy, including 1-stage oral graft urethroplasty versus staged urethroplasty with nongenital skin or oral mucosa (Potts et al., 2016). Outcomes of these often morbid and invasive procedures are far from perfect with reported success rates of 70% to 85% for substitution urethroplasty and 70% to 100% for diversion with perineal urethrostomy (Belsante, Selph, & Peterson, 2015).
In China, the differences of economic and medical conditions of different regions and patients’ embarrassment and strong persistence and endurance made it difficult for the early diagnosis and treatment of LS and USCC. Many studies have implied that LS is a premalignant condition and an independent risk factor for pSCC with a reported lag time from onset of LS to diagnosis of malignancy of 18 years (Fistarol & Itin, 2013; Potts et al., 2016). While this has been definitively shown in the female population with LS, most of these reports in men tend to be anecdotal and retrospective (Memon et al., 2011; Peng, Guo, Jin, Wang, & Sa, 2018). The reported risk of pSCC in male patients with genital LS is estimated at between 2% and 12.5% (Bunker & Shim, 2015). The present study demonstrates that the majority of LS who have been recognized and treated early were not progress to USCC (524/542, 96.68%), whereas the majority of USCC were accompanied by a long history of LS and urethral strictures (18/20, 90.00%). So it can be hypothesized that early aggressive treatment of LS may relieve high pressure voiding and may prevent the progress of LS.
Furthermore, Pietrzak, Hadway, Corbishley, and Watkin (2006) reported that 22% of patients presenting with pSCC had a histological diagnosis of LS. Carlson et al. (2013) have reported that increased P53 and Ki-67 expression in pSCC and vulva SCC was associated with LS, especially in cases of chronic, long-standing LS. P16 over expression was significantly associated with human papillomavirus (HPV) infection and it was useful for distinguishing HPV-associated pSCC and vulva SCC from HPV-independent SCC. HPV infection and P16 expression were also reported in the context of LS. In this study, all LS accompanied by USCC patients were positive for P53 and Ki-67, and P16 was positive in four cases of HPV-associated USCC and negative in 14 cases of HPV-independent USCC, which is in accordance with the findings of protein markers of malignant potential in LS associated with pSCC and vulva SCC. Taken together, these results implied that USCC may also have some associations with LS.
USCC generally occurs in the fifth decade of life. At initial presentation, visible hematuria or bloody urethral discharge is reported in up to 62% of the cases. Further symptoms of locally advanced disease include an extraurethral mass (52%), bladder outlet obstruction (48%), pelvic pain (33%), urethrocutaneous fistula (10%), abscess formation (5%), or dyspareunia (Gakis et al., 2013). In this study, 11 cases of LS accompanied by USCC (61.11%) occur in the fifth decade of life and four cases of early stages (T1N0M0) presented visible hematuria or bloody urethral discharge, which are in accordance with USCC. However, the majority of present cases with locally advanced disease presented as periurethral abscess, extraurethral mass, and pelvic pain, which may be attributed to the coexistence of LS, the differences of locations and stages of USCC, and the small size of the patients.
Management of USCC is a particularly challenging field due to rarity of the disease and the lack of randomized evidence to inform practice and detailed comparative studies in the literature. Treatment of distal UC previously followed the procedure for penile cancer and has become the focus of clinicians to improve functional outcome and quality of life while preserving oncologic safety (Gakis et al., 2013; Karnes, Breau, & Lightner, 2010). By contrast, patients with noninvasive proximal UC or carcinoma in situ of the prostatic urethra and prostatic ducts can be treated with a urethra-sparing approach with transurethral resection and bacillus Calmette-Guerin (BCG; Gakis et al., 2013). Cystoprostatectomy with extended pelvic lymphadenectomy should be reserved for patients not responding to BCG or a primary treatment option in patients with extensive ductal or stromal involvement (Gakis et al., 2013). More proximal tumors often required a combination of radiotherapy and radical surgery.
Although the treatment of urethral carcinoma is not uniform, most urologists recognize two groups of patients with differing survival characteristics. Those with distal tumors are more similar to pSCC, in that local surgical control is achievable and appears to be effective in many patients (Smith et al., 2007). A retrospective series found no evidence of local recurrence, even with <5-mm resection margins (median follow-up: 17–37 months), in men with pT1–3 N0–2 anterior UC treated with well-defined penis-preserving surgery and additional iliac/inguinal lymphadenectomy for clinically suspected lymph node disease (Smith et al., 2007). Similar results for the feasibility of penile-preserving surgery were reported in other retrospective series (Gheiler et al., 1998; Hakenberg, Franke, Froehner, & Wirth, 2001). In localized posterior USCC, a bladder, prostate, penile sparing approach may be an alternative to primary radical urethrectomy, provided negative surgical margins can be achieved. However, proximal lesions tend to present with high stage and usually difficult for a negative surgical margin. Radiation usually fails in the central portion of the tumors and the pelvic LN. Furthermore, micrometastatic disease may have happened at the time of primary intervention (Dalbagni, Zhang, Lacombe, & Herr, 1999). All these factors lead to the poor prognosis of proximal urethral tumors even if chemoradiation and surgery were performed.
In contrast to penile cancer, in which clinically enlarged lymph nodes at initial diagnosis are not uncommon due to inflammatory conditions (Heyns et al., 2010), enlarged lymph nodes in UC often represent metastatic disease (Dayyani et al., 2013). Currently, no clear evidence supports prophylactic bilateral inguinal and/or pelvic lymphadenectomy in all patients with UC (Gakis et al., 2013; Karnes et al., 2010). The terrible outcomes of patients with clinically apparent nodal involvement at presentation further confirmed this. However, in patients with clinically enlarged inguinal/pelvic lymph nodes at an early stage, regional lymphadenectomy still should be considered for initial treatment (Karnes et al., 2010).
Risk factors for survival include age, tumor stage and grade, nodal stage, presence of distant metastasis, histological type, tumor size, tumor location, and modality of treatment. Distal UC tends to present at a lower stage and has a better prognosis, with 5-year survival rates of >50% after treatment (Dalbagni et al., 1999; Gheiler et al., 1998). Results from treatment with surgery alone for proximal USCC have been disappointing, with 5-year disease-free survival of 0% to 15% (Dalbagni et al., 1999). More recently, combined treatment with surgery and chemoradiotherapy was suggested to improve local control and survival (Cohen et al., 2008). However, the patients with bulbar USCC, bulbomembranous USCC, and entire USCC present a higher stage in this study and all cases died of distant metastasis despite treatment with surgery, chemotherapy, and radiation therapy. Similarly, Memon (Memon et al., 2011) have reported a case of LS accompanied by bulbar USCC presented with a recurrent urinary tract infection. The tumor abutted the anterior rectal wall and infiltrated through the levator ani into the perineum. Although the patient received high dosages of chemoradiotherapy and a radical surgery with extensive clear margins which required abdominoperineal resection, right inguinal superficial lymphadenectomy, cystoprostatectomy with ileal conduit, penectomy, scrotal excision, and bilateral orchidectomy, PET still showed extensive pelvic nodal recurrence and distal soft tissue and bony metastases 5 months later after surgery.
With a small sample size and the long interval between diagnosis of LS and treatment for USCC, it is difficult to interpret whether there is a definite association between male genital LS and USCC. It seems prudent to advocate a more extensive follow-up of patients with LS. Instructing patients to perform regular self-examination may be a practical universal measure.
Conclusions
LS should be treated early to prevent the disease progression. It probably has some associations with USCC. Distal USCC has a better prognosis than proximal USCC and the prognosis of LS accompanied by proximal USCC remains poor despite treatment with surgery and chemoradiotherapy. It is imperative that the effective therapeutic method should be developed.
Acknowledgments
We would like to acknowledge the contributions of the participants who made this work possible.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Algaba F., Horenblas S., Pizzocaro-Luigi Piva G., Solsona E., Windahl T. & European Association of Urology. (2002). EAU guidelines on penile cancer. European Urology, 42(3), 199–203. [DOI] [PubMed] [Google Scholar]
- Belsante M. J., Selph J. P., Peterson A. C. (2015). The contemporary management of urethral strictures in men resulting from lichen sclerosus. Translational Andrology and Urology, 4(1), 22–28. doi: 10.3978/j.issn.2223-4683.2015.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker C. B., Shim T. N. (2015). Male genital lichen sclerosus. Indian Journal of Dermatology, 60(2), 111–117. doi: 10.4103/0019-5154.152501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson B. C., Hofer M. D., Ballek N., Yang X. J., Meeks J. J., Gonzalez C. M. (2013). Protein markers of malignant potential in penile and vulvar lichen sclerosus. Journal of Urology, 190(2), 399–406. doi: 10.1016/j.juro.2013.01.102 [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Triaca V., Billmeyer B., Hanley R. S., Girshovich L., Shuster T., … Zinman L. (2008). Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. Journal of Urology, 179(2), 536–541. doi: 10.1016/j.juro.2007.09.068 [DOI] [PubMed] [Google Scholar]
- Dalbagni G., Zhang Z. F., Lacombe L., Herr H. W. (1999). Male urethral carcinoma: Analysis of treatment outcome. Urology, 53(6), 1126–1132. [DOI] [PubMed] [Google Scholar]
- Dayyani F., Pettaway C. A., Kamat A. M., Munsell M. F., Sircar K., Pagliaro L. C. (2013). Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urologic Oncology, 31(7), 1171–1177. doi: 10.1016/j.urolonc.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fistarol S. K., Itin P. H. (2013). Diagnosis and treatment of lichen sclerosus: An update. American Journal of Clinical Dermatology, 14(1), 27–47. doi: 10.1007/s40257-012-0006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakis G., Witjes J. A., Comperat E., Cowan N. C., De Santis M., Lebret T. … European Association of Urology. (2013). EAU guidelines on primary urethral carcinoma. European Urology, 64(5), 823–830. doi: 10.1016/j.eururo.2013.03.044 [DOI] [PubMed] [Google Scholar]
- Gatta G., van der Zwan J. M., Casali P. G., Siesling S., Dei Tos A. P., Kunkler I., … Licitra L. (2011). Rare cancers are not so rare: The rare cancer burden in Europe. European Journal of Cancer, 47(17), 2493–2511. doi: 10.1016/j.ejca.2011.08.008 [DOI] [PubMed] [Google Scholar]
- Gheiler E. L., Tefilli M. V., Tiguert R., de Oliveira J. G., Pontes J. E., Wood D. P., Jr. (1998). Management of primary urethral cancer. Urology, 52(3), 487–493. [DOI] [PubMed] [Google Scholar]
- Hakenberg O. W., Franke H. J., Froehner M., Wirth M. P. (2001). The treatment of primary urethral carcinoma—The dilemmas of a rare condition: Experience with partial urethrectomy and adjuvant chemotherapy. Onkologie, 24(1), 48–52. doi: 10.1159/000050282 [DOI] [PubMed] [Google Scholar]
- Heyns C. F., Fleshner N., Sangar V., Schlenker B., Yuvaraja T. B., van Poppel H. (2010). Management of the lymph nodes in penile cancer. Urology, 76(2 Suppl 1), S43–57. doi: 10.1016/j.urology.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Karnes R. J., Breau R. H., Lightner D. J. (2010). Surgery for urethral cancer. Urologic Clinics of North America, 37(3), 445–457. doi: 10.1016/j.ucl.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Liu J. S., Walker K., Stein D., Prabhu S., Hofer M. D., Han J., … Gonzalez C. M. (2014). Lichen sclerosus and isolated bulbar urethral stricture disease. Journal of Urology, 192(3), 775–779. doi: 10.1016/j.juro.2014.03.090 [DOI] [PubMed] [Google Scholar]
- Memon S., Craig Lynch A., Cleeve L., Murphy D. G., Pohl M. J., Heriot A. G. (2011). Squamous cell carcinoma of the bulbar urethra. Journal of Clinical Oncology, 29(28), e733–e735. doi: 10.1200/JCO.2011.36.5890 [DOI] [PubMed] [Google Scholar]
- Nelson D. M., Peterson A. C. (2011). Lichen sclerosus: Epidemiological distribution in an equal access health care system. Journal of Urology, 185(2), 522–525. doi: 10.1016/j.juro.2010.09.107 [DOI] [PubMed] [Google Scholar]
- Peng X., Guo H., Jin C., Wang L., Sa Y. (2018). Squamous cell carcinoma of the bulbar urethra accompanied by lichen sclerosus: A case report. American Journal of Men’s Health, 12(2), 493–497. doi: 10.1177/1557988317743386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak P., Hadway P., Corbishley C. M., Watkin N. A. (2006). Is the association between balanitis xerotica obliterans and penile carcinoma underestimated? BJU International, 98(1), 74–76. doi: 10.1111/j.1464-410X.2006.06213.x [DOI] [PubMed] [Google Scholar]
- Potts B. A., Belsante M. J., Peterson A. C. (2016). Intraurethral steroids are a safe and effective treatment for stricture disease in patients with biopsy proven lichen sclerosus. Journal of Urology, 195(6), 1790–1796. doi: 10.1016/j.juro.2015.12.067 [DOI] [PubMed] [Google Scholar]
- Powell J. J., Wojnarowska F. (1999). Lichen sclerosus. Lancet, 353(9166), 1777–1783. [DOI] [PubMed] [Google Scholar]
- Pugliese J. M., Morey A. F., Peterson A. C. (2007). Lichen sclerosus: Review of the literature and current recommendations for management. Journal of Urology, 178(6), 2268–2276. doi: 10.1016/j.juro.2007.08.024 [DOI] [PubMed] [Google Scholar]
- Smith Y., Hadway P., Ahmed S., Perry M. J., Corbishley C. M., Watkin N. A. (2007). Penile-preserving surgery for male distal urethral carcinoma. BJU International, 100(1), 82–87. doi: 10.1111/j.1464-410X.2007.06901.x [DOI] [PubMed] [Google Scholar]
- Tausch T. J., Peterson A. C. (2012). Early aggressive treatment of lichen sclerosus may prevent disease progression. Journal of Urology, 187(6), 2101–2105. doi: 10.1016/j.juro.2012.01.071 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Fu Q., Zhang X. (2016). The presence of human papillomavirus and Epstein-Barr virus in male Chinese lichen sclerosus patients: A single center study. Asian Journal of Andrology, 18(4), 650–653. doi: 10.4103/1008-682X.160261 [DOI] [PMC free article] [PubMed] [Google Scholar]