Significance
Tobacco smoke (TS) contains numerous carcinogens. Intriguingly, while TS itself is a weak carcinogen in animal models, many of the TS components, such as 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) and polycyclic aromatic hydrocarbons (PAHs), are strong carcinogens. We found that TS induces mainly aldehyde-DNA adducts in mice and humans. TS reduces DNA repair activity and repair proteins in mouse lung. All of these TS-induced effects can be reduced by diet polyphenols. Aldehydes prevent PAHs and NNK from inducing DNA damage in human cells. We propose that, because they act to damage DNA, reduce DNA repair activity, and inhibit NNK and PAHs from becoming DNA-damaging agents, aldehydes are the major TS carcinogens. These insights allow for better TS cancer risk assessment and the design of effective preventive measures.
Keywords: tobacco smoke carcinogenesis, aldehydes, DNA damage, DNA repair, polyphenols
Abstract
Tobacco smoke (TS) contains numerous cancer-causing agents, with polycyclic aromatic hydrocarbons (PAHs) and nitrosamines being most frequently cited as the major TS human cancer agents. Many lines of evidence seriously question this conclusion. To resolve this issue, we determined DNA adducts induced by the three major TS carcinogens: benzo(a)pyrene (BP), 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanoe (NNK), and aldehydes in humans and mice. In mice, TS induces abundant aldehyde-induced γ-hydroxy-propano-deoxyguanosine (γ-OH-PdG) and α-methyl-γ-OH-PdG adducts in the lung and bladder, but not in the heart and liver. TS does not induce the BP- and NNK-DNA adducts in lung, heart, liver, and bladder. TS also reduces DNA repair activity and the abundance of repair proteins, XPC and OGG1/2, in lung tissues. These TS effects were greatly reduced by diet with polyphenols. We found that γ-OH-PdG and α-methyl-γ-OH-PdG are the major adducts formed in tobacco smokers’ buccal cells as well as the normal lung tissues of tobacco-smoking lung cancer patients, but not in lung tissues of nonsmokers. However, the levels of BP- and NNK-DNA adducts are the same in lung tissues of smokers and nonsmokers. We found that while BP and NNK can induce BPDE-dG and O6-methyl-dG adducts in human lung and bladder epithelial cells, these inductions can be inhibited by acrolein. Acrolein also can reduce DNA repair activity and repair proteins. We propose a TS carcinogenesis paradigm. Aldehydes are major TS carcinogens exerting dominant effect: Aldehydes induce mutagenic PdG adducts, impair DNA repair functions, and inhibit many procarcinogens in TS from becoming DNA-damaging agents.
Tobacco smoke (TS) is the major cause of human cancer. More than 80% of lung cancers and 50% of bladder cancers are TS related (1). Recently, it has been found that both lung and bladder tumors carry numerous mutations, including those in oncogenes and tumor suppressor genes, indicating that TS induces mutagenic DNA damage in lung and bladder cells (2, 3). Indeed, it has been found that TS contains more than 60 documented cancer-causing agents that can induce DNA damage and mutations in human cells (4, 5). However, the identity of and cancer-causing mechanisms of the TS agents responsible for the induction of lung and bladder cancer remain controversial (6–8). Numerous studies have linked polycyclic aromatic hydrocarbons (PAHs), aromatic amines, and nitrosamines to human cancers, including lung and bladder cancer, and indeed, many of the PAHs and nitrosamines in TS per cigarette (cig) are potent carcinogens in animal models (9–12). However, the amounts of PAHs (up to 2 µg) and nitrosamines (up to 0.2 µg) in TS are relatively minute (13, 14). The TS lung carcinogenicity of PAHs has also been seriously challenged by the findings that the so-called TS-induced DNA adducts detected in the thin-layer chromatography (TLC) diagonal zone are, in fact, aldehyde-induced DNA adducts, rather than PAHs-related adducts (15). It is worth noting that the amount of aldehydes including acrolein (Acr) in TS is more than a 1,000 times greater than those of PAHs and nitrosamines (15–17). Furthermore, it has been found that, in human bronchial epithelial cells, the distribution of DNA damage in the p53 gene induced by both the TS Acr and PAHs coincides with the p53 mutation spectrum in lung cancer (8).
Although TS nitrosamines such as 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) and N′-nitrosonornicotine (NNN) are potent carcinogens in animal models that can induce cancer in different tissues including lung tissue, these nitrosamines induce mainly G-to-A transition mutations (18, 19), whereas the major mutations observed in TS-related human lung cancer are G-to-T mutations (20, 21).
These results raise the possibility that the role of various carcinogens in TS may be different from that of the individual carcinogens in isolation because of the effects of the over 6,000 TS chemicals on each other’s absorption, metabolism, and deposition. It is also likely that components in TS may interact with each other, resulting in attenuating or enhancing their carcinogenic potentials. If this is the case, then, to understand the carcinogenic mechanisms of TS, assess cancer risk of TS, and design effective TS-related cancer prevention measures, investigations should be focused on the determination of DNA damage, mutagenicity, and carcinogenicity of total TS, rather than on the individual carcinogens found in TS.
To address these important questions, we have determined the major types of DNA adducts in different organs of mice exposed to mainstream TS (MTS). Specifically, we quantified cyclic 1,N2-propano-dG (PdG), benzo(a)pyrene diol epoxide (BPDE)-dG, and O6-methyl-dG (O6-medG) adducts induced by three major DNA-damaging carcinogens in TS—namely, aldehydes, benzo(a)pyrene (BP), and NNK (22–24). We also determined the effects of MTS on DNA repair capacity and DNA repair protein expression in lung tissues. We found that Acr-derived γ-hydroxy-1,N2-PdG (γ-OH-PdG) is the major type of adduct, and acetaldehyde (Acet)- and crotonaldehyde (Cro)-derived (6R,8R)-α-methyl-γ-hydroxy-PdG (α-meth-γ-OH-PdG) is the minor adduct in lung. Only γ-OH-PdG is formed in bladder, and these PdG adducts do not form in heart or liver. TS does not enhance BPDE-dG or O6-medG DNA adduct formation in any of these organs, even though the amount of these compounds found in TS when isolated is sufficient to induce DNA damage in mice (25–28). MTS inhibits both nucleotide and base excision repair (NER and BER) and reduces the levels of the DNA repair proteins XPC and OGG1/2. We found that diet Polyphenon E (PPE) could prevent these TS effects: induction of aldehyde-DNA adducts, inhibition of DNA repair, and reduction of DNA repair proteins. Significantly, we found that γ-OH-PdG and α-meth-γ-OH-PdG, but not BPDE-dG and O6-medG, are the two major adducts formed in buccal cells and lung tissues of tobacco smokers. We found that Acr can prevent BP and NNK from becoming DNA-damaging agents in human lung and bladder epithelial cells. Based on these results, we propose that aldehydes, rather than PAHs and nitrosamines, are the major TS carcinogens and that aldehyde carcinogenic effects can be effectively prevented by diet polyphenols.
Results
MTS Induces PdG in Lung and Bladder in Mice.
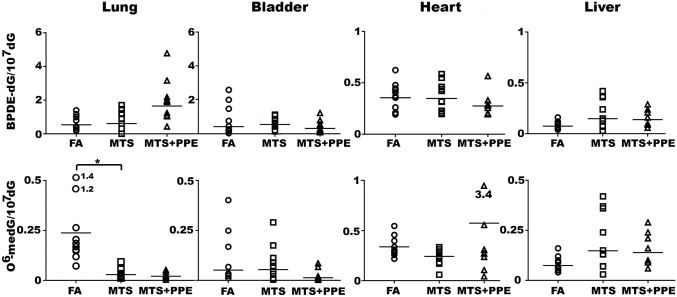
The carcinogenicities of aldehydes, PAHs, and nitrosamines have been well established (28–30), and results from cultured cell studies clearly demonstrate that TS aldehydes, such as Acr, Acet, and Cro, as well as metabolically activated PAHs and nitrosamines, can induce mutagenic DNA damage (8, 31, 32). Therefore, a crucial factor determining the roles of these different carcinogens in TS-induced human cancer is their efficiency in inducing DNA damage in different tissues of animals and humans exposed to TS. To address this question, we measured PdG, BPDE-dG, and O6-medG adducts in lung, bladder mucosa, heart, and liver tissues of mice exposed to TS. Mice were exposed to MTS at the level of ∼75 mg/m3 for 12 wk, which is equivalent to the TS exposure of a habitual smoker with 40 pack-year history. PdG adducts were first measured by an immunochemical method, using a monoclonal antibody against PdG adducts (33–35). The results in Fig. 1 A and B show that MTS induced significant levels of PdG adducts in lung (P < 0.0001) and bladder (P < 0.0001) tissues, but not in heart or liver tissues. The PdG adducts formed in lung and bladder tissues were further analyzed by a 32P-postlabeling and 2D-TLC/HPLC method (34, 35). The results in Fig. 1 C and D show that two types of PdG adducts, γ-OH-PdG and α-meth-γ-OH-PdG, were formed in lung tissue and that the level of γ-OH-PdG adducts was eightfold higher than the level of α-meth-γ-OH-PdG adducts. In contrast, only γ-OH-PdG adducts were formed in bladder tissue.
Fig. 1.
Mainstream tobacco smoke (MTS) induces γ-OH-PdG and α-meth-γ-OH-PdG in lung and γ-OH-PdG in bladder, but not in heart and liver. Polyphenol E (PPE) can prevent this TS effect. Two groups of mice (20 mice per group) were fed a control diet or diet containing 0.1% PPE. Ten mice from each group were either exposed to filtered air (FA) or MTS for 12 wk, as described in the text. Genomic DNAs from lung, heart, liver, and bladder tissues were prepared as described (34). PdG formation in these tissues was analyzed by an immunochemical method using a monoclonal antibody against PdG and quantum dot-labeled secondary antibody, as previously described (33). Each sample was measured two to four times. Typical slot blot hybridization results (Upper, antibody reaction; Lower, input DNA) are shown in A, and the quantification results are shown in B. Lines represent the geometric average values. ****P < 0.0001, ***P < 0.001, and *P < 0.05. PdG adduct formation in the lung and bladder tissues was further analyzed by a 32P-postlabeling 2D-TLC/HPLC method, as previously described (34). (C) Typical 2D-TLC chromatographic autoradiograms. (D) The spots circled in C were extracted and further analyzed by an HPLC method (34). The elution positions of the standard γ-OH-PdG adduct and the α-meth-γ-OH-PdG adducts are indicated by the arrows. Note: MTS induced γ-OHPdG and α-meth-γ-OH-PdG in lung and γ-OH-PdG in bladder tissues, but not in heart and liver tissues. PPE prevented both types of DNA adduct formation in lung and bladder tissues in mice exposed to MTS, but PPE did not affect PdG adduct formation in lung and bladder tissues of the control mice. FA, mice exposed to FA; MTS, mice exposed to MTS; MTS + PPE, mice fed with diet with PPE and exposed to MTS; Std, standard DNA with different PdG levels.
Since TS contains up to 500 μg per cig Acr, 40–50 μg per cig Cro, and up to 2,000 μg per cig Acet (16, 27), and while Acr induces γ-OH-PdG, both Acet and Cro can induce different isoforms of α-meth-γ-OH-PdG adducts (33, 36), it is puzzling as to why TS induces a higher level of γ-OH-PdG adducts than α-meth-γ-OH-PdG adducts in lung and only γ-OH-PdG in bladder. One possibility could be that, due to the volatility of Acet, with boiling point (16 °C) below ambient temperature, it is likely that the majority of Acet in MTS may not be inhaled as efficiently as Acr by mice during the whole-body exposure. We found that Acr is more efficient at inducing γ-OH-PdG adducts than Cro is in inducing α-meth-γ-OH-PdG adducts in lung epithelial cells (SI Appendix, Fig. S1). Taken together, these two factors may contribute to the lower levels of α-meth-γ-OH-PdG adducts than γ-OH-PdG adducts formed in lung tissues and bladder mucosa of mice exposed to TS.
MTS Exposure Does Not Significantly Induce BPDE-dG and O6-medG in Lung and Bladder.
We measured BPDE-dG adducts using an immunochemical method and the 3D-TLC method, and we quantified O6-medG adducts through both an immunochemical method and HPLC analysis (33, 34, 37, 38). The results in Fig. 2 show that MTS does not induce BPDE-dG and O6-medG significantly in lung, heart, liver, or bladder tissues. It is worth noting that the basal levels of BPDE-dG and O6-medG adducts detected in lung, bladder, liver, and heart are 20- to 100-fold lower than the levels of PdG adducts in these organs (Fig. 1B vs. Fig. 2).
Fig. 2.
Mainstream tobacco smoke (MTS) does not induce BPDE-dG and O6-meth-dG formation in lung, bladder, heart, or liver tissues. The same genomic DNAs isolated from different organs of mice exposed to MTS and fed a diet with and without PPE, as described in Fig. 1, were used for BPDE-dG and O6-medG adduct detection using an immunochemical method (35, 67). For simplicity, only the quantitation of DNA adduct levels is shown. Abbreviations are the same as in Fig. 1. The typical slot blot hybridization results are shown in SI Appendix, Fig. S4 A and B. Note: MTS did not induce a significant difference in BPDE-dG and O6-medG adduct formation in lung, bladder, heart, or liver tissues of mice compared with the FA group. PPE did not affect BPDE-dG and O6-medG adducts formation in these organs. MTS exposure significantly reduces the background O6-medG level in lung (FA vs. MTS, P = 0.044).
MTS Inhibits DNA Repair Function in Mouse Lung Tissue.
Previously, we have found that Acr, Cro, and Acet not only can damage genomic DNA but also can modify repair proteins causing repair dysfunction in cultured lung and bladder epithelial cells (8, 33, 35). Since MTS contains an abundance of these aldehydes, it is possible that MTS may inhibit DNA repair function. To test this possibility, using the in vitro DNA-dependent repair synthesis assay, we measured the repair activity in cell-free cell lysates from lung and liver of mice exposed to MTS (8, 33, 35). The results in Fig. 3 A and B show that both NER and BER activity in lung tissues of MTS-exposed mice were much lower than in lung tissues of control mice. In contrast, the results in SI Appendix, Fig. S2 A and B show no significant differences in NER and BER repair activity in liver tissues between mice with and without MTS exposure. Due to limited amounts of tissue, we were unable to similarly determine DNA repair activity in bladder mucosa.
Fig. 3.
Mainstream tobacco smoke (MTS) causes a reduction of DNA repair capacity and levels of repair proteins, XPC, and OGG1 in lung tissues. Polyphenon E (PPE) can prevent these TS effects. Mice fed control diet and diet with PPE were exposed to filtered air (FA) and MTS, as described in Fig. 1. Cell-free cell lysates of lung tissues from these mice were prepared. The nucleotide excision repair (NER) and base excision repair (BER) capacities of the cell lysates were determined by methods previously described (8, 34, 78). (A) Typical autoradiograms (Lower panels), DNA staining (Upper panels) of the electrophoresed gels, and relative repair capacity for individual mouse (Bottom). (B) Quantitation of relative NER and BER activity. (C) The XPC and OGG1 proteins were detected by Western blots (Upper), and the relative protein levels were quantified (Lower). Abbreviations are the same as in Figs. 1 and 2. ****P < 0.0001, ***P < 0.001, and **P < 0.01.
MTS Causes a Reduction of DNA Repair Proteins XPC and OGG1/2 in Mouse Lung Tissue.
Previously, we have found that Acr, Cro, and Acet can modify DNA repair proteins such as XPC, hOGG1/2, MLH1, and PMS2, and that the modified DNA repair proteins are degraded via an autophagosome pathway in cultured lung and bladder epithelial cells (33, 35). These findings raised the possibility that the reduction of NER and BER activity in lung tissues of TS-exposed mice is due to reduction of repair proteins caused by TS aldehydes. To test this possibility, we measured XPC and OGG1/2 levels in lung tissues of mice with and without MTS exposure. XPC is a major NER protein for repair of bulky DNA adducts, such as UV-induced photodimers, BPDE-dG, and PdG adducts, in genomic DNA (39); OGG1/2 is a major enzyme for repair of 8-oxo-dG DNA damage (40). The results in Fig. 3C show that MTS exposure caused a significant reduction of XPC and OGG1/2 protein levels in lung tissues. However, MTS exposure did not affect XPC and OGG1/2 levels in liver tissues (SI Appendix, Fig. S2 C and D). These results indicate that either TS aldehydes do not enter into liver cells and/or liver cells have the capacity to inactivate MTS aldehydes.
PPE Prevents MTS-Induced PdG Formation and DNA Repair Inhibition in Lung and PdG Formation in Bladder Tissues.
The results presented above indicate that aldehydes in MTS, mainly Acr, induce PdG adducts in lung and bladder tissues in vivo. MTS also inhibits NER and BER capacity in lung tissues, and this effect is most likely due to MTS aldehydes. We propose that these two outcomes of MTS exposure contribute to lung and bladder carcinogenesis. If this is correct, then it should be possible to prevent TS-induced lung and bladder cancer by neutralizing sufficient amount of TS aldehydes in vivo. It has long been recognized that the carbonyl group and the olefinic bond in Acr are the active moieties that can interact with DNA and proteins to form DNA and protein adducts (41, 42). These reactions can be prevented by numerous antioxidants and reducing agents with molecules that have sulfhydryl groups (43–45). We found that PPE and PP-60, polyphenols from tea extracts, which are potent antioxidants and appropriate for human consumption, can effectively prevent Acr-induced DNA adduct formation in cultured lung epithelial cells and bladder epithelial cells (SI Appendix, Fig. S3 A, C, D, and F). The results in SI Appendix, Fig. S3 B, C, E, and F show that PPE and PP-60 can also considerably reduce the inhibitory effects of Acr on DNA repair in cultured lung epithelial and urothelial cells.
Armed with these encouraging results, we then determined the effect of diet PPE on TS-induced PdG formation and DNA repair inhibition in mouse models. Four groups of mice, each with 10 mice, were fed with control diet (AIN93M) or diet containing 0.1% PPE (NIA93M). These mice were exposed to either MTS or filtered air (FA) for 12 wk, the same exposure protocol as described in Fig. 1. The PdG formation (Fig. 1) in lung and bladder tissue and DNA repair activity in lung tissue (Fig. 3 A and B) were determined. The results were compared with the groups that were fed the control diet without PPE but subjected to the same MTS exposure. The results in Fig. 1 show that PdG levels in lung tissues of MTS-exposed mice fed with PPE (MTS + PPE) were significantly lower than in MTS-exposed mice on control diets (MTS) (P < 0.001). PdG levels in bladder tissues of MTS-exposed mice fed with PPE were also significantly lower than in MTS-exposed mice on control diet (P = 0.0423). These results indicate that PPE can neutralize MTS effects on PdG induction in lung and bladder tissues. SI Appendix, Fig. S4C shows that there was no significant difference in PdG levels in lung tissue of mice fed with control diet and PPE-enriched diet without MTS exposure, indicating that PPE does not induce PdG adducts in lung and bladder tissues in mice.
We further tested whether or not PPE can prevent the MTS-caused reduction DNA repair activity and of XPC and OGG1/2 expression. The results in Fig. 3 A and B show that PPE can also prevent MTS-induced inhibition of both NER and BER activity in lung tissue. The results in Fig. 3C show that, whereas the levels of XPC and OGG1/2 are significantly lower in lung tissues of MTS-exposed mice than in FA mice (MTS vs. FA), the levels of these two proteins in the lung tissue of MTS-exposed mice fed with the diet containing PPE showed no significant difference compared with mice fed the control diet without MTS exposure [(MTS + PPE) vs. FA]. Together, these results indicate that the PPE prevention of TS-induced DNA repair inhibition is through neutralizing TS’s effects on the reduction of XPC and OGG1/2, the two crucial factors for NER and BER.
α-Meth-γ-OH-PdG and γ-OH-PdG Adducts Are the Major DNA Damage Detected in Buccal Cells and Lung Tissues of Smokers.
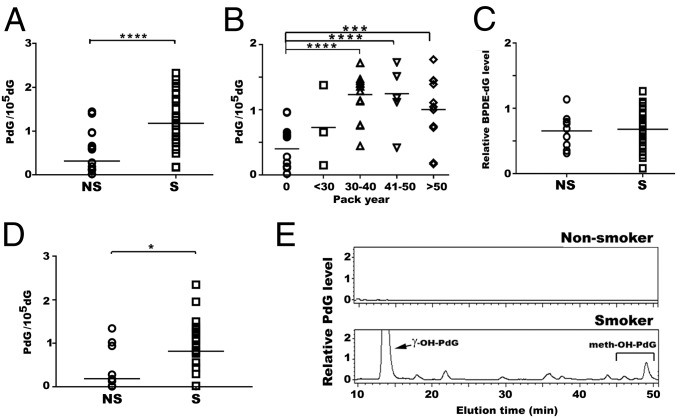
The aforementioned results demonstrate that, in a mouse model, aldehyde-derived γ-OH-PdG and α-meth-γ-OH-PdG adducts, rather than commonly believed PAH- and nitrosamine-derived DNA adducts such as BPDE-dG and O6-medG, are the major MTS-induced DNA adducts in the lung and bladder in vivo, and that MTS also inhibits DNA repair and reduces the levels of DNA repair proteins. These results raise an important question: Does TS induce these effects in human lungs in the same manner as it does in the mouse model? Simply put, does TS induce PdG rather than BPDE-dG and O6-medG adducts in human lung tissue? An insurmountable hurdle in addressing this question is the inability to obtain lung tissue, bronchoalveolar lavage, or bronchial brushing from smokers with different tobacco smoking consumption in a representative population with appropriate nonsmoking controls. We chose to use buccal cells and sputum as surrogates for lung cells. Buccal cells are the first line of cells to encounter TS exposure and have similar molecular responses to TS as airway epithelial cells and bronchial epithelial cells. Numerous studies have established a positive relationship between TS and cytological and molecular markers in lung cancer (46), and buccal cells have also been used for the early diagnosis of oral and lung cancer (47–49). Sputum consists of bronchial epithelial cells and macrophages in the lung (48). DNA adduct levels obtained from these two types of samples may thus reflect TS effects in different regions of the airway. Furthermore, a sufficient amount of buccal cells and sputum for analyzing DNA adduct formation is obtainable via oral brushing and sputum induction, which are both relatively minor procedures. Using the same immunochemical method and 32P-postlabeling and 2D-TLC/HPLC method as described above, we determined the PdG and BPDE-dG formation in buccal cells of individuals with different smoking and nonsmoking histories. The results in Fig. 4 show that (i) the levels of BPDE-dG adducts in buccal cells from smokers (S) and nonsmokers (NS) show no significant differences (Fig. 4C); (ii) the levels of PdG adducts in buccal cells were significantly higher in smokers (S) than in nonsmokers (NS) (Fig. 4A, NS vs. S, P < 0.0001) and are related to 40 smoking pack-year (Fig. 4B, 0 vs. 30–40, P < 0.0001; 0 vs. 41–50, P < 0.0001; 0 vs. >50, P = 0.0007); and (iii) γ-OH-PdG and α-meth-γ-OH-PdG adducts were the two major types of PdG adducts detected in buccal cells from smokers (Fig. 4E). The results also show that the levels of PdG adducts in sputum were significantly higher in smokers than in nonsmokers (Fig. 4D, NS vs. S, P = 0.0193). In summary, these results indicate that aldehydes are the major TS agents that cause DNA damage in buccal cells and lung tissues of smokers.
Fig. 4.
The levels of γ-OH-PdG and α-meth- γ-OH-PdG adducts, but not BPDEdG, were higher in buccal cells of tobacco smokers than nonsmokers. γ-OHPdG, α-meth-γ-OH-PdG, and BPDE-dG in buccal cells of nonsmokers (NS) (n = 17) and smokers (S) (n = 33) with different smoking history (pack-year) were determined by methods described in Figs. 1 and 2. (A) The PdG adducts was detected by the immunochemical methods. (B) The PdG levels in individuals with different smoking history. (C) Relative levels of BPDE-dG in smokers vs. nonsmokers. The PdG adducts in sputum samples of nonsmokers (NS) (n = 8) and smokers (S) (n = 22) with different smoking history were detected by the immunochemical method as described in Fig. 1, and the results were shown in D. (E) HPLC profiles of PdG adduct formed in buccal cells. Genomic DNA from buccal cells of smokers and nonsmokers were pooled for 32P-postlabeling and 2D-TLC/HPLC analysis as in Fig. 1. Note: (i) Due to the limited number of sputum samples collected, the PdG adducts were not analyzed by 32P-postlabeling, 2D-TLC/HPLC. (ii) The ratio of γ-OH-PdG to α-meth-γ-OHPdG detected in buccal cells of smokers is similar to that detected in lung tissues of mice exposed to MTS (8-9:1). ****P < 0.0001, ***P < 0.001, and *P < 0.05.
We also determined the DNA adduct formed in noncancerous lung tissues obtained from lung lobectomy of lung cancer patients of tobacco smokers (n = 41) and lung tissues from nonsmokers (n = 13). The results in Fig. 5 show that the levels of γ-OH-PdG and α-meth-γ-OH-PdG adducts in lung tissues of smokers are significantly higher than in nonsmokers, and that the levels of α-meth-γ-OH-PdG are higher than γ-OH-PdG. However, the levels of BPDE-dG and O6-medG are similar in the lung tissues of smokers and nonsmokers. It is worth noting that the basal levels of BPDE-dG and O6-medG are significantly lower than the levels of γ-OH-PdG and α-meth-γ-OH-PdG in lung tissues of both smokers and nonsmokers. Similar results were observed in TS-exposed mice (Figs. 1 and 2). These results indicate that TS induces mainly γ-OH-PdG and α-meth-γ-OH-PdG in smokers’ lung tissues.
Fig. 5.
Quantitations of α-OH-PdG, γ-OH-PdG, α-meth-γ-OH-PdG, BPDEdG, and O6-medG adduct in human lung tissues. Lung tissue samples were obtained from 13 nonlung cancer patients of nonsmokers (N) and 41 lung cancer patients who were smokers (S). Only nontumor lung tissue samples from smoker lung patients were used for DNA adduct detection. Methods for DNA adduct detection are the same as described in Figs. 1, 2, and 4.
Acr Exerts Dominant and Inhibitory Effect on BP- and NNK-DNA Adduct Induction in Lung and Bladder Epithelial Cells.
Lung and bladder epithelial cells contain a variety of cytochrome p450s (CYPs) that can metabolically activate PAHs, including BP, into epoxide forms that can effectively adduct DNA (11, 50–52). CYPs in lung and urothelial cells can also metabolize nitrosamines into metabolites that can spontaneously degrade into pyridyl-butanoic acid derivatives, formaldehyde, as well as methyldiazohydroxide, which can methylate DNA (11, 53). Why then did MTS not induce BPDE-dG and O6-medG adducts in the lung tissue and bladder mucosa of mice and in human lung tissue? Aldehydes such as Acr, Cro, and Acet can cause protein dysfunction by modifying the proteins (50–52). Perhaps TS aldehydes inhibit the activation of BP and nitrosamines via modification of CYP proteins. To test this possibility, we determined the effect of Acr exposure on BP- and NNK-induced DNA adduct formation in human lung epithelial and urothelial cells. The results in Fig. 6 show that, by themselves, BP can induce BPDE-dG and NNK can induce PdG and O6-medG adducts. However, in the presence of Acr, the ability of BP to induce BPDE-dG adducts and the ability of NNK to induce O6-medG adducts were greatly reduced. In fact, only PdG adducts were observed in cells treated with the combination of BP and Acr (Fig. 6 C, D, G, and H). These results indicate that Acr can inhibit BP and NNK from becoming DNA-damaging agents. Therefore, our results suggest that the most likely reason MTS does not induce BPDE-dG and O6-medG adducts in lung and bladder tissues of exposed mice and in human lung tissues is that, in these tissues, Acr in MTS inhibits the CYP enzymes, which are necessary for the activation of PAHs and nitrosamines to become DNA-damaging agents.
Fig. 6.
Acr prevents BP and NNK from inducing DNA damage. Human lung epithelial BEAS-2B cells (A–D) and urothelial UROtsa cells (E–H) were treated with (A and E) NNK (1× = 75 μM and 2× = 150 μM) or (C and G) BP (1× = 25 μM and 2× = 50 μM) in the presence and absence of Acr (1× = 75 μM) for 1 h. (A, C, E, and G) The relative levels of BPDE-dG, O6-medG, and PdG adducts were determined by the immunochemical method shown in Fig. 1. (B, D, F, and H) Quantification results. Symbols: (I), (II), (III), (IV), (V), and (VI) on the x axis of B, D, F, and H represent different treatments as shown in A, C, E, and G. Note: Acr significantly reduced BP- and NNK-induced BPDE-dG and O6-medG adduct formation. ***P < 0.001, **P < 0.01, and *P < 0.05. NNK also reduced Acr-induced PdG formation (SI Appendix, Fig. S6).
Discussion
TS contains more than 60 known human carcinogens including PAHs, heterocyclic hydrocarbons, aromatic amines, and nitrosamines (12, 14). The mutagenicity, as well as carcinogenicity, in animal and cultured cell models of many TS carcinogens, particularly BP and NNK, are well established (12, 14). BP is a ubiquitous contaminant and a strong carcinogen in animal models (29, 30, 39). BPDE, a major electrophilic metabolite of BP, is a potent DNA-damaging agent and mutagen (29, 30, 39). It induces G-to-T transversion mutations similar to TS-induced mutations in the p53 gene (6, 54). BPDE, as well as other epoxide forms of PAHs, induce DNA damage in the p53 gene in human lung cells, preferentially at the TS-related lung cancer p53 mutational hot spots (55, 56). NNK by itself is a strong carcinogen in animal models; it induces tumors in different organs including the lungs (14). Hence, PAHs and nitrosamines, particularly BP and NNK, have been accepted as major causes for TS-related cancers (11–14, 24, 30, 50).
It is therefore puzzling that no BPDE-dG and O6-medG adducts were found in the lung and bladder tissue of mice exposed to MTS (Figs. 1 and 2). Similar results have been found in mice exposed to side-stream TS, which is responsible for 20–30% of TS-related cancers and heart diseases (57–60). However, BPDE-dG and O6-medG adducts were observed in lung tissues of mice exposed to BP or NNK alone exhibiting higher levels than those exposed to MTS (25, 61). We found that, indeed, BP and NNK can induce PdG and O6-medG, respectively, in human lung and bladder epithelial cells. Therefore, induction of BPDE-dG and O6-medG adducts are most likely a major carcinogenic mechanism in BP and NNK carcinogenesis. However, we also found that Acr can greatly inhibit this DNA damage induction effect of BP and NNK in human cells (Fig. 6). The total amount of TS aldehydes including Acr, Cro, Acet, and formaldehyde, is 10,000-fold more than PAHs and nitrosamines (14). We propose that aldehydes in TS, because of their abundance and capability to adduct proteins, inhibit CYP enzyme activities, which are necessary for the metabolism of PAHs and NNK to reactive forms that are able to adduct genomic DNA (Fig. 6). In fact, several reports have shown that Acr can inhibit the function of CYP enzymes (62–64).
It is well established that γ-OH-PdG and α-meth-γ-OH-PdG adducts are mutagenic, and that aldehydes can reduce DNA repair capacity (8, 34, 41). Furthermore, it has been found that Acet, Cro, and formaldehyde can induce tumors in animal models (65–67). Although the extremely high cardiopulmonary toxicity of Acr to mice has hampered the full evaluation of its lung carcinogenicity, it has been shown that, in combination with uracil, Acr also can induce bladder tumors in animals (68). Based on these results, we propose a paradigm for TS carcinogenesis: TS aldehydes such as Acr, Acet, formaldehyde, and Cro are the major TS lung and bladder carcinogens; their carcinogenicity is via induction of DNA damage, mainly γ-OH-PdG and α-meth-γ-OH-PdG adducts, and inhibition of DNA repair mechanisms including NER, BER, and mismatch repair. The abundance of aldehydes in TS also effectively prevents the metabolic activation process of the relatively small amount of TS procarcinogens such as PAHs, nitrosamines, aromatic amines, heterocycle hydrocarbons, and benzene, all of which require metabolic activation by CYPs to become carcinogenic DNA-damaging agents. Consequently, TS carcinogenicity is mainly a manifestation of aldehyde carcinogenicity, rather than an additive result of all TS carcinogens.
It is worth noting that the distribution of Acr-induced DNA damage in the p53 gene of human bronchial epithelial cells coincides with the p53 mutational spectrum of TS-related lung cancer and that the percentage of G-to-T and G-to-A mutations induced by Acr is similar to those found in the p53 gene of human lung cancer patients (8, 41, 54). It has been found that the level of γ-OH-PdG adducts is 30–40 times higher than 4-aminobiphenyl-DNA adducts in normal human urothelial mucosa (35).
Our results raise the intriguing question of why the PdG adducts are not formed in heart and liver, but only in lung and bladder. The lung is the first major organ exposed to TS; therefore, lung cells are subjected to damage from all aldehydes in inhaled TS. Upon entering the bloodstream, we believe that the aldehydes are quickly conjugated with proteins in blood and that these protein-conjugated aldehydes are no longer able to enter cells in other organs such as the heart and liver. The protein-conjugated aldehydes in the bloodstream eventually are reversed into aldehydes and protein in the renal system and are then excreted in the urine. Therefore, bladder tissue is also exposed to the concentrated aldehydes in urine, which induce PdG in bladder cells. It should be noted that only γ-OH-PdG adducts are detected in human bladder mucosa (35). Why are only γ-OH-PdG adducts formed in bladder tissue? We found that the ability of Cro to induce PdG in urothelial cells in urine is less than one-third that in the Tris-buffer condition. In contrast, Acr’s ability to induce PdG in urine is similar, if not identical, to its ability in Tris-buffer conditions (SI Appendix, Fig. S5).
Since MTS is inhaled directly into the aerodigestive system of smokers, human buccal cells and lung tissues are exposed to Acet and Cro, as well as Acr, which induce α-meth-γ-OH-PdG and γ-OH-PdG adducts, respectively (8, 32, 36). TS contains a fourfold higher amount of Acet and Cro than Acr (69). However, we found that the levels of γ-OH-PdG are eightfold to ninefold higher than the levels of α-meth-γ-OH-PdG in buccal cells of smokers, as well as in lung tissue of mice subjected to whole-body MTS exposure. We believe that since the boiling point of Acet is much lower than ambient temperature, a major portion of Acet in TS escapes from absorption by smokers, and that Cro is less efficient than Acr in inducing DNA adducts. We found that the levels of α-meth-γ-OH-PdG are slightly higher than the levels of γ-OH-PdG in lung tissues of smokers, although the reason is unclear. We found that the relative levels of these two types of PdG in buccal cells and lung tissues of smokers are different. The cause for this difference is unclear.
Our results that TS does not induce PdG, BPDE-dG, or O6-medG adducts in liver tissue raise two important possibilities: First, TS-related liver cancer is not caused by aldehydes, BP, or NNK; and second, TS-related cancers in different organs may be caused by different carcinogens in TS. If these possibilities are correct, then it is necessary to determine the types of DNA damage induced by TS in different organs to design effective cancer preventive measures.
Last, but not least, our results indicate not only that PPE is effective in eliminating the effect of TS on inducing DNA damage in mouse lung and bladder tissues but also that it is effective in inhibiting DNA repair activity and reducing the abundance of repair protein in lung tissues. Similarly, polyphenols can greatly reduce the effect of Acr in DNA adduct induction and repair inhibition in cultured human lung and bladder epithelial cells. These results raise the possibility that polyphenols may be able to prevent TS-induced lung and bladder cancer. It is worth noting that epidemiology studies have suggested that tea consumption reduces lung cancer risk (70–72).
In 2015, there were 36.5 million tobacco smokers in the United States alone (73). Smokers are not the only people who are exposed to TS; innocent bystanders and family members of smokers are also exposed to TS. We found that side-stream TS also induces DNA damage and inhibition of DNA repair the same as MTS does (34). For the foreseeable future, TS will remain a major cause of human cancer. Our findings that aldehydes, rather than the PAHs and nitrosamines, are the major agents that induce DNA damage and inhibit DNA repair, the two carcinogenic mechanisms, provide crucial insights not only for cancer risk assessment but also for the design of clinically effective preventive measures for reducing TS-related cancers.
Similar to TS effects, we recently have found that electronic-cigarette smoke (ECS) induces γ-OH-PdG and O6-medG in lung, heart, and bladder tissues, but not in liver tissue, and reduces DNA repair activity in lung tissue in the same mouse model with the same 12-wk exposure time (74). It should be noted that, whereas TS-induced DNA damage and repair inhibition are via the aldehydes in TS, which result from the incomplete combustion of tobacco leaves, ECS-induced DNA damage and repair inhibition are via aldehydes resulting from nitrosation, metabolism, and degradation of nicotine (74). These results indicate that the amount of TS and ECS consumption is most likely an important factor in determining the levels of DNA damage induction and DNA repair inhibition. These two effects, however, can serve as valuable parameters for assessing the relative harmful effects of TS and ECS.
Materials and Methods
Mice and Diet Supplement with PPE.
Forty mice (male FVBN mice; 8 wk old; purchased from Charles River) were randomized into four groups (A, B, C, and D) with 10 mice in each group. Two groups (A and B) of mice were fed the AIN93M diet containing 0.1% PPE (NIA93M), and the other two groups (C and D) were fed the AIN93M diet as control. PPE was a gift from Dr. Yukihiko Hara (Mitsui Norin Company, Tokyo, Japan). The diet was prepared by Research Diets.
Exposure of Mice to MTS.
The details of MTS and FA exposure method are as previously described (75–77). In brief, mice were exposed to MTS (groups A and D) or FA (groups B and C) 6 h/d, 5 d/wk for 12 wk. The MTS (∼75 mg/m3) was generated with an automated cigarette-smoking machine (CH Technologies), using 2R4F cigarettes (Kentucky Tobacco Research and Development Center). Three cigarettes were lit at a time, with an automatically regulated piston pump (2-s puff of 35-mL volume once per min) to produce MTS. The MTS produced from the cigarette was diluted by FA and introduced into a 1.3-m3 stainless-steel chamber for animal exposure (75–77). Mice were killed 24 h after the last exposure. The lung, heart, liver, and bladder were collected from each animal and immediately frozen at −80 °C.
Genomic DNA Isolation and Cell Lysate Preparation.
Genomic DNAs were isolated from lung, bladder, liver, and heart, as previously described (34). Cell-free cell lysates were prepared from lung tissues, as previously described (8, 33, 78).
DNA Damage and DNA Repair Function.
The levels of DNA damage (PdG, BPDE-dG, and O6-medG adducts) in lung, liver, heart, and bladder and the DNA repair capacity (NER and BER) in the lung tissues of all four groups of mice were determined. PdG adducts were determined by an immunochemical method and 32P postlabeling, followed by 2D-TLC/HPLC analysis, as described (32–34) (SI Appendix, Supplementary Methods). BPDE-dG adducts were determined by an immunochemical method and a 3D-TLC method (33, 34) (SI Appendix, Supplementary Methods). O6-medG adducts were determined by an immunochemical method and confirmed by an HPLC method (37, 38). The NER and BER capacity in cell lysates isolated from lung tissues was measured by their capacity to carry out DNA damage-dependent repair synthesis using 32P-labeled dNTP as precursors and supercoiled DNA with UV- or H2O2-induced DNA damage as substrates as previously described (8, 33, 78).
Detection of DNA Repair Proteins.
The effects of MTS exposure on the NER- and BER-related XPC and OGG1 protein levels in lung tissues were determined as previously described (33, 34).
Treatment of Human Bronchial Epithelial and Urothelial Cells with Acr and Polyphenols.
Immortalized human bronchial epithelial cells BEAS-2B and urothelial cells UROtsa were pretreated with different concentrations of PPE (0, 20, 80, and 160 µg/mL) and PP-60 (0, 20, 40, and 80 µg/mL; Sigma) for 1 h at 37 °C. The medium was removed and replaced with fresh medium containing Acr (10 µM), and the cells were incubated for 1 h at 37 °C. The DNA repair activity and PdG formation in these cells were determined as described above (33–35, 78).
Effect of Acr on BP-Induced BPDE-dG Formation and on NNK-Induced O6-medG Formation in Human Lung Epithelial Cells and Urothelial Cells.
Human bronchial epithelial cells, BEAS-2B, and urothelial cells, UROtsa, with and without preincubation with Acr (75 µM) were incubated with BP (25 or 50 µM) or NNK (75 or 150 µM) for 1 h. BPDE-dG, PdG, and O6-medG adducts formed in the genomic DNA were detected by the immunochemical method as described (33–35).
Human Buccal Cells and Sputum.
Buccal mucosa were collected using a cytologic brush, as described (79), per an approved IRB protocol. Sputum samples were collected by the method described (80). Collected buccal cells and sputum samples were immediately frozen at −80 °C. All subjects were free of lung cancer at the time of the initial screening. The smoking histories (pack-year) of these patients were based on patients’ report. The method for purifying genomic DNA from buccal cells and sputum was the same as previously described (81).
Lung Tissues and Genomic DNA Isolation.
The “normal lung tissue” samples from lung cancer patients of smokers (n = 41) were obtained from the marginal tissues that were free of tumors, as determined by surgeons, of surgical resected lung tumor. The lung tissue samples of non-lung cancer patients (n = 13) were obtained from the Lung Tissue Research Consortium of the National Heart Lung and Blood Institute under the National Institutes of Health (NIH). The lung tissues were dissected into small pieces on ice and used Dounce-type homogenizer with cell lysis buffer to loosen tissue cells. The method for purifying genomic DNA from these tissues was the same as previously described (34).
Effect of NNK on Acr-Induced PdG Formation.
To determine the effect of NNK on Acr-induced PdG formation, different concentrations of NNK (0, 37.5, 75, 150, and 225 µM) were incubated with Acr (0.1 mM) in the presence of human genomic DNA in Tris buffer; the PdG adducts were detected by the immunochemical method, as described above (33).
Statistical Analysis.
All statistical analyses were performed using Microsoft Excel and the statistical software GraphPad Prism 7. The geometric means were compared between different sample groups using two-sided Student’s t tests.
Supplementary Material
Acknowledgments
We thank Drs. Frederic Beland and Catherine B. Klein for reviewing this manuscript. This work was supported by NIH Grants 1P01CA165980, R01CA190678, P30CA16087, and ES00260.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804869115/-/DCSupplemental.
References
- 1.Howlader NNA, et al. In: SEER Cancer Statistics Review, 1975–2013. Cronin KA, editor. National Cancer Institute; Bethesda: 2016. [Google Scholar]
- 2.Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network 2014 Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:Supplementary Information. [Google Scholar]
- 4.Yang Q, Hergenhahn M, Weninger A, Bartsch H. Cigarette smoke induces direct DNA damage in the human B-lymphoid cell line Raji. Carcinogenesis. 1999;20:1769–1775. doi: 10.1093/carcin/20.9.1769. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Albino AP, Jorgensen E, Traganos F, Darzynkiewicz Z. DNA damage response induced by tobacco smoke in normal human bronchial epithelial and A549 pulmonary adenocarcinoma cells assessed by laser scanning cytometry. Cytometry A. 2009;75:840–847. doi: 10.1002/cyto.a.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandrov LB, et al. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354:618–622. doi: 10.1126/science.aag0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeifer GP, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 8.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding YS, et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev. 2008;17:3366–3371. doi: 10.1158/1055-9965.EPI-08-0320. [DOI] [PubMed] [Google Scholar]
- 10.Straif K, et al. Exposure to high concentrations of nitrosamines and cancer mortality among a cohort of rubber workers. Occup Environ Med. 2000;57:180–187. doi: 10.1136/oem.57.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50:307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 14.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 15.Arif JM, et al. Lung DNA adducts detected in human smokers are unrelated to typical polyaromatic carcinogens. Chem Res Toxicol. 2006;19:295–299. doi: 10.1021/tx0502443. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- 17.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer 2004. Tobacco Smoke and Involuntary Smoking, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (WHO International Agency for Research on Cancer, Lyon, France), Vol 83.
- 18.Ronai ZA, Gradia S, Peterson LA, Hecht SS. G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis. 1993;14:2419–2422. doi: 10.1093/carcin/14.11.2419. [DOI] [PubMed] [Google Scholar]
- 19.Lozano JC, Nakazawa H, Cros MP, Cabral R, Yamasaki H. G→A mutations in p53 and Ha-ras genes in esophageal papillomas induced by N-nitrosomethylbenzylamine in two strains of rats. Mol Carcinog. 1994;9:33–39. doi: 10.1002/mc.2940090107. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Boussard TM, Hainaut P. A specific spectrum of p53 mutations in lung cancer from smokers: Review of mutations compiled in the IARC p53 database. Environ Health Perspect. 1998;106:385–391. doi: 10.1289/ehp.98106385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 22.Chung FL, et al. Endogenous formation and significance of 1,N2-propanodeoxyguanosine adducts. Mutat Res. 1999;424:71–81. doi: 10.1016/s0027-5107(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 23.Gräslund A, Jernström B. DNA-carcinogen interaction: Covalent DNA-adducts of benzo(a)pyrene 7,8-dihydrodiol 9,10-epoxides studied by biochemical and biophysical techniques. Q Rev Biophys. 1989;22:1–37. [PubMed] [Google Scholar]
- 24.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 25.de Vries A, et al. Induction of DNA adducts and mutations in spleen, liver and lung of XPA-deficient/lacZ transgenic mice after oral treatment with benzo[a]pyrene: Correlation with tumour development. Carcinogenesis. 1997;18:2327–2332. doi: 10.1093/carcin/18.12.2327. [DOI] [PubMed] [Google Scholar]
- 26.Thomson NM, Kenney PM, Peterson LA. The pyridyloxobutyl DNA adduct, O6-[4-oxo-4-(3-pyridyl)butyl]guanine, is detected in tissues from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-treated A/J mice. Chem Res Toxicol. 2003;16:1–6. doi: 10.1021/tx025585k. [DOI] [PubMed] [Google Scholar]
- 27.International Agency for Research on Cancer 1983. Polynuclear Aromatic Compounds, Part 1: Chemical, Environmental and Experimental Data, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans (International Agency for Research on Cancer, Lyon, France), Vol 32, pp 33–451.
- 28.International Agency for Research on Cancer 2007. Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (International Agency for Research on Cancer, Lyon, France), Vol 89.
- 29.International Agency for Research on Cancer 1985. Allyl Compounds, Aldehydes, Epoxides and Peroxides, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans (International Agency for Research on Cancer, Lyon, France)
- 30.International Agency for Research on Cancer 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (International Agency for Research on Cancer, Lyon, France)
- 31.Weng MW, et al. AFB1 hepatocarcinogenesis is via lipid peroxidation that inhibits DNA repair, sensitizes mutation susceptibility and induces aldehyde-DNA adducts at p53 mutational hotspot codon 249. Oncotarget. 2017;8:18213–18226. doi: 10.18632/oncotarget.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung FL, Young R, Hecht SS. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 33.Wang HT, et al. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J Biol Chem. 2012;287:12379–12386. doi: 10.1074/jbc.M111.329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HW, et al. Cigarette side-stream smoke lung and bladder carcinogenesis: Inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget. 2015;6:33226–33236. doi: 10.18632/oncotarget.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HW, et al. Acrolein- and 4-aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget. 2014;5:3526–3540. doi: 10.18632/oncotarget.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Villalta PW, Wang M, Hecht SS. Analysis of crotonaldehyde- and acetaldehyde-derived 1,N2-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography electrospray ionization tandem mass spectrometry. Chem Res Toxicol. 2006;19:1386–1392. doi: 10.1021/tx060154d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engelbergs J, Thomale J, Galhoff A, Rajewsky MF. Fast repair of O6-ethylguanine, but not O6-methylguanine, in transcribed genes prevents mutation of H-ras in rat mammary tumorigenesis induced by ethylnitrosourea in place of methylnitrosourea. Proc Natl Acad Sci USA. 1998;95:1635–1640. doi: 10.1073/pnas.95.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang HI, et al. Highly sensitive, specific detection of O6-methylguanine, O4-methylthymine, and O4-ethylthymine by the combination of high-performance liquid chromatography prefractionation, 32P postlabeling, and immunoprecipitation. Cancer Res. 1992;52:5307–5312. [PubMed] [Google Scholar]
- 39.Sugasawa K, et al. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell. 1998;2:223–232. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 40.Radicella JP, Dherin C, Desmaze C, Fox MS, Boiteux S. Cloning and characterization of hOGG1, a human homolog of the OGG1 gene of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:8010–8015. doi: 10.1073/pnas.94.15.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang MS, et al. Acrolein induced DNA damage, mutagenicity and effect on DNA repair. Mol Nutr Food Res. 2011;55:1291–1300. doi: 10.1002/mnfr.201100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomes R, Meek ME, Eggleton M. 2002. Acrolein (WHO, Geneva)
- 43.Taylor MJ, Richardson T. Antioxidant activity of skim milk: Effect of sonication. J Dairy Sci. 1980;63:1938–1942. doi: 10.3168/jds.S0022-0302(80)83161-4. [DOI] [PubMed] [Google Scholar]
- 44.Tanel A, Averill-Bates DA. Inhibition of acrolein-induced apoptosis by the antioxidant N-acetylcysteine. J Pharmacol Exp Ther. 2007;321:73–83. doi: 10.1124/jpet.106.114678. [DOI] [PubMed] [Google Scholar]
- 45.Schoenike SE, Dana WJ. Ifosfamide and mesna. Clin Pharm. 1990;9:179–191. [PubMed] [Google Scholar]
- 46.Yu L, et al. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int J Cancer. 2010;127:2870–2878. doi: 10.1002/ijc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proia NK, Paszkiewicz GM, Nasca MA, Franke GE, Pauly JL. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer—a review. Cancer Epidemiol Biomarkers Prev. 2006;15:1061–1077. doi: 10.1158/1055-9965.EPI-05-0983. [DOI] [PubMed] [Google Scholar]
- 48.Thunnissen FB. Sputum examination for early detection of lung cancer. J Clin Pathol. 2003;56:805–810. doi: 10.1136/jcp.56.11.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mashberg A, Samit A. Early diagnosis of asymptomatic oral and oropharyngeal squamous cancers. CA Cancer J Clin. 1995;45:328–351. doi: 10.3322/canjclin.45.6.328. [DOI] [PubMed] [Google Scholar]
- 50.Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol Sci. 2015;145:5–15. doi: 10.1093/toxsci/kfv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sankhwar M, Sankhwar SN. Variations in CYP isoforms and bladder cancer: A superfamily paradigm. Urol Oncol. 2014;32:28.e33-40. doi: 10.1016/j.urolonc.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Hecht SS. Human urinary carcinogen metabolites: Biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 53.Hecht SS, Carmella SG, Foiles PG, Murphy SE, Peterson LA. Tobacco-specific nitrosamine adducts: Studies in laboratory animals and humans. Environ Health Perspect. 1993;99:57–63. doi: 10.1289/ehp.939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang HT, et al. Effect of CpG methylation at different sequence context on acrolein- and BPDE-DNA binding and mutagenesis. Carcinogenesis. 2013;34:220–227. doi: 10.1093/carcin/bgs323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 56.Denissenko MF, Pao A, Pfeifer GP, Tang M. Slow repair of bulky DNA adducts along the nontranscribed strand of the human p53 gene may explain the strand bias of transversion mutations in cancers. Oncogene. 1998;16:1241–1247. doi: 10.1038/sj.onc.1201647. [DOI] [PubMed] [Google Scholar]
- 57.US Department of Health and Human Services . The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2006. [Google Scholar]
- 58.International Agency for Research on Cancer 2012. Personal Habits and Indoor Combustions, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (International Agency for Research on Cancer, Lyon, France)
- 59.Glantz SA, Parmley WW. Passive smoking and heart disease. Mechanisms and risk. JAMA. 1995;273:1047–1053. [PubMed] [Google Scholar]
- 60.Kritz H, Schmid P, Sinzinger H. Passive smoking and cardiovascular risk. Arch Intern Med. 1995;155:1942–1948. [PubMed] [Google Scholar]
- 61.Peterson LA, Hecht SS. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–5564. [PubMed] [Google Scholar]
- 62.Kandagatla SK, et al. Inhibition of human cytochrome P450 2E1 and 2A6 by aldehydes: Structure and activity relationships. Chem Biol Interact. 2014;219:195–202. doi: 10.1016/j.cbi.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raner GM, Chiang EW, Vaz AD, Coon MJ. Mechanism-based inactivation of cytochrome P450 2B4 by aldehydes: Relationship to aldehyde deformylation via a peroxyhemiacetal intermediate. Biochemistry. 1997;36:4895–4902. doi: 10.1021/bi9630568. [DOI] [PubMed] [Google Scholar]
- 64.Cooper KO, Witz G, Witmer C. The effects of alpha, beta-unsaturated aldehydes on hepatic thiols and thiol-containing enzymes. Fundam Appl Toxicol. 1992;19:343–349. doi: 10.1016/0272-0590(92)90172-e. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi M, et al. Effects of ethanol, potassium metabisulfite, formaldehyde and hydrogen peroxide on gastric carcinogenesis in rats after initiation with N-methyl-N′-nitro-N-nitrosoguanidine. Jpn J Cancer Res. 1986;77:118–124. [PubMed] [Google Scholar]
- 66.International Agency for Research on Cancer 1999. Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide, Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Acetaldehyde (International Agency for Research on Cancer, Lyon, France), Vol 77.
- 67.Pan J, et al. Proceedings of the 104th Annual Meeting of the American Association for Cancer Research. Am Assoc Cancer Res; Philadelphia: 2013. Detection of acrolein-derived cyclic DNA adducts in human cells by monoclonal antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen SM, Garland EM, St John M, Okamura T, Smith RA. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992;52:3577–3581. [PubMed] [Google Scholar]
- 69.Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. 2nd Ed CRC Press; Boca Raton, FL: 2013. [Google Scholar]
- 70.Yuan JM. Green tea and prevention of esophageal and lung cancers. Mol Nutr Food Res. 2011;55:886–904. doi: 10.1002/mnfr.201000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seeram NP, et al. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J Agric Food Chem. 2006;54:1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- 72.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 73.Jamal A, et al. Current cigarette smoking among adults—United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 74.Lee HW, et al. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA. 2018;115:E1560–E1569. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Repace JL, Lowrey AH. An enforceable indoor air quality standard for environmental tobacco smoke in the workplace. Risk Anal. 1993;13:463–475. doi: 10.1111/j.1539-6924.1993.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 76.Repace JL, Lowrey AH. Indoor air pollution, tobacco smoke, and public health. Science. 1980;208:464–472. doi: 10.1126/science.7367873. [DOI] [PubMed] [Google Scholar]
- 77.Chen LC, et al. Atherosclerosis lesion progression during inhalation exposure to environmental tobacco smoke: A comparison to concentrated ambient air fine particles exposure. Inhal Toxicol. 2010;22:449–459. doi: 10.3109/08958370903373845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood RD, Robins P, Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988;53:97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- 79.Osswald K, Mittas A, Glei M, Pool-Zobel BL. New revival of an old biomarker: Characterisation of buccal cells and determination of genetic damage in the isolated fraction of viable leucocytes. Mutat Res. 2003;544:321–329. doi: 10.1016/j.mrrev.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 80.Fahy JV, Liu J, Wong H, Boushey HA. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147:1126–1131. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- 81.Richards B, et al. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.