Abstract
Stably elevated behavioural inhibition (BI) is an established risk factor for internalizing disorders. This stability may be related to genetic factors, including a valine-to-methionine substitution on codon 66 (val66met) of the brain-derived neurotrophic factor (BDNF) gene. Past work on the BDNF met variant has inconsistently linked it to vulnerability to internalizing problems; some of this inconsistency may stem from the failure to consider gene-trait interactions in shaping the course of early BI. Toward elucidating early pathways to anxiety vulnerability, we examined gene-by-trait interactions in predicting the course of BI over time in 476 children, assessed for BI using standardized laboratory methods. We found that children with the met allele showed lower stability of BI between ages 3 and 6 than those without this allele. While the mechanisms that underlie this effect are unclear, our findings are consistent with the notion that the met variant, in the context of early BI, influences the stability of this trait in early development.
Keywords: BDNF, behavioural inhibition, val66met, children, laboratory assessment, Gene-trait interaction
Introduction
Behavioural Inhibition (BI) is a temperament style characterized by reticence and withdrawal when exposed to novel situations or people (Kagan, Reznick, Clarge, Snidman, & Garcia-Coll, 1984). As befitting a temperament construct, BI is relatively stable across time (Caspi, 2000; De Pauw & Mervielde, 2010). Indeed, ample evidence supports the moderate stability of children’s BI (Gest, 1997; Scarpa, Raine, Venables, & Mednick, 1995); however, there is also evidence for change in BI during childhood (Essex, Klein, Slattery, Goldsmith, & Kalin, 2010).
BI is of interest to developmental psychopathologists due to its associations with internalizing disorders (Clauss & Blackford, 2012; Fox, Henderson, Marshall, Nichols, & Ghera, 2005a; Schwartz, Snidman, & Kagan, 1999). However, children with stably elevated BI, rather than high BI at any one time, appear to be at greatest risk for anxiety. For example, Prior, Smart, Sanson, and Oberklaid (2000) found that maternally reported persistent child shyness, a trait that shows conceptual overlap with BI in that it captures reticence with unfamiliar persons, was associated with children’s anxiety in early adolescence. With respect to BI more specifically, Chronis-Tuscano and colleagues (2009) found that children with stable, elevated BI across early to middle childhood, indexed via maternal report, were at almost four-fold increased risk for social anxiety disorder in adolescence. In another longitudinal investigation, persistently high observed BI across grades 1, 3, 5, 7, and 9 was associated with significantly higher lifetime rates of internalizing disorders (Essex et al., 2010). It is also noteworthy that related traits, such as disinhibition, are tied to externalizing problems (Hirshfeld-Becker et al., 2007; Iacono, Malone, & McGue, 2008).
Given the risk associated with stable BI, understanding factors that contribute to its stability in childhood may help identify youth at greatest risk for psychopathology, potentially informing prevention and early intervention efforts. That said, relatively little work has been done investigating factors that contribute to the stability of BI over time, particularly from a biological standpoint (Fox et al., 2005a); most extant work has focused on caregiving styles (Park, Belsky, Putnam, & Crnic, 1997; Rubin, Burgess, & Hastings, 2002; Rubin, Hastings, Stewart, Henderson, & Chen, 1997) and maternal personality (Degnan, Henderson, Fox, & Rubin, 2008). Whether stability and change in BI are related to genetic variation implicated in neurodevelopment is unclear.
While genetic factors are consistently implicated in shaping human behavioral traits (Turkheimer, 2000), including BI (Dilalla, Kagan, & Reznick, 1994; Plomin et al., 1993; Robinson, Kagan, Reznick, & Corley, 1992), the search for specific polymorphisms important to the development of such traits has yielded inconclusive results. DeYoung and Clark (2012) argued that this may be due in part to the failure to consider gene-trait interactions (GXT) in trait development, as traits themselves are not only shaped by genetic influences, they also provide a broad and important context in which genetic influences unfold. Put differently, traits serve to shape the environment in which the expression of genetic influences on individual differences subsequently unfold (DeYoung & Clark, 2012). For example, genetic influences on neurodevelopment in inhibited children are expressed in a context where novelty is presumably avoided. This concept is related to, but slightly different from, the more familiar model of gene-environment interaction (GXE), where genetic influence of a trait occurs only under certain exogenous, presumably environmental conditions. In the case of GXT, an endogenous trait becomes the context that shapes genetic influences on development.
Of the small literature on GXT, most work has focused on the interaction between genotype and a given trait in predicting another behavior, usually symptoms (Bau, Almeida, & Hutz, 2000; Caspi et al., 2008; DeYoung & Clark, 2012; Monteleone et al., 2006). However, it is possible that specific traits may interact with genotype to predict the ontogeny of that same trait over time. Put differently, given that traits and environmental exposures are nonrandom (e.g., due to evocative processes or other gene-environment correlations), early individual differences (e.g., BI) are related to certain environments that may interact dynamically with genes for neurodevelopment, setting the context for trajectories of that same individual difference factor. An understanding of such processes may prove useful in the context of better understanding early developmental trajectories that may eventuate in disorder later in development, such as adolescence or adulthood.
In a review of gene-trait interactions (2012), DeYoung and Clark emphasize the importance of understanding how specific genetic polymorphisms interact with traits, including temperament, in shaping subsequent psychological development. Such interactions have received little attention, even though they may shed light on how single polymorphisms interact with neural and behavioural traits that are manifested in individual differences. As noted by DeYoung and Clark, traction may be gained in our understanding of the effects of specific genes on outcomes by examining how the gene in question interacts with the broader context of the brain, as captured by biologically influenced traits (i.e., the gene in its “natural habitat” pp. 2, DeYoung & Clark, 2012), such as BI. More specifically, BI is thought to mark the functioning of several neurobiological processes, including amygdalar (Kagan & Snidman, 1991; Perez-Edgar et al., 2007), striatal (Guyer et al., 2006), thalamic (Kalin, Shelton, Fox, Oakes, & Davidson, 2005), and hypothalamic-pituitary-adrenal (HPA) axis reactivity (Fox et al., 2005a; Tops & Boksem, 2011). To the extent that GXT capture interplay between a given genetic variant and broad characteristics of the individual as shaped by both genes and environment, studying GXT may yield new hypotheses regarding gene-neural systems interactions. In this manner, DeYoung and Clark (2012) posit that GXT is a proxy for both epistasis (gene-gene interaction) and gene–environment interaction, permitting a “more proximal and specific characterization of the features of the organism that … have consequences for the effects of a given genetic variant” (p. 3). Thus, identifying GXT allows us to capitalize on what is known about the neurobiology of the trait in question, which may in turn guide the development of hypotheses about the underlying biological systems with which the specific gene interacts.
There are few extant studies of GXT, with relevant previous work focusing on the interplay between genetic influences and personality traits in associations with psychiatric diagnoses in adults (Caspi et al., 2008; Monteleone et al., 2006). For example, Monteleone and colleagues (2006) reported that the serotonin transporter linked polymorphic region (5-HTTLPR) genotype predicted harm avoidance only in those diagnosed with bulimia nervosa. Additionally, Bau et al. (2000) found the Taql A polymorphism of the dopamine D2 receptor gene (D2D2) interacted with several personality traits (e.g., stress vulnerability and harm avoidance) to predict alcoholism severity and antisocial personality disorder symptoms. Relevant to our study, Bau and colleagues found support for the notion that the temperament trait harm avoidance, a marker of individual differences relevant to fearfulness, provided a stressful context that influenced the impact of the Taq1 allele on psychopathology. However, existing GXT studies have not considered the developmental trajectories of emergent trait vulnerability to psychopathology, which could shed new light on pathways by which risk evolves. In particular, in young children, it may be less useful to apply GXT to examine psychiatric outcomes, as base rates of many disorders are low in early childhood (Wichstrøm et al., 2012); however, examining GXT in the context of children’s developmental trajectories implicated in risk may shed light on factors contributing to risk for adverse outcomes.
Toward this goal, in the current study, we were interested in whether early trait BI and Brain-Derived Neurotrophic Factor (BDNF) val66met polymorphisms contributed to the stability of BI in early childhood. There are multiple reasons to consider the role of BDNF polymorphisms in the development of temperament traits, particularly those important to developmental psychopathology. BDNF is a member of a protein family known as neurotrophins, which contribute to the differentiation and survival of neurons (Schinder & Poo, 2000), as well as neural plasticity (Cotman & Berchtold, 2002; Schinder & Poo, 2000). Located on chromosome 11p13, the BDNF gene has a guanine-to-adenine single nucleotide polymorphism (SNP) at nucleotide 196 (rs6265) that causes a valine (val) to methionine (met) substitution at codon 66 (val66met). The met substitution modifies the BDNF protein, lowering BDNF secretion and decreasing its neural availability, in turn causing atrophy of structures in the brain. This may lead to potentially negative neurobiological effects downstream (e.g., functional impairments in the central nervous system [Chen et al., 2004], memory and hippocampal dysfunction [Egan et al., 2003], and reduced amygdalar and hippocampal volume [Gatt et al., 2009]).
The BDNF gene is a plausible contributor to BI and psychopathology risk based on several lines of research. Specifically, the met allele appears to contribute to risk for internalizing disorders (Brunoni, Lopes, & Fregni, 2008), including anxiety disorders (Suliman, Hemmings, & Seedat, 2013), albeit in an inconsistent manner, with some studies implicating the met (Enoch, White, Waheed, & Goldman, 2008) in increased risk, and others suggesting this variant is associated with decreased risk (Zhang et al., 2006). More recent work suggests that the met variant may not be a marker of pathology per se; rather, it may reflect an increased responsivity to environments of all stripes (Nilsson, Comasco, Hodgins, Oreland, & Åslund, 2015). Thus, although the val66met polymorphism appears to be important in understanding vulnerability, its mechanisms of risk are unclear. Recently, BDNF variation has been related to the stability of behavioral disinhibition (Drury et al., 2012), a marker of vulnerability to externalizing disorders risk (Iacono et al., 2008). Drury and colleagues (2012) investigated the role of BDNF genotype in abandoned children’s disinhibited behaviour (i.e., lack of reticence in unfamiliar situations, inappropriate boundaries and affection with strangers, failure to check in with familiar caregivers in unfamiliar settings). They found that the BDNF met variant was associated with reductions in disinhibited behaviour in children assigned to high-quality foster care, whereas children who remained institutionalized or were homozygous for the val allele showed less change in disinhibition over time (i.e., a GXT interaction moderated further by the environment). While this study did not test this specifically, one possibility is that child disinhibition sets the context for the influence of BDNF genetic variability, driving how this trait unfolds in different environments.
In line with models of GXT (DeYoung & Clark, 2012), we posit that the val66met genotype may interact with behavioural traits to predict the development of children’s BI over time. In the current study, we addressed the question of whether the stability of early BI varied depending on children’s BDNF genotype. More specifically, in the context of a GXT model, and toward the goal of tracing a developmental pathway for BI, and hence psychopathology risk, we tested whether BI in preschool was more stable, and hence, a greater vulnerability for later psychopathology, in children with or without the met variant. While previous work suggests that the met variant of the val66met BDNF SNP interacts with the environment in shaping children’s responsivity to their environments (Drury et al., 2012; Hayden et al., 2010; 2013), these studies did not test GXT. Additionally, past work (Bau et al., 2000) suggests that individual differences in fear responding provide a context that moderates associations between genetic variants and behavioral outcomes. Given these data, we hypothesized that the BDNF met variant of the val66met SNP would interact with trait BI to influence the stability of BI over time, if trait BI itself serves as a marker of biological and environmental factors that shape expression of the met variant. We tested this model in a large sample of typically developing children from the community assessed for BI using observational methods.
Method
Participants
Participants were 476 children (254 boys) from the community, recruited through a commercial mailing list. Eligible families had a child between 3 and 4 years of age with no significant medical conditions or developmental disabilities, and at least one English-speaking biological parent. The ethnicity of the sample was mostly White (87.3%), with smaller proportions of Hispanic (8.3%) and Asian (3.2%) participants; the remaining participants were Black, mixed race, or “other.” At baseline (age 3) participants were an average of 42.1 months old (SD = 3.1). At age 6 follow-up, 382 (204 boys) children remained in the study (19.7% attrition rate) with an average age of 73.1 months (SD = 9.6). Most participants were from middle-class families, as measured by Hollingshead’s Four Factor Index of Social Status (M = 44.3, SD = 10.9; Hollingshead, 1975). Children who did not participate in the follow-up visit did not differ from those who remained based on age, sex, race, social status, age 3 BI level, or BDNF genotype (all p > .059).
Genetic Assessment
Buccal swabs were collected from each child during the age 3 visit. Qiagen’s DNA Micro-Kit (Qiagen, Valencia, CA) was used to isolate genomic DNA (gDNA) from individual buccal cells, according to the manufacturer’s instructions. Individual gDNA isolates were used to genotype the val66met polymorphism using the polymerase chain reaction, followed by the restriction fragment length polymorphism analysis method (Bueller et al., 2006; Sheikh, Hayden, Kryski, Smith & Singh, 2010). In the current sample, 188 participants (49.2%) were homozygous for the val/val genotype, 174 were heterozygous (45.5%), and 20 participants (5.2%) were homozygous for the met/met genotype. Chi-square tests confirmed that the sample was in Hardy-Weinberg equilibrium (χ2 = 6.4, p = .17; Rodriguez, Gaunt, & Day, 2009). Given previous research suggesting phenotypic differences between individuals homozygous for the val genotype and those carrying the met variant (Bath & Lee, 2006; Chen et al., 2006), and in keeping with previous behavioural studies considering the val66met SNP (Dougherty, Klein, Congdon, Canli, & Hayden, 2010; Hayden et al., 2010; Hayden et al., 2013), we compared participants homozygous for the val genotype to participants with at least one met allele (met/xxx).
Laboratory Assessment of BI
Each child participated in a standardized lab visit based on the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995) and an age-adapted version of the Lab-TAB (Durbin, Hayden, Klein, & Olino, 2007). At age 3, children participated in tasks drawn directly from the preschool-aged version of the Lab-TAB. In order to ensure laboratory tasks were both novel and developmentally appropriate for participants at age 6, we used adapted versions of tasks from the Lab-TAB. Our group has used these tasks extensively and shown their associations with analogous behaviors drawn from the preschool-aged version (Durbin et al., 2007; Kotelnikova, Mackrell, Jordan, & Hayden, 2015). The Lab-TAB consisted of 12 tasks at age 3; 9 tasks were used at age 6. A subset of the tasks was designed to elicit BI at both the age 3 and age 6 assessment (three and two tasks, respectively). Each episode lasted approximately 3 minutes, except for Risk Room and Exploring New Objects, which lasted approximately 5 minutes. All episodes were led by a female experimenter and videotaped through a one-way mirror for coding. During BI-relevant tasks (described below), children were either alone in the room (without an experimenter or parents), with an experimenter only, with a confederate, or with a caregiver. When a caregiver was in the room with the child, they were given paperwork to complete and instructed to refrain from interacting with the child.
Observational data collected from the aforementioned tasks at both time points was coded by trained undergraduate and graduate research assistants using a coding system based on that of Goldsmith and colleagues (1995), which uses widely used methods for coding laboratory observations of BI (e.g., Durbin, Klein, Hayden, Buckley, & Moerk, 2005; Olino, Klein, Dyson, Rose, & Durbin, 2010; Pfeifer, Goldsmith, Davidson, & Rickman, 2002). Coders were blind to other study data and had to reach at least 80% agreement with a master coder on individually coded participants before independently coding. In addition to formal reliability checks, every 10th coded episode was reviewed with master coders to reduce rater drift. In this coding system, each task is separated into 20–30 second “epochs” from which specific BI-relevant emotions and behaviours are coded. For each epoch, raters coded the maximum intensity of every relevant emotion and behaviour (described below). Although the Lab-TAB is designed to elicit a wide range of temperamental characteristics, only those tasks used in the present study (i.e., those designed to elicit BI), along with their coding procedures, are described below.
Age three BI assessment
Assessment of BI at age three consisted of three Lab-TAB tasks: Risk Room, Stranger Approach, and Exploring New Objects. In Risk Room, the experimenter invited the child to explore a set of novel and ambiguous stimuli (e.g., a cloth tunnel, balance beam, Halloween mask, etc.) and then left the child in the main experimental area (with the parent present but occupied with questionnaires). After 5 minutes of free play, the experimenter returned to the room and asked the child to touch each of the stimuli. In Stranger Approach, the experimenter left the child alone in the main experimental area. Following a brief delay, an unfamiliar male research assistant entered the room and spoke to the child in a neutral tone while gradually walking closer to the child. Finally, in Exploring New Objects, the child was left alone to freely explore a set of novel and ambiguous stimuli, including a mechanical spider, a mechanical bird, and soft, sticky gel balls. After 5 minutes of free play, the experimenter returned to the room and asked the child to touch each of the stimuli.
In the process of scale development, coded items that negatively impacted internal consistency were dropped. Thus, during all three episodes, fearful facial (e.g., eyebrows raised in distress, mouth drawn back in fear), bodily (e.g., wary gait, bodily tension, nervous fidgeting), and vocal (e.g., timid tone of voice, comments with fearful content) affect, as well as latency to first fear response were coded and included in the final scale. In both Risk Room and Exploring New Objects, codes included on the final scale were: latency to touch objects, total number of objects touched, tentative play, references and proximity to parent, references to experimenter (e.g., timid glances toward experimenter or fearful questioning before complying with the experimenter’s request to touch objects), time spent playing, and latency to verbalize. During Exploring New Objects, a startle variable was also coded and included on the final scale. During Stranger Approach gaze aversion, latency to vocalize, approach to and avoidance of stranger, and verbal/nonverbal interactions with the stranger (e.g., talking with the stranger, nodding in response to the stranger) were included on the final scale. Latency scores were reverse coded to ensure that they were in the same direction of other variables making up the composite BI scores. Age 3 BI scores were a composite score based on the average of z-scores for coded variables (listed above) for all the above episodes. These procedures for computing BI composite scores are consistent with other studies using observational coding (e.g., Johnson et al., 2016; Williams et al., 2009). BI composite scores at age 3 (α = .80; ICC = .88; n = 28) were log-transformed to achieve normality.
Age six BI assessment
Assessment of BI at age 6 consisted of two Lab-TAB tasks: Story Time and Object Fear. In Story Time, the child was asked to tell a story to an unfamiliar research assistant, described as a “story expert,” using the picture book “A Boy, A Dog, and a Frog.” The child was told that the story expert would assign them a grade based on how well they told the story. In Object Fear, the child was left alone and instructed to explore a room filled with fear-eliciting objects, including a box filled with plastic insects from which cricket sounds were emitted, a cage with plastic rats inside it, and a large, fuzzy, black spider covered in a cloth.
Age 6 BI tasks were coded using similar coding procedures as those described for age 3 BI. Coded items that negatively impacted internal consistency were dropped. Thus, during both episodes fearful facial, bodily, and vocal affect, as well as stilling/freezing were coded and included in the final scale. During Story Time distress vocalizations, latency to vocalize, approach to the ‘story expert’, and verbal/nonverbal interactions with the ‘story expert’ were also coded and included in the final scale. Finally, during Object Fear latency to touch objects, total number of objects touched, tentative play, references to the experimenter (e.g., timid glances toward experimenter or fearful questioning of experimenter before complying with the experimenter’s request to touch objects), time spent playing, time spent talking, fearful or wary comments and questions, latency to comply, and non-compliance were all coded and included in the composite BI score. Latency scores were reverse coded to ensure that they were in the same direction of other variables making up the composite BI scores. Behaviors coded during BI episodes that decreased the reliability of the BI composite were dropped. Similar to age 3 BI, age 6 BI scores were a composite score based on the averaged z-scored variables for the above coded behaviours. Similar procedures for computing BI composite scores are consistent with other studies using observational coding (e.g., Johnson et al., 2016; Williams et al., 2009). BI composite scores at age 6 (α = .72; ICC = .64, n = 35) were log-transformed to achieve normality.
Results
Bivariate correlations between all major study variables are in Table 1. A modest positive correlation between BI at age 3 and age 6 was found with a magnitude consistent with estimates of childhood BI stability previously reported (e.g., Gest, 1997), albeit higher than that reported in other studies (e.g., Scarpa et al., 1995). Consistent with past research indicating sex differences in BI (Doey, Coplan, & Kingsbury, 2014; Fox et al., 2005a), an independent t-test found that girls (M = .66, SD = .20) had significantly higher BI than boys (M = .59, SD = .18), t(380) = -3.80, p < .001 at age 3 (g = .37). Girls (M = .61, SD = .05) also had significantly higher BI than boys (M = .59, SD = .05), t(380) = -3.51, p < .001 at age 6 (g = .40). Neither BI at age 3 (t(380) = .18, p = .86) nor age 6 (t(380) = .74, p = .55) significantly differed between those with the val/val genotype and those with the met/xxx genotype.
Table 1.
Bivariate correlations of variables of interest
| M (SD) | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| 1. Age at age 3 (months) | 42.1 (3.1) | – | ||||
| 2. Age at age 6 (months) | 73.1 (9.6) | .12* | – | |||
| 3. Age 3 BI | .62 (.19) | −.09 | .02 | – | ||
| 4. Age 6 BI | .60 (.05) | −.02 | .00 | .57*** | – | |
| 5. BDNF genotype | – | −.09 | −.03 | −.01 | .03 | – |
Note:
p < .05;
p < .01;
p < .001.
Ns = 379–382. BI = behavioural inhibition; BDNF = brain derived neurotrophic factor.
BI stability was operationalized as the strength of association between BI at age 3 and BI at age 6. To examine the moderating role of BDNF genotype on BI stability, we performed a regression analysis with BI at age 3 as the predictor, BDNF genotype as the moderator, and BI at age 6 as the outcome (Aiken & West, 1991). BDNF genotype was dummy-coded into a binary variable (i.e., val/val and met/xxx). Given previous work suggesting sex differences in BI (Doey et al., 2014; Fox et al., 2005a), as well as the association between child sex and BI in our sample, child sex was entered as a covariate. Additionally, to control for the possibility of population stratification due to the different ethnic groups that comprised our sample, ethnicity was also entered as a covariate. As there were no main effects of sex nor ethnicity and they did not interact with other variables, both were dropped from final analyses.
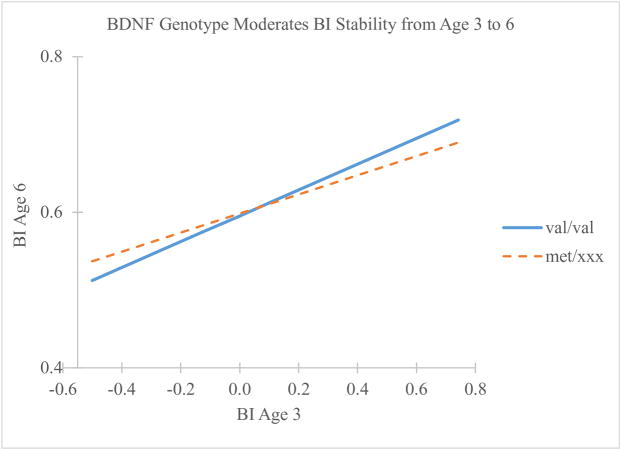
Regression analysis showed a main effect of BI at age 3 on age 6 BI but no main effect of BDNF genotype (Table 2). However, the interaction between age 3 BI and BDNF was significant, indicating that BDNF genotype moderated the relationship between BI at age 3 and BI at age 6. Overall, this model accounted for 33% of the variance in age 6 BI, with the interaction between age 3 BI and BDNF genotype accounting for approximately 1% of this variance. This interaction effect is plotted in Figure 1. The dummy-coded BDNF genotype variable was reversed to probe for simple slopes. Simple slopes analysis indicated a significant positive relationship between BI at age 3 and age 6 for individuals with the val/val genotype (b = .17, sr = .42, p < .001) and the met/xxx genotype (b = .12, sr = .40, p < .001). The lower slope for individuals with at least one met allele indicates that these children demonstrate lower BI stability from age 3 to 6, relative to children without a met variant.
Table 2.
Stepwise multiple regression analysis with BI age 6 as outcome (n = 379).
| Variables | B | SE(B) | β | ΔR2 | adjusted R2 |
|---|---|---|---|---|---|
| Step 1 | |||||
| BI age 3 | .139 | .010 | .574*** | .330** | .328 |
| Step 2 | |||||
| BI age 3 | .140 | .010 | .574*** | .001 | .327 |
| BDNF genotype | .003 | .004 | .033 | ||
| Step 3 | |||||
| BI age 3 | .166 | .017 | .594*** | .007* | .333 |
| BDNF genotype | .003 | .004 | .032* | ||
| BI age 3 X BDNF genotype | −.043 | .021 | −.088* | ||
Note:
p < .05;
p < .01.
BI = behavioural inhibition; BDNF = brain derived neurotrophic factor.
Figure 1.
Discussion
We examined whether BDNF genotype interacted with BI to moderate the stability of BI in children from age 3 to age 6. More specifically, in the context of GXT models, and toward the goal of tracing a developmental pathway for BI, we tested whether BI in preschool was more stable, and hence, a greater vulnerability for later internalizing problems, in children with or without the met variant. Given work implicating the met variant of the val66met BDNF SNP in gene-environment interaction (Hayden et al., 2010; 2013), and in the stability of related traits (i.e., disinhibition, Drury et al., 2012), as well as past work showing that individual differences in traits related to BI moderate genetic influences (Bau et al., 2000), we hypothesized that presence of this variant would interact with trait BI to confer lower BI stability over time. Although the effects obtained were small, our results corroborated this hypothesis: children with a met allele demonstrated lower BI stability from age 3 to 6 than their homozygous val counterparts. This finding is consistent with previous findings suggesting presence of a met allele was associated with greater change in behaviour related to inhibition (i.e., less stability; Drury et al., 2012).
Traits such as BI in GXT models are posited to mark activity within specific neural systems, specifically fear-processing regions of the limbic system such as the amygdala (Kagan & Snidman, 1991). Neuroimaging research reports correlations between BI and amygdalar activity in response to novel stimuli (Schwartz et al., 2003) and emotional faces (Perez-Edgar et al., 2007). If BI in childhood marks amygdalar reactivity during novel situations, it is conceivable that such patterns of reactivity are related to the likelihood of seeking out novel environments. Our work raises the possibility that children with the BDNF met allele will, in turn, be particularly impacted by the novelty of the environments they experience, thus contributing to BI stability.
We did not test specific environments that might have contributed to change in young children’s BI, an important direction for future work. It is relevant to note that some work suggests that the val66met SNP serves as a marker of differential susceptibility to the environment, increasing reactivity to both positive and negative environmental contexts (Belsky & Pluess, 2009). While not the focus of the current study, differential susceptibility posits that, rather than conferring vulnerability to pathology, some genetic polymorphisms confer increased plasticity such that those with these variants are rendered more susceptible to the influence of environmental factors, both negative and positive. Such plasticity could potentially account for the effects obtained in the present study, although we did not test this specifically; specifically, to the extent that traits such as BI predict environmental experience (e.g., children higher in BI systematically selecting environments low in novelty), the met allele may render those with this variant more susceptible to such environments, either consolidating or weakening initial tendencies toward inhibited behavior potentially through alterations in gene expression. We acknowledge that such possibilities are speculative yet may be worthy of future study.
Parenting such as maternal overprotection (Johnson et al., 2016) and maternal intrusiveness and low paternal affection (Park et al., 1997) may warrant integration into broader models of how genetic influences interact with early BI to shape subsequent trait development. For example, it is possible that early BI elicits specific caregiving styles that differentially impact later BI based on whether children have a met allele. Future investigations drawing on larger samples of children should explore the mediating influence of these and other environmental factors in val66met’s moderation of BI stability. Complex models such as these will require large samples of children and their parents.
Children homozygous for the val allele tended to show greater stability of BI over time, relative to children with one or more met allele. Lower BI stability among those with the met/xxx genotype is consistent with previous studies suggesting it is a marker of plasticity (Nilsson et al., 2015). As previously mentioned, stably high BI acts as a marker of risk for internalizing disorders (Chronis-Tuscano et al., 2009; Essex et al., 2010; Prior et al., 2000). Similarly, stably high disinhibition, a trait with conceptual similarity to low BI, is associated with an increased risk of externalizing disorders (Hirshfeld-Becker et al., 2007; Iacono et al., 2008). These findings, combined with our results, suggest the most important implications of BDNF genotype are for those children at the extremes of BI. Specifically, individuals at high or low age 3 BI are more likely to remain at the relative extremes of trait BI into middle childhood if they are homozygous for the val allele, relative to met/xxx peers, potentially putting them at higher risk for internalizing or externalizing disorders, respectively. In analyses not reported here, we did not identify a significant interaction between age 3 BI and BDNF genotype in predicting age 6 psychopathology symptoms. We contend that this is unsurprising as this is a community sample of young children, yielding fairly low psychopathology symptoms. Seeing whether BI and BDNF interact to predict trajectories that ultimately result in psychopathology is an important next step in testing our model.
The current study has both strengths and limitations. To our knowledge, this is the only study investigating the role of BDNF genotype in predicting BI stability. Our use of observational measures of BI reduces the effect of rater biases (Durbin et al., 2007). With regard to weaknesses, although the sample used in the current study is relatively large for a study using laboratory measures of behaviour, it is still small for a genetic association study, which limited our ability to do more extensive analyses of the role of the environment. Although we did find that BDNF genotype significantly moderated BI stability over time, the effect was small; having said that, small effects may be expected when considering the impact of a single gene on a relatively complex behavior such as BI. Multiple genes almost certainly contribute to BI (Fox et al., 2005b), while we considered only one in the current study. Also, experts disagree on the extent to which population stratification, which can produce false positive associations, is a concern in studies such as ours using an ethnically homogenous sample (Hutchison, Stallings, McGeary, & Bryan, 2004). Also, it is possible that the BDNF gene is in linkage disequilibrium with another gene that accounts for the associations with BI found in the present study.
In conclusion, we found the met variant of the val66met BDNF gene SNP was associated with decreased stability of BI from age 3 to age 6. While preventative efforts may be best targeted toward children who are homozygous val, given their increased probability of higher stability of BI during childhood, those with the met variant may be more amenable to preventative efforts aimed at either promoting greater constraint in uninhibited children or decreasing high inhibition, a possibility to be tested in future research.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications Inc; 1991. [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (val66met) impact on brain structure and function. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Bau CHD, Almeida S, Hutz MH. The Taql A1 allele of the dopamine D2 receptor gene and alcoholism in Brazil: Association and interaction with stress and harm avoidance on severity prediction. American Journal of Medical Genetics. 2000;96:302–306. doi: 10.1002/1096-8628(20000612)96:3<302::aid-ajmg13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. International Journal of Neuropsychopharmacology. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF val66met allele is associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Caspi A. The child is father of the man: Personality continuities from childhood to adulthood. Journal of Personality and Social Psychology. 2000;78:158–172. doi: 10.1037//0022-3514.78.1.158. [DOI] [PubMed] [Google Scholar]
- Caspi A, Langley K, Milne B, Moffitt TE, O’Donovan M, Owen MJ, … Williams B. A replicated molecular genetic basis for subtyping antisocial behavior in children with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2008;65(2):203–210. doi: 10.1001/archgenpsychiatry.2007.24. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, … Hempstead BL. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF)(Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. The Journal of Neuroscience. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, … Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of American Academy of Child and Adolescent Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of American Academy of Child and Adolescent Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco E, Åslund C, Oreland L, Nilsson KW. Three-way interaction effect of 5-HTTLPR, BDNF Val66Met, and childhood adversity on depression: a replication study. European Neuropsychopharmacology. 2013;23:1300–1306. doi: 10.1016/j.euroneuro.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Dilalla LF, Kagan J, Reznick JS. Genetic etiology of behavioral inhibition among 2-year-old children. Infant Behavior & Development. 1994;17(4):405–412. [Google Scholar]
- De Pauw SSW, Mervielde I. Temperament, personality, and developmental psychopathology: A review based on the conceptual dimensions underlying childhood traits. Child Psychiatry and Human Development. 2010;41:313–329. doi: 10.1007/s10578-009-0171-8. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Henderson HA, Fox NA, Rubin KH. Predicting social wariness in middle childhood: The moderating roles of child care history, maternal personality and maternal behavior. Social Development. 2008;17:471–487. doi: 10.1111/j.1467-9507.2007.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Clark R. The gene in its natural habitat: The importance of gene--trait interactions. Development and Psychopathology. 2012;24(04):1307–1318. doi: 10.1017/S0954579412000727. [DOI] [PubMed] [Google Scholar]
- Doey L, Caplan RJ, Kingsbury M. Bashful boys and coy girls: A review of gender differences in childhood shyness. Sex Roles. 2014;70:255–266. [Google Scholar]
- Dougherty LR, Klein DN, Congdon E, Canli T, Hayden EP. Interaction between 5-HTTLPR and BDNF val66met polymorphisms on HPA axis reactivity in preschoolers. Biological Psychology. 2010;83:93–100. doi: 10.1016/j.biopsycho.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Gleason MM, Theall KP, Smyke AT, Nelson CA, Fox NA, Zeanah CH. Genetic sensitivity to the caregiving context: The influence of 5httlpr and BDNF val66met on indiscriminate social behavior. Physiology & Behavior. 2012;106:728–735. doi: 10.1016/j.physbeh.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin CE, Hayden EP, Klein DN, Olino TM. Stability of laboratory-assessed temperamental emotionality traits from ages 3 to 7. Emotion. 2007;7:388–399. doi: 10.1037/1528-3542.7.2.388. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, … Lu B. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Waheed J, Goldman D. Neurophysiological and genetic distinctions between pure and comorbid anxiety disorders. Depression and Anxiety. 2008;25(5):383–392. doi: 10.1002/da.20378. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MD, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. American Journal of Psychiatry. 2010;167:40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005a;56:245–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, … Pine DS. Evidence for a gene-environment interaction in predicting behavioral inhibition in predicting behavioural inhibition in middle childhood. Psychological Science. 2005b;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, … Williams LM. Interactions between BDNF Val66Met polymorphism and early stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gest SD. Behavioral inhibition: Stability and associations with adaptation from childhood to early adulthood. Journal of personality and social psychology. 1997;72:467–475. doi: 10.1037//0022-3514.72.2.467. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Laboratory Temperament Assessment Battery: Preschool version. University of Wisconsin-Madison; 1995. Unpublished manuscript. [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, … Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. The Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DK, Dougherty LR, Olino TM, Dyson MW, Durbin CE, … Singh SM. The role of brain-derived neurotrophic factor genotype, parental depression, and relationship discord in predicting early-emerging negative emotionality. Psychological Science. 2010;21:1678–1685. doi: 10.1177/0956797610385357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Olino TM, Bufferd SJ, Miller A, Dougherty LR, Sheikh HI, … Klein DN. The serotonin transporter linked polymorphic region and brain-derived neurotrophic factor valine to methionine at position 66 polymorphisms and maternal history of depression: Associations with cognitive vulnerability to depression in childhood. Development and Psychopathology. 2013;25:587–598. doi: 10.1017/S0954579413000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Micco JA, van Grondelle A, … Rosenbaum JF. Clinical outcomes of laboratory-observed preschool behavioral disinhibition at five-year follow-up. Biological Psychiatry. 2007;62:565–572. doi: 10.1016/j.biopsych.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975. Unpublished manuscript. [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Johnson VC, Olino TM, Klein DN, Dyson MW, Bufferd SJ, Durbin CE, … Hayden EP. A longitudinal investigation of predictors of the association between age 3 and age 6 behavioural inhibition. Journal of Research in Personality. 2016;63:51–61. doi: 10.1016/j.jrp.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. [Google Scholar]
- Kagan J, Snidman N. Temperamental factors in human development. The American Psychologist. 1991;46(8):856–862. doi: 10.1037//0003-066x.46.8.856. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological psychiatry. 2005;58(10):796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelnikova Y, Mackrell SVM, Jordan PL, Hayden EP. Longitudinal associations between reactive and regulatory temperament traits and depressive symptoms in middle childhood. Journal of Clinical Child and Adolescent Psychology. 2015;44(5):775–786. doi: 10.1080/15374416.2014.893517. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Santonastaso P, Mauri M, Bellodi L, Erzegovesi S, Fuschino A, … Maj M. Investigation of the serotonin transporter regulatory region polymorphism in bulimia nervosa: relationships to harm avoidance, nutritional parameters, and psychiatric comorbidity. Psychosomatic medicine. 2006;68(1):99–103. doi: 10.1097/01.psy.0000195746.52074.63. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Hodgins S, Oreland L, Åslund C. Genotypes do not confer risk for delinquency but rather alter susceptibility to positive and negative environmental factors: Gene-environment interactions of BDNF val66met, 5-HTTLPR, and MAOA-uVNTR. International Journal of Neuropsychopharmacology. 2015;18:1–10. doi: 10.1093/ijnp/pyu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. Journal of Abnormal Psychology. 2010;119:468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Belsky J, Putnam S, Crnic K. Infant emotionality, parenting, and 3-year inhibition: Exploring stability and lawful discontinuity in a male sample. Developmental Psychology. 1997;33:218–227. doi: 10.1037//0012-1649.33.2.218. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Development. 2002;73:1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- Plomin R, Emde RN, Braungart JM, Campos J, Corley R, Fulker DW, … Zahn-Waxler C. Genetic change and continuity from fourteen to twenty months: the MacArthur Longitudinal Twin Study. Child Development. 1993;64(5):1354–1376. [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Oberklaid F. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:461–468. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Kagan J, Reznick JS, Corley R. The heritability of inhibited and uninhibited behavior: A twin study. Developmental Psychology. 1992;28(6):1030. [Google Scholar]
- Rodriguez S, Gaunt TR, Day INM. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. American Journal of Epidemiology. 2009;169(4):505–514. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, Hastings PD. Stability and social-behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Development. 2002;73:483–495. doi: 10.1111/1467-8624.00419. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Hastings PD, Stewart SL, Henderson HA, Chen X. The consistency and concomitants of inhibitions: Some of the children, all of the time. Child Development. 1997;68:467–483. [PubMed] [Google Scholar]
- Scarpa A, Raine A, Venables PH, Mednick SA. The stability of inhibited/uninhibited temperament from ages 3 to 11 years in Mauritian children. Journal of Abnormal Child Psychology. 1995;23:607–618. doi: 10.1007/BF01447665. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for neuroplasticity. Trends in Neuroscience. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome if inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Hayden EP, Kryski KR, Smith HJ, Singh S. Genotyping the BDNF rs6265 (val66met) polymorphism by one-step amplified refractory mutation system PCR. Psychiatric Genetics. 2010;20:109–112. doi: 10.1097/YPG.0b013e32833a2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman S, Hemmings SMJ, Seedat S. Brand-derived neurotrophic factor (BDNF) protein levels in anxiety disorders: Systematic review and meta-regression analysis. Frontiers in Integrative Neuroscience. 2013;7(55):1–11. doi: 10.3389/fnint.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Boksem MA. Cortisol involvement in mechanisms of behavioral inhibition. Psychophysiology. 2011;48(5):723–732. doi: 10.1111/j.1469-8986.2010.01131.x. [DOI] [PubMed] [Google Scholar]
- Turkheimer E. Three laws of behavior genetics and what they mean. Current Directions in Psychological Science. 2000;9:160–164. [Google Scholar]
- Wichstrøm L, Berg-Nielsen TS, Angold A, Egger HL, Solheim E, Sveen TH. Prevalence of psychiatric disorders in preschoolers. Journal of Child Psychology and Psychiatry. 2012;53(6):695–705. doi: 10.1111/j.1469-7610.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- Williams LR, Degnan KA, Perez-Edgar KE, Henderson HA, Rubin KH, Pine DS, … Fox NA. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. Journal of Abnormal Child Psychology. 2009;37(8):1063–1075. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, … Gelernter J. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2006;141B(4):387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]