Plant domestication is regarded as one of the most pivotal events in human history, as it allowed the growth and development of human civilization by providing the surplus of food needed for its expansion. Occurring independently and almost simultaneously in several parts of the world, plant domestication involved only a few species. Humans selected genetic variants of these species with favorable traits, such as increased fruit size (1). In turn, the expansion of human settlements promoted the spread of the newly domesticated crop species across the continents. This expansion was much quicker in Eurasia than in other regions, which likely contributed to the domination of the world by the cultures originating in this region. The faster spread of domesticated crops throughout Eurasia was most likely due to its predominant east–west axis: distant locations in Eurasia share similar latitudes, day lengths, and climates, and therefore, crops did not require additional genetic changes to adapt to new places (1). By contrast, large changes in latitude, day length, and climate are associated with the spread of plants in the Americas or Africa, where north–south axes predominate. Therefore, adaptation to new locations in these continents did require additional genetic changes that adjusted the growth and development of the plants to the different daily and seasonal environmental cycles that result in contrasting climates. In PNAS, Müller et al. (2) report that the expansion of the area of tomato (Solanum lycopersicum) cultivation from Central America to Europe and North America involved the selection of genetic variants associated with a large deletion within the clock gene LNK2, which synchronizes internal rhythms with the external light/dark cycle and, therefore, adjusts the timing of multiple biological processes in response to the long-day characteristics of high latitudinal regions.
Most organisms possess endogenous timing devices, known as circadian clocks, which align internal rhythms in physiology with periodic changes in the environment. This alignment not only is crucial for human health, as is clear from the jet lag experienced after transmeridian flights (3), but also enhances fitness and survival in plants (4). Synchronization of endogenous rhythms with the environment is achieved through entraining these internal clocks by daily light/dark transitions, and/or changes in light intensity, a phenomenon that is mediated by specific photoreceptors. Circadian clocks are also key components of the mechanism that contributes to measuring day length and adjusting the timing of key developmental transitions to the most appropriate seasons of the year (5).
Therefore, it is not surprising that natural variations in clock-associated genes played a critical role in enabling the cultivation of domesticated plants far away from their original places of domestication. So far, most examples of clock-associated gene variants affecting latitudinal adaptation of crop plants were associated with the regulation of seasonal rhythms in key developmental transitions, such as flowering time or tuberization, rather than with the fine-tuning of daily rhythms in common physiological processes (6). Evidence for the adaptive importance of circadian rhythms and their synchrony with environmental cycles is provided by the observation that some plant species, including the model plant Arabidopsis thaliana, grow well under constant light and temperature conditions, whereas others, such as tomato, fail to thrive under prolonged periods of continuous light (7). This observation, together with additional experimental data, has provided strong support for the idea that the damage caused by continuous light exposure in tomato is, at least in part, due to circadian asynchrony, that is, a misalignment of the timing of internal physiological processes in relation to diurnal changes in environmental conditions (7). If this is indeed the case, one would expect humans to have selected genetic variants that adjust the timing of key biological processes in relation to the longer days of the European summers, compared with the shorter days experienced year-round in Central America. Tomato plants are also an excellent model organism to study the adjustment of daily, rather than seasonal, rhythms to the different photoperiods present along latitudinal gradients, as the floral transition in this species is not affected by day length.
Two years ago, the group of José M. Jiménez-Gómez showed that domestication of tomato was associated with the selection of variants with both a delayed phase of circadian rhythms and a slower clock. The locus responsible for the delayed-phase phenotype was identified; domesticated tomatoes carry a single-amino acid substitution in the EID1 protein, the Arabidopsis homolog of which has been shown to play a role in light signaling through the phytochrome family of photoreceptors (8). The same group now reports the identification and characterization of the locus conferring the long-period phenotype, which encodes an ortholog of the Arabidopsis night light-inducible and clock-regulated 2 (LNK2) gene (2), previously shown to be a key component of the light signaling pathway that modulates clock function (9). Thus, two genes manipulated during tomato domestication are involved in light signaling, consistent with the fact that light perceived by different photoreceptors entrains the clock and controls both its phase and period.
To identify the causative gene for the long-period phenotype, the authors first used a classical mapping approach, which allowed them to narrow down the region containing the candidate quantitative trait locus to a genomic region containing 245 annotated genes. However, no obvious candidates were identified. The authors then considered the possibility that the causative gene may be present only in wild species and was lost during domestication. With this idea in mind, they screened for genes that appeared to be deleted in tomato compared with its wild ancestors (Solanum pennellii and Solanum pimpinellifolium), and focused on one candidate located within the genomic interval, which turned out to be a truncated version of an ortholog of Arabidopsis LNK2. The authors present several lines of evidence that LNK2 was the causative gene variant associated with the long-clock period phenotype. First, transforming cultivated tomatoes with the complete LNK2 coding sequence from the wild ancestor S. pimipinellifolium, but not with a truncated version, shortened the circadian period. Second, the LNK2 deletion occurs only within S. lycopersicum; indeed, mutations in both EID1 and LNK2 were absent in wild species and appeared first in the most ancient domesticated tomatoes. Third, the frequency of the lnk2 mutation increased in a linear manner and only became fixed in modern cultivars, which originated after tomato was brought to Europe. Finally, positive selection signatures were present in the genomic region surrounding this gene.
In Arabidopsis, the four members of the LNK gene family are induced by light treatments that reset the clock at times of the day when the clock is most sensitive to light (9). LNK2 and its paralog LNK1 are expressed in the morning and promote the expression of clock genes such as PRR5 and ELF4 in the afternoon, acting as molecular bridges between the clock-regulated DNA binding proteins of the RVE family and RNA pol II (10, 11). Interestingly, lnk2 mutants in Arabidopsis exhibit a long-period phenotype (9, 10). Supporting the idea that domestication influenced light signaling pathways to the clock, tomato phyB1 mutants show an advanced phase and a shortened period phenotype, as well as down-regulation of the orthologs of PRR5 and ELF4 at dusk. Furthermore, the phyB1 mutation in tomato is epistatic to the eid1 and lnk2 alleles, whose phenotypes are only evident under continuous-light conditions. Whereas LNK2 and EID1 affect clock function independently in tomato, the activity of both genes depends on light signals perceived by phytochrome B1 (2). Taken together, these results support the idea that gene variants that altered light input to the clock and thus delayed its pace were selected during domestication, possibly so that the timing of daily internal rhythms could better match the long-day conditions present at elevated latitudes.
Although the possibility of this mutation being selected because of random demography-associated effects cannot be excluded, the increase in allele frequency, together with the genomic signatures of selection, strongly suggests that the deletion in LNK2, and the resulting lengthening of circadian period, have been targeted by selection during tomato domestication. However, some details suggest that other processes might also be at work. For example, the phase-shifting mutation in EID1 appears to have been fixed in landraces around the equator (2, 8), where the argument for latitudinal adaptation is weaker. A certain delay in the clock pace may have been initially selected for its effects on phenotypic outputs not necessarily related to adaptation to higher latitudes, such as branching patterns or carbohydrate allocation to fruits, or as local adaptations to particular agricultural landscapes around the equator, which in that area of western South America range, in relatively short distances, from coastal desert oases to high-altitude humid valleys (12). Intriguingly, the clock period in S. pimpinellifolium accessions is also longer than in other sympatric species (Fig. 1B). Both S. pimpinellifolium and the so-called wild varieties of tomato (S. lycopersicum var. cerasiforme) behave as weeds and are associated with locally disturbed agricultural landscapes. For many wild species, the adaptation to agricultural settings and/or human activities should not be overlooked. One hypothesis states that tomatoes spread from the equator to central Mexico, where they entered a defined phase of domestication and selection for bigger fruits, as feral companions of farmed plots (13). Even Arabidopsis may have followed the disturbed fields of neolithic farmers in their northbound expansion from southern Europe. Although neither EID1 nor LNK2 show signs of selection in S. pimpinellifolium, variants in other genes might still have been selected that enabled the appearance of the eid1 or lnk2 mutations. The introgression lines and local varieties developed and analyzed in these studies should provide a bountiful supply of genetic tools to test the adaptive contribution of clock differences in local settings.
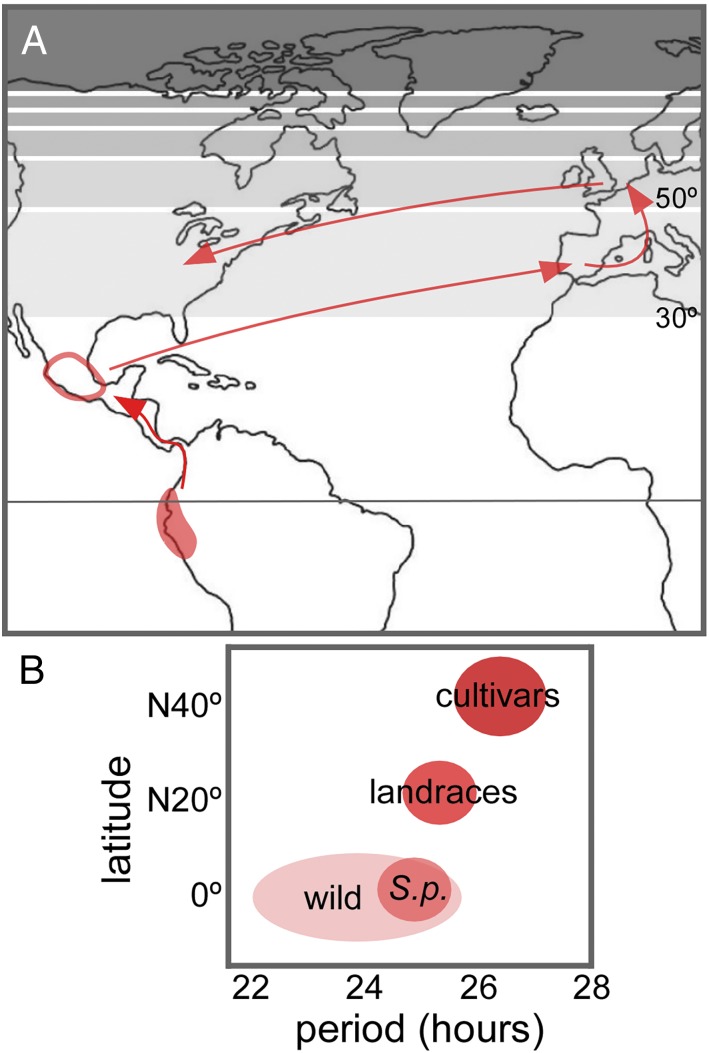
Fig. 1.
Latitudinal wanderings of tomatoes. (A) Tomato suffered a mild process of domestication in its center of origin (filled area around the equator) but was later domesticated and cultivated in central Mexico. Starting from the mid-16th century, it started to be grown in Europe, as a curiosity by herbalists and as a dietary complement in the Mediterranean area, to be later cultivated in higher latitudes in Europe. Most modern varieties are the result of breeding programs during the late 19th and 20th centuries both in Europe and North America. The shaded bands in the map represent 2-h latitudinal differences in day length, from about 12 h near the equator to permanent light in the North Pole, as observed in the summer solstice. (B) A progressive lengthening of clock period (and phase, not shown) is evident in tomato. S.p., Solanum pimpinellifolium.
The findings by Müller et al. not only highlight the power of selection in domesticated crops but also provide some clues as to how to further improve crop productivity across latitudes, at least in species such as tomato and potato (Solanum tuberosum) where circadian asynchrony is detrimental. Future work should examine whether fully operative LNK variants would increase tomato productivity in the tropics, whether the circadian clock can be manipulated through other gene variants to further increase productivity, and whether changes in LNK function in other crops, such as potato, would also contribute to increased productivity at high latitudes.
Footnotes
The authors declare no conflict of interest.
See companion article on page 7135.
References
- 1.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418:700–707. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- 2.Müller NA, Zhang L, Koornneef M, Jiménez-Gómez JM. Mutations in EID1 and LNK2 caused light-conditional clock deceleration during tomato domestication. Proc Natl Acad Sci USA. 2018;115:7135–7140. doi: 10.1073/pnas.1801862115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354:994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- 4.Yerushalmi S, Green RM. Evidence for the adaptive significance of circadian rhythms. Ecol Lett. 2009;12:970–981. doi: 10.1111/j.1461-0248.2009.01343.x. [DOI] [PubMed] [Google Scholar]
- 5.Yanovsky MJ, Kay SA. Living by the calendar: How plants know when to flower. Nat Rev Mol Cell Biol. 2003;4:265–275. doi: 10.1038/nrm1077. [DOI] [PubMed] [Google Scholar]
- 6.Nakamichi N. Adaptation to the local environment by modifications of the photoperiod response in crops. Plant Cell Physiol. 2015;56:594–604. doi: 10.1093/pcp/pcu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velez-Ramirez AI, van Ieperen W, Vreugdenhil D, Millenaar FF. Plants under continuous light. Trends Plant Sci. 2011;16:310–318. doi: 10.1016/j.tplants.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Müller NA, et al. Domestication selected for deceleration of the circadian clock in cultivated tomato. Nat Genet. 2016;48:89–93. doi: 10.1038/ng.3447. [DOI] [PubMed] [Google Scholar]
- 9.Rugnone ML, et al. LNK genes integrate light and clock signaling networks at the core of the Arabidopsis oscillator. Proc Natl Acad Sci USA. 2013;110:12120–12125. doi: 10.1073/pnas.1302170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Gil S, Grasser KD, Mas P. Targeted recruitment of the basal transcriptional machinery by LNK clock components controls the circadian rhythms of nascent RNAs in Arabidopsis. Plant Cell. 2018;30:907–924. doi: 10.1105/tpc.18.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Q, et al. LNK1 and LNK2 are transcriptional coactivators in the Arabidopsis circadian oscillator. Plant Cell. 2014;26:2843–2857. doi: 10.1105/tpc.114.126573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakazato T, Housworth EA. Spatial genetics of wild tomato species reveals roles of the Andean geography on demographic history. Am J Bot. 2011;98:88–98. doi: 10.3732/ajb.1000272. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins JA. The origin of the cultivated tomato. Econ Bot. 1948;2:379–392. [Google Scholar]