Abstract
Background:
Efforts to improve adherence by reducing copayments through value-based insurance design are spreading despite limited evidence of improved health outcomes. The objective of this study was to determine whether eliminating patient copayments for blood pressure medications improves blood pressure control.
Methods:
The Collaboration to Reduce Disparities in Hypertension (CHORD) was a randomized controlled trial with 12 months follow-up conducted among patients from the Philadelphia and Pittsburgh VA Medical Centers. We enrolled 479 patients with poorly controlled systolic blood pressure. Participants were randomly assigned to receive reductions in copayments from $8 to $0 per medication per month for each anti-hypertensive prescription filled, a computerized behavioral intervention (CBI), both copay reduction and CBI, or usual care. Our main outcome measure was change in systolic blood pressure from enrollment to 12 months post-enrollment.
Results:
There were no significant interactions between the copayment interventions and the CBI interventions. Blood pressure decreased among all participants, but to a similar degree between the financial incentive and control groups. Systolic pressure within the incentive group dropped 13.2 mm vs. 15.2 mm for the control group (difference = 2.0, [95% CI = −2.3 to 6.3], p=0.36.) The proportion of patients with blood pressure under control at 12 months was 29.5% in the incentive vs. 33.9 in the control group (OR = 0.8; [95% CI = 0.5 to 1.3], p=0.36).
Conclusions:
Among patients with poorly controlled blood pressure, financial incentives that effectively eliminated copayments for blood pressure medications did not improve blood pressure control.
Precis:
This study tests the impact of a reward that lowered copayments for blood pressure medication for blood pressure to zero on blood pressure control.
Introduction
Hypertension, especially within socio-economically disadvantaged communities, remains a leading cause of cardiovascular morbidity and mortality in the US, affecting nearly 50 million Americans.1 Although there are effective medications to treat hypertension, nearly two-thirds of Americans with hypertension have poorly controlled blood pressure.2 Lack of adherence to antihypertensive medications is considered a critically important factor in blood pressure management. Medication adherence for chronic diseases such as hypertension and hypercholesterolemia is extremely low,3–7 limiting the potential for highly efficacious medications to improve population health.
Value-based Insurance Designs (VBID), an approach based on the premise that reductions in copayments will significantly increase utilization of beneficial and cost-effective services, are being widely adopted.8,9 As part of the Affordable Care Act, elimination of cost sharing for preventive services is being mandated to increase utilization of such services. While observational studies have shown increases in copayments are associated with both decreased medication use and worsened health outcomes,10–18 the impact of decreasing copayments seen in observational studies has been more modest19–25 and studies have generally focused exclusively on medication use and not measured health outcomes. The underlying psychology of how people process changes in payments as losses compared to gains suggests that increases and decreases in copayments may not be equivalent.26
Only two randomized trials examining the relationship between copayments and health have been published. The first, the RAND Health Insurance Experiment (HIE), was conducted in the 1970s and varied cost sharing for all services, not just that for medications; the second, the recently published MI FREEE study, was a test of copayment reduction following discharge for myocardial infarction in an insurer-based intervention. 27
To examine whether reducing copayments from $8 to $0 per medication per month for all anti-hypertensive medications significantly improves blood pressure control, we conducted a clinic-based randomized controlled trial of elimination of copayments among patients with poorly controlled blood pressure at two medical centers in Pennsylvania.
Methods
Study Population
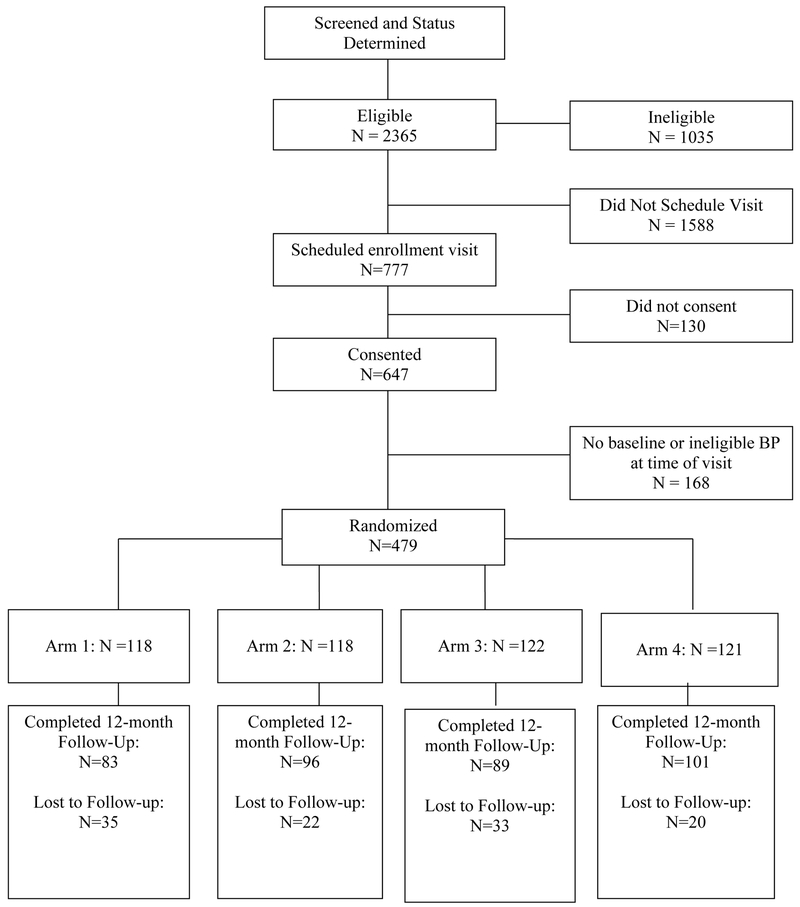
Study participants were drawn from patients at two hospitals in Pennsylvania: the Philadelphia Veterans Affairs Medical Center (PVAMC) and the VA Pittsburgh Healthcare System (VAPitt), with recruitment occurring between March, 2005 and July, 2007. Eligible patients were 21 years of age or older, with one or more active prescriptions for an anti-hypertensive medication, systolic blood pressure (SBP) of at least 140 (130 in diabetic patients), and who paid a copayment for their medications. Exclusion criteria included: participation in another experimental study; markedly shortened life expectancy (due to diagnosis of metastatic cancer, end-stage renal disease on dialysis, NYHA class IV CHF, or dementia); or atrial fibrillation (because of concerns with accuracy of BP measurement). Details on enrollment are given in Figure 1.
Figure 1.
Flow diagram of trial participation
Study Protocol
The protocol was approved by the Institutional Review Boards of the PVAMC, VAPitt, and the University of Pennsylvania, and all participants provided written informed consent prior to randomization. The study was registered at http://clinicaltrials.gov as ID # NCT00133068. Participants were randomized to receive either: 1) a financial incentive equal to their copayments for all anti-hypertensive medications that effectively lowered copayments from $8 to $0 per medication per month (on January 1, 2006, 9 months after initiation of enrollment, copayments were raised from $7 to $8 per month per VHA Directive 2005–052 and incentives were adjusted accordingly); 2) a computerized behavioral intervention (CBI) that was provided immediately following enrollment and repeated at the 6-month follow-up visit; 3) both the financial incentives and the CBI; or 4) usual care, which consisted only of their existing medical care. After an initial visit, all participants were requested to return for follow-up blood pressure readings and completed surveys at 3, 6, 9, and 12 months. The financial reimbursements were paid as soon as study staff received confirmation of prescription fills using either the VA’s computer records, prescription bottles, or receipts.
Randomization Procedures
Randomization was carried out using a random number generator and via permuted block randomization with a block size of four. Randomization was stratified by site, income (<100%, 100–200%, 200–300%, and >300% of the federal poverty line), and baseline blood pressure (SBP < 160 mmHg or SBP ≥ 160 mmHg). Allocation assignments were concealed, with staff unable to access randomization assignment for each subject until all eligibility criteria were entered in an electronic tracking system and consent forms were completed. Neither staff nor study participants could be blinded due to the nature of the intervention; investigators and analysts, however, remained blinded to intervention assignments until unblinding occurred, in coordination with the Data Safety Monitoring Board, once follow-ups were complete.
Outcome Assessments
The primary outcome variable was change in systolic and diastolic blood pressure (SBP and DBP) from enrollment to 12 months post-enrollment. Secondary outcome variables included change in SBP and DBP 6 months post-enrollment, the percentage of patients with well-controlled blood pressure at 6 and 12 months post-enrollment, self-reported medication adherence, and prescription refill data from the VA electronic medical record system. Blood pressure control was defined as SBP below 140 mmHg and DBP below 90 mmHg for non-diabetic patients and as SBP below 130 mmHg and DBP below 85 mmHg for diabetic patients.
Measurement of blood pressure was done following a standardized protocol using an automated blood pressure cuff (Omron HEM-90R) ensuring that the correct cuff size was used.28 Participants were instructed to relax while seated for five minutes before their blood pressure was taken; the patient’s arm was supported on a chair or desk and blood pressures were measured three times, each two minutes apart, averaged, but not revealed to the study participants. Although the study nurses could not be blinded to the randomization, the use of an automated blood pressure cuff and a standardized protocol protected against differences in blood pressure measurement.
Medication adherence was measured using self-report based on the Hill Bone Scale,29 with supplemental assessment using electronic prescription fill records where available. For patients with these records, we calculated the proportion of days covered (PDC) measure (number of days with antihypertensive drug supply on hand divided by the number of days in the observation period). Patients with a PDC>=0.80 were considered adherent. In addition, continuous medication gap measures were calculated as at least 1 continuous episode with no antihypertensive medication for a minimum of 30, 60, or 90 days.30
Study Covariates
Other factors assessed at baseline included height, weight, serum creatinine level, income, health status, health history, medication use, age, gender, and self-reported race or ethnicity. We used information on income and family size to calculate income as a percent of the federal poverty line.
Statistical analysis
To evaluate the similarity of the treatment groups with respect to baseline covariates, we compared groups using Student’s t test for continuous variables and χ2 test for categorical variables, with Fisher’s Exact tests used for analyses with five or fewer subjects per cell. Because of the factorial design, we first assessed whether receipt of CBI affected the impact of incentive payments. We then collapsed the arms to compare all subjects receiving incentive payments to all subjects receiving no payments; the primary unadjusted analyses tested the mean differences in the degree of change in SBP and DBP between the incentive and control groups from baseline to 12 months post-enrollment using Student’s t-test. We similarly calculated differences in change in SBP and DBP from baseline to 6 months post-enrollment. Missing values for 6-month and 12-month SBP and DBP readings were handled using the Markov Chain Monte Carlo (MCMC) multiple imputation method, utilizing 10 imputations.31 Separate imputation regression models were implemented for SBP and DBP. Unadjusted odds ratios for achieving in-control blood pressure were estimated via logistic regression using the imputed data in the same manner.
We estimated regression coefficients and their 95% confidence intervals from an unadjusted linear regression model that incorporated only a factor indicating receipt of incentives vs. control; we then compared these with regression coefficients estimated from a model adjusted for the stratification variables (site, high SBP, and income), in all cases using the imputed data. In addition to pre-specified subgroup analyses on race and income, we examined changes in SBP and DBP in subgroups defined by study site, initial SBP (>=160 mmHg vs. below 160 mmHg), presence of diabetes, and education level (high school or lower, some college or college degree, beyond college). Homogeneity of the association between treatment groups and blood pressure change across subgroups was tested by assessing the significance of appropriate interaction terms included in the linear regression models described above.
The trial was powered to ensure that clinically meaningful differences in SBP of 10 mm Hg and of DBP of 5 mm Hg32–34 could be detected in any of the contrasts discussed above, assuming an interaction between the CBI and incentive interventions. We used an α of 0.01 to account for multiple comparisons and standard deviations of change in SBP and DBP of 20 and 10, respectively (based on the upper limit of standard deviation directly measured in clinical trials).35,36 Based on these estimates, we estimated that we would need 93 subjects per arm to detect the clinically meaningful difference in blood pressures discussed above. Recruitment goals for each arm for this study were increased to 116 subjects to accommodate an estimated 20% loss to follow-up, for a target of 464 subjects.
Results
Baseline Sample Characteristics
Study sample characteristics were generally balanced across the arms of the study (Table 1). Average age was 69, with a predominantly male population (97%) that was about 56% white and 42% black. Fifteen percent of the study population had incomes < 100% of the federal poverty line (FPL), 32% between 100% and 200% of the FPL, and the rest fairly evenly divided between the 200–300% FPL and >300% FPL ranges. The baseline mean SBP and DBP were 158 and 81 mm Hg, respectively.
Table 1.
Socio-Demographic and Clinical Characteristics of Study Sample.
| Variable | Copay Elimination n = 239 |
Control n = 240 |
p -value |
|---|---|---|---|
| Demographics | |||
| Average age (years) (std dev) |
68.9 (10.4) |
68.8 (11.3) |
0.89 |
| Male (%) | 98.3 | 97.1 | 0.36 |
| White (%) | 55.2 | 55.8 | 0.99 |
| Black (%) | 42.7 | 42.1 | |
| Other race (%) | 2.1 | 2.1 | |
| Hispanic Ethnicity (%) | 1.3 | 2.1 | 0.72 |
| Site | |||
| Philadelphia VA (%) | 73.6 | 69.6 | 0.32 |
| Pittsburgh VA (%) | 26.4 | 30.4 | |
| Education | |||
| High School or lower (%) | 45.0 | 52.1 | 0.06 |
| Some College or College (%) | 44.1 | 42.4 | |
| Beyond college (%) | 10.9 | 5.5 | |
| Poverty | |||
| <100% Poverty line (%) | 15.9 | 13.8 | 0.86 |
| 100-200% Poverty line (%) | 32.2 | 30.8 | |
| 200-300% Poverty line (%) | 25.5 | 26.7 | |
| >300% Poverty line (%) | 26.4 | 28.8 | |
| Baseline Blood pressure | |||
| Systolic blood pressure (mm Hg) (std dev) |
156.5 (15.4) |
159.0 (17.9) |
0.09 |
| Diastolic blood pressure (mm Hg) (std dev) |
80.7 (14.1) |
80.3 (13.6) |
0.77 |
| Medication taking | |||
| % with MPR>=80% | 47.4% | 61.5% | 0.34 |
| # anti-hypertensive medications (std dev) |
2.5 (1.2) |
2.6 (1.3) |
0.31 |
| # medications overall (std dev) |
4.8 (2.7) |
4.7 (2.8) |
0.78 |
| Comorbidities* | |||
| Diabetes (%) | 54.8 | 52.1 | 0.55 |
| Congestive Heart Failure (%) | 8.8 | 12.1 | 0.24 |
| Heart Attack or AMI (%) | 15.5 | 18.3 | 0.41 |
| Kidney Failure (%) | 2.9 | 0.8 | 0.11 |
| Stroke (%) | 8.8 | 6.7 | 0.38 |
| TIA or Mini Stroke (%) | 10.5 | 14.2 | 0.22 |
| High Cholesterol (%) | 71.1 | 68.3 | 0.51 |
Subjects may have had multiple comorbidities, thus these percentages do not sum to 100.
Follow-up rates at 12 months were slightly higher among incentive arm participants, with rates of 82.4% and 71.6% for incentive arm and control arm participants, respectively (p<0.01). Baseline SBP, number of anti-hypertensive medications, and number of medications overall did not differ between those who were lost to follow-up and those participants in whom we had data at 12 months.
Mean changes in systolic blood pressure were −12.9 mm Hg [95% CI −17.4, −8.4] in the control group, −12.6 [95% CI −17.0, −8.3] in the copayment reduction group, −17.4 [95% CI −21.7, −13.0] in the CBI group, and −13.7 [95% CI −18.0, −9.4] in the combined copayment/CBI group. There were no significant interactions between incentive payments and receipt of the CBI with respect to 12-month outcomes (p-value = 0.45); therefore, the primary and all subsequent analyses are combined by CBI status to focus on the impact of copayment reduction on blood pressure and adherence (hereafter comparison of ‘incentive’ vs ‘control’).
Primary and Secondary Outcomes
We found no significant difference in systolic and diastolic blood pressure reduction between the incentive and control groups (Table 2). SBP decreased an average of 13.2 mm Hg from baseline to 12 months post-enrollment in the incentive group compared to an average of 15.2 mm Hg in the control group (p=0.36). At the end of 12 months, there were no differences in the proportion of participants with blood pressure in control (29.5% of the incentive group vs. 33.9% for the control group, [OR = 0.8, 95% CI 0.5, 1.3]; p=0.36). Results of sensitivity analyses in which we assumed that the blood pressure in all patients lost to follow-up was equal to their last measured value or their baseline blood pressure (as opposed to the imputed blood pressure value) were qualitatively similar.
Table 2.
Change in blood pressure from baseline to 12 months for Copay Elimination arm (‘Incentive arm’) compared to control arm.
| Outcome measures | Copay Elimination n = 239 |
Control n = 240 |
Difference Between Groups (95% CI) |
p -value |
|---|---|---|---|---|
| Change in blood pressure | ||||
| Systolic blood pressure (mm Hg) | −13.2 | −15.2 | 2.0 (−2.3, 6.3) |
0.36 |
| Diastolic blood pressure (mm Hg) | −7.0 | −7.1 | 0.1 (−2.5, 2.7) |
0.94 |
|
Copay Elimination n = 239 |
Control n = 240 |
Odds Ratio (95% CI) |
p -value | |
| % who reached goal | 29.5 | 33.9 | 0.8 (0.5, 1.3) |
0.36 |
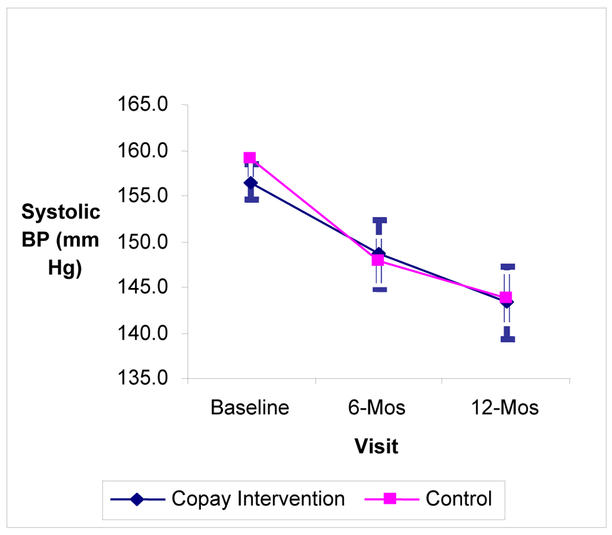
The pattern of changes in blood pressure from baseline to 6 months was similar, with no significant differences observed between the incentive and control group participants (Figure 2). Adjusted estimates of changes in SBP indicated no significant differences between incentive and control groups in the degree of change in blood pressure (Table 3).
Figure 2.
Change in Systolic Blood Pressure over Time Comparing the Intervention and Control Groups.
Table 3.
Change in Systolic Blood Pressure at 12 months from Multivariable Models
| Group | Parameter | BP Change (95% CI) |
|---|---|---|
| Unadjusted Model | ||
| Intercept | No Intervention | −15.2 (−18.1, −12.2) |
| Study Arm | Copay Intervention |
2.0 (−2.3, 6.3) |
| Adjusted for Stratification Variables | ||
| Intercept | No Intervention Site = Philadelphia VA SBP at baseline <160 Income >300% Poverty Line |
−8.5 (−13.2, −3.7) |
| Study Arm | Copay Intervention |
1.6 (−2.4, 5.6) |
| Site | Pittsburgh | 0.9 (−3.9, 5.6) |
| SBP at baseline |
SBP >=160 | −16.7 (−21.0, −12.5) |
| Income | <100% Poverty line | −4.5 (−11.1, 2.1) |
| 100-200% Poverty line | 0.4 (−5.3, 6.2) |
|
| 200-300% Poverty line | 1.6 (−4.0, 7.1) |
|
Changes in adherence as measured by PDC indicated no relative change in the proportion of participants who had PDC≥0.8 between baseline and 12 months (OR = 0.8, [95% CI 0.5, 1.3], p-value 0.49). Changes as measured by continuous medication gaps of 30 (OR = 1.0, [95% CI 0.6, 1.7], p-value 0.98), 60 (OR = 1.2, [95% CI 0.6, 2.6, p-value 0.61], or 90 days (OR = 0.6, [95% CI 0.3, 1.5], p-value 0.27) indicated no detectable differences between the control and incentive groups.
Subgroup analyses
The degree of change in SBP between the incentive and control group was compared among several subgroups of the populations in the study, including those with MPRs at baseline above and below 80%, those with and without diabetes, those with a baseline SBP above or below 160 mmHg, and subgroups determined by race, income, and education. There were no statistically significant differences in change in blood pressure between the incentive and control groups 12 months post-enrollment in any of the subgroups tested.
Discussion
In a sample of 479 primary care patients with uncontrolled hypertension receiving care at two Pennsylvania VA medical centers, we found that a financial incentive that essentially eliminated copayments for anti-hypertensive medications had no effect on blood pressure control. This is the first clinic-based experiment to test the impact of reduced prescription cost-sharing while holding constant the level of coverage for other services.
Our findings inform ongoing discussions about value-based insurance design and the national effort to improve patient outcomes through reduction in copayments for high-value prescription medications.9,37 Strategies to modify the degree of patient cost sharing based on the value of the prescriptions provided have garnered extensive publicity and are of great interest nationally.38,39 Reducing the price of cost-effective treatments or preventive services encourages utilization of highly cost-effective preventive services that could potentially save money by reducing the rate of adverse events from poorly controlled health.40,41 While increases in prescription copayments have been associated with decreases in medication adherence and increases in poor health outcomes in numerous studies,16,18,42,43 previously published studies on copayment reduction had not examined change in health outcomes until the MI-FREEE study, which indicated reductions in the rate of total major vascular events or revascularization without any increase in total health care spending.27 The RAND Health Insurance Experiment (HIE), the most definitive study conducted to date on the relationship between patient cost-sharing and expenditures found sizable effects of patient cost-sharing on health care utilization but more modest effects on health status. Of note, however, among low-income persons with high blood pressure, free care resulted in significant improvements in blood pressure. That study, however, was performed more than 25 years ago when fewer effective medications were available, excluded the elderly, comprised over 80% Caucasian participants, and included a population with relatively few comorbidities,44 populations for whom prescription drug coverage is more likely to be cost-effective. Most importantly, the HIE did not isolate the effect of prescription drug cost-sharing.45,46
Observational studies that have conducted robust empirical evaluations of a VBID approach to lowering copayments have generally found that when copayments were lowered to zero for generics and by about 30% for brand name medication among employees or insurance beneficiaries, adherence as measured by medication possession ratios (MPR) increases by about 1 to 4 percentage points on a baseline MPR of 60–80%. This means that for every employee who was completely non-adherent (MPR=0%) who became fully adherent (MPR≥80%) there would be 20–25 employees who would now receive copayment reductions but whose adherence didn’t change.2319-22 It is unlikely that changes in adherence of this magnitude would produce any significant changes in clinical outcomes.
Reductions in copayments may have less of an impact on patient outcomes than increases in copayments for several reasons. First, increases in copayments affect utilization among adherent patients, whereas decreases in copayments are targeted at affecting utilization among non-adherent patients, in whom a change in copayment of a given magnitude is likely to have less impact. Second, increases in copayments are likely processed as a loss by patients, and behavioral economists have demonstrated that losses are felt much more strongly than equivalent gains.26 Third, copayment reductions may be a bit like the ‘dog that didn’t bark’; for a non-adherent patient who doesn’t come to the pharmacy or fill prescriptions, communications about a reduction in copayments may be largely ignored. Fourth, studies have indicated that unbundling rewards from other payments make the rewards more effective;47 for this reason, in this study we provided post-hoc rebates rather than up-front cost reductions where the discounted amount received might appear diluted by the simultaneous filling of other prescriptions.
There are several limitations to consider. First, because prescriptions for chronic medications within the VA system are typically filled by mail and billed at the end of the month as opposed to immediately upon filling, our use of post-hoc rebates could be partly responsible for the absence of an effect, since people tend to have strong present-biased preferences,48 making rewards provided in the future less valuable. Second, patients may be non-adherent for many reasons and the magnitude of the reduction in copayments may have been too small to induce changes in behavior, though previous work has found copayment increases as low as 50 cents to 1 dollar per prescription (approximately $2–3 adjusting for inflation) in low income population can reduce drug utilization.49 Third, our study sample was predominantly male veterans from two VA hospitals and the findings may not generalize to women or patients who receive care in the private sector. However, there are no obvious reasons to believe that intervention effectiveness would be different in women. Finally, there may have been contemporaneous efforts to improve blood pressure control within VA facilities through quality improvement initiatives, and the effectiveness of these efforts may have limited our ability to measure a differential impact on blood pressure of copayment reduction.
In conclusion, we found that incentives that effectively eliminated copayments for high-value medications to treat blood pressure had no impact on blood pressure control. Reducing barriers to high-value care in and of itself makes sense; future studies should examine whether other incentive designs are more successful in improving blood pressure control.
Take-away points:
Small rewards that lower copayments to zero did not improve blood pressure control significantly more than in a control group which simply had blood pressure measured. This may be because of the way in which this program was administered with delays in providing rebates for copayments following prescription filling. The exact nature of the program implementation may have a significant impact on program effectiveness and should be carefully considered in VBID program design and assessment of impact.
Acknowledgements
Other coauthors include Wei Yang, John H. Holmes, Dominick L. Frosch, Katrina Armstrong, Kjell Enge, Raymond R. Townsend. They were not listed on the cover sheet because of the limitations to how many authors could be listed.
The work in this paper was primarily supported by a grant from the Commonwealth of Pennsylvania, titled Collaboration to Reduce Disparities in Hypertension, grant number ME-02–382. Supplemental support was received from Pfizer, Inc. The sponsors/funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Volpp and Dr. Kimmel had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Volpp has received funding from the Aetna Foundation, Aramark, Discovery (South Africa), Horizon Blue Cross and Blue Shield, Humana, Mckinsey, and Weight Watchers (all unrelated to the topic of this paper), research funding and consulting income from CVS Caremark, and consulting income from VALHealth. Dr. Kimmel has received funding from the Aetna Foundation and from several pharmaceutical companies and has done consulting for several pharmaceutical companies, including Pfizer, all unrelated to the topic of this paper. Other than what is listed above, there are no known financial conflicts of interest among any of the authors including but not limited to employment/affiliation, all grants or funding, honoraria, paid consultancies, expert testimony, and patents filed, received or pending.
Footnotes
Trial registration: http://Clinicaltrials.gov ID # NCT00133068
REFERENCES
- 1.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997;157:2413–46. [DOI] [PubMed] [Google Scholar]
- 2.Malik S, Lopez V, Chen R, Wu W, Wong ND. Undertreatment of cardiovascular risk factors among persons with diabetes in the United States. Diabetes Res Clin Pract 2007;77:126–33. [DOI] [PubMed] [Google Scholar]
- 3.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842–7. [DOI] [PubMed] [Google Scholar]
- 4.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 5.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA : the journal of the American Medical Association 2002;288:462–7. [DOI] [PubMed] [Google Scholar]
- 6.Haynes RB. Improving Patient Adherence: State of the art, with a special focus on medication taking for cardiovascular disorders In: Burke LE, Ockene IS, eds. Compliance in Healthcare and Research. Armonk, NY: Futura Publishing Company, Inc.; 2001:3–21. [Google Scholar]
- 7.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–41. [DOI] [PubMed] [Google Scholar]
- 8.Chernew ME, Rosen AB, Fendrick AM. Value-based insurance design. Health Affairs 2007;26:195–203. [DOI] [PubMed] [Google Scholar]
- 9.Fendrick AM, Chernew ME. Value-based insurance design: a “clinically sensitive” approach to preserve quality of care and contain costs. The American journal of managed care 2006;12:18–20. [PubMed] [Google Scholar]
- 10.Goldman DP, Joyce GF, Escarce JJ, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA : the journal of the American Medical Association 2004;291:2344–50. [DOI] [PubMed] [Google Scholar]
- 11.Hsu J, Price M, Huang J, et al. Unintended consequences of caps on Medicare drug benefits. N Engl J Med 2006;354:2349–59. [DOI] [PubMed] [Google Scholar]
- 12.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P. Effects of a limit on Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med 1994;331:650–5. [DOI] [PubMed] [Google Scholar]
- 13.Soumerai SB, Ross-Degnan D, Avorn J, McLaughlin T, Choodnovskiy I. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med 1991;325:1072–7. [DOI] [PubMed] [Google Scholar]
- 14.Tamblyn R, Laprise R, Hanley JA, et al. Adverse Events Associated with Prescription Drug Cost-sharing Among Poor and Elderly Persons. JAMA : the journal of the American Medical Association 2001;285:421–9. [DOI] [PubMed] [Google Scholar]
- 15.Roblin DW, Platt R, Goodman MJ, et al. Effect of increased cost-sharing on oral hypoglycemic use in five managed care organizations: how much is too much? Med Care 2005;43:951–9. [DOI] [PubMed] [Google Scholar]
- 16.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. Health Economics 2007;298:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson TB, McLaughlin CG, Smith DG. A copayment increase for prescription drugs: the long-term and short-term effects on use and expenditures. Inquiry 2005;42:293–310. [DOI] [PubMed] [Google Scholar]
- 18.Trivedi AN, Moloo H, Mor V. Increased ambulatory care copayments and hospitalizations among the elderly. N Engl J Med 2010;362:320–8. [DOI] [PubMed] [Google Scholar]
- 19.Choudhry NK, Fischer MA, Avorn J, et al. At Pitney Bowes, value-based insurance design cut copayments and increased drug adherence. Health affairs (Project Hope) 2010;29:1995–2001. [DOI] [PubMed] [Google Scholar]
- 20.Gibson TB, Wang S, Kelly E, et al. A value-based insurance design program at a large company boosted medication adherence for employees with chronic illnesses. Health affairs (Project Hope) 2011;30:109–17. [DOI] [PubMed] [Google Scholar]
- 21.Gibson TB, Mahoney J, Ranghell K, Cherney BJ, McElwee N. Value-based insurance plus disease management increased medication use and produced savings. Health affairs (Project Hope) 2011;30:100–8. [DOI] [PubMed] [Google Scholar]
- 22.Maciejewski ML, Farley JF, Parker J, Wansink D. Copayment reductions generate greater medication adherence in targeted patients. Health affairs (Project Hope) 2010;29:2002–8. [DOI] [PubMed] [Google Scholar]
- 23.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Affairs 2008;27:103–12. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney JJ. Reducing patient drug acquisition costs can lower diabetes health claims. The American journal of managed care 2005;11:S170–6. [PubMed] [Google Scholar]
- 25.N KV, M K, S J, W P, RR A, J P. Prescription Copay Reduction Program for Diabetic Employees: Impact on Medication Compliance and Healthcare Costs and Utilization. American Health and Drug Benefits 2009;2:14–24. [PMC free article] [PubMed] [Google Scholar]
- 26.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica 1979;47:263–91. [Google Scholar]
- 27.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088–97. [DOI] [PubMed] [Google Scholar]
- 28.Perloff D, Grim C, Flack J, et al. Human Blood Pressure Determination by Sphygmomanometry. Circulation 1993;88:2460–70. [DOI] [PubMed] [Google Scholar]
- 29.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog Cardiovasc Nurs 2000;15:90–6. [DOI] [PubMed] [Google Scholar]
- 30.Doshi JA, Zhu J, Lee B, Kimmel S, Volpp KG. Impact of a Prescription Copayment Increase on Lipid Lowering Medication Adherence in Veterans. Circulation 2009;119:365–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley; 2002. [Google Scholar]
- 32.Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000;355:865–72. [DOI] [PubMed] [Google Scholar]
- 33.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827–38. [DOI] [PubMed] [Google Scholar]
- 34.MacMahon S, Peto R, Cutler JA, et al. Blood Pressure, Stroke, and Coronary Heart Disease Part 1: Prolonged Differences in Blood Pressure: Prospective Observational Studies Corrected for the Regression Dilution Bias. Lancet 1990;335:765–74. [DOI] [PubMed] [Google Scholar]
- 35.Hooper L, Bartlett C, Davey Smith G, Ebrahim S. Systematic review of long term effects of advice to reduce dietary salt in adults. BMJ (Clinical research ed) 2002;325:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma AM, Golay A. Effect of orlistat-induced weight loss on blood pressure and heart rate in obese patients with hypertension. Journal of hypertension 2002;20:1873–8. [DOI] [PubMed] [Google Scholar]
- 37.Fendrick AM, Smith DG, Chernew ME, Shah SN. A benefit-based copay for prescription drugs: patient contribution based on total benefits, not drug acquisition cost. The American journal of managed care 2001;7:861–7. [PubMed] [Google Scholar]
- 38.Freudenheim M Some Employers Are Offering Free Drugs. New York Times 2007. [Google Scholar]
- 39.V F. New Tack on Copays: Cutting Them. Wall Street Journal 2007. May 8. [Google Scholar]
- 40.Coffield AB, Maciosek MV, McGinnis JM, et al. Priorities among recommended clinical preventive services. Am J Prev Med 2001;21:1–9. [DOI] [PubMed] [Google Scholar]
- 41.Pauly MV, Held PJ. Benign moral hazard and the cost-effectiveness analysis of insurance coverage. J Health Econ 1990;9:447–61. [DOI] [PubMed] [Google Scholar]
- 42.Gibson TB, Ozminkowski RJ, Goetzel RZ. The effects of prescription drug cost sharing: a review of the evidence. The American journal of managed care 2005;11:730–40. [PubMed] [Google Scholar]
- 43.Rice DP. The economic impact of schizophrenia. The Journal of clinical psychiatry 1999;60 Suppl 1:4–6; discussion 28–30. [PubMed] [Google Scholar]
- 44.Lohr KN, Brook RH, Kamberg CJ, et al. Use of medical care in the Rand Health Insurance Experiment. Diagnosis- and service-specific analyses in a randomized controlled trial. Med Care 1986;24:S1–87. [PubMed] [Google Scholar]
- 45.Newhouse JP. Free for all? Lessons from the RAND health insurance experiment Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- 46.Keeler EB, Brook RH, Goldberg GA, Kamberg CJ, Newhouse JP. How free care reduced hypertension in the health insurance experiment. JAMA : the journal of the American Medical Association 1985;254:1926–31. [PubMed] [Google Scholar]
- 47.Thaler RH. Mental Accounting and Consumer Choice. Marketing Science 1985;4:199–214. [Google Scholar]
- 48.O’Donoghue T, Rabin M. Doing it now or later. American Economic Review 1999;89:103–24. [Google Scholar]
- 49.Soumerai SB, Ross-Degnan D, Fortess EE, Abelson J. A critical analysis of studies of state drug reimbursement policies: research in need of discipline. Milbank Q 1993;71:217–52. [PubMed] [Google Scholar]