Significance
Bone formation occurs through two distinct pathways, namely, endochondral ossification (EO) and intramembranous ossification (IMO). While significant effort has gone into understanding the role of various soluble signals in EO and IMO, the impact of the bone inorganic interface in triggering these ossification pathways has remained unexplored. Herein, we report the discovery that the bone-like mineral phase promotes formation of bone by mesenchymal stem/stromal cells (MSCs) exclusively via IMO even in the presence of soluble signals that promote the EO paradigm. Furthermore, we provide mechanistic insights into our observations and illustrate a previously unidentified role for extracellular calcium-sensing receptor (CaSR) in dictating the choice of ossification pathway in MSCs. These findings have significant implications for developing new strategies for bone repair and understanding bone homeostasis.
Keywords: bone remodeling, calcium phosphate, regenerative medicine, stem cell niche, Wnt signaling
Abstract
In adult bone injuries, periosteum-derived mesenchymal stem/stromal cells (MSCs) form bone via endochondral ossification (EO), whereas those from bone marrow (BM)/endosteum form bone primarily through intramembranous ossification (IMO). We hypothesized that this phenomenon is influenced by the proximity of MSCs residing in the BM to the trabecular bone microenvironment. Herein, we investigated the impact of the bone mineral phase on human BM-derived MSCs’ choice of ossification pathway, using a biomimetic bone-like hydroxyapatite (BBHAp) interface. BBHAp induced hyperstimulation of extracellular calcium-sensing receptor (CaSR) and temporal down-regulation of parathyroid hormone 1 receptor (PTH1R), leading to inhibition of chondrogenic differentiation of MSCs even in the presence of chondroinductive factors, such as transforming growth factor-β1 (TGF-β1). Interestingly rescuing PTH1R expression using human PTH fragment (1–34) partially restored chondrogenesis in the BBHAp environment. In vivo studies in an ectopic site revealed that the BBHAp interface inhibits EO and strictly promotes IMO. Furthermore, CaSR knockdown (CaSR KD) disrupted the bone-forming potential of MSCs irrespective of the absence or presence of the BBHAp interface. Our findings confirm the expression of CaSR in human BM-derived MSCs and unravel a prominent role for the interplay between CaSR and PTH1R in regulating MSC fate and the choice of pathway for bone formation.
Mesenchymal stem/stromal cells (MSCs) play a prominent role in the development of skeleton through two distinct pathways, namely, endochondral ossification (EO) (1) and intramembranous ossification (IMO) (2, 3). Development of long bones via EO starts with the formation of cartilaginous template through differentiation of MSCs to chondrocytes, followed by invasion of matrix by osteoprogenitors and mineralization of matrix (4). However, development of flat bones occurs through direct differentiation of MSCs to osteoblasts (3). Many paracrine factors released from bone extracellular matrix (ECM) and bone cells during bone remolding, such as calcium [which can increase up to 40 mM (5)] and transforming growth factor-β1 (TGF-β1) (6), modulate the functionality of cells residing in the marrow. Several in vitro and in vivo studies have demonstrated that cells residing in the bone environment, such as osteoblasts and osteoclasts, are able to sense the local changes in extracellular calcium concentration via calcium-sensing receptor (CaSR) (7). CaSR is a member of class C of G protein-coupled receptors that senses extracellular calcium (8–11) via large extracellular N-terminal domain binding to calcium. CaSR plays a major role in calcium homeostasis and bone turnover and other pathophysiology (11, 12). The consequence of the elevated extracellular calcium concentration and stimulation of CaSR on growth plate chondrocytes (GPCs) (13, 14) or osteoblasts differentiation (15–17) has been investigated in various in vitro studies, all of which alluded to a critical role for CaSR in regulating bone growth and turnover (18). However, studies using CaSR mutant mouse models have focused more on embryogenesis and skeletal development, and have largely ignored the role of CaSR in adult bone development (19–22). Recently, Chang et al. (19) found that chondrocyte-specific deletion of CaSR is fatal and deletion of CaSR in bone cells leads to extreme bone defects. Similar observations were made when CaSR was conditionally knocked out in osteoblasts (23). Despite this wide cohort of studies, whether adult human bone marrow (BM) MSCs express CaSR is not known.
In this study, we hypothesized that the bone-like microenvironment, recapitulated primarily using biomimetic apatite (i.e., bone-like apatite) and prominent soluble signals, provides a biophysically heterogeneous environment that could play an important role in modulating human MSC fate. We have found that human BM-derived MSCs express CaSR and that bone-like biomimetic hydroxyapatite (BBHAp) hyperstimulates CaSR. Additionally, we made a counterintuitive finding that the bone-like physicochemical microenvironment inhibits MSC chondrogenic differentiation and bone formation via EO even in presence of chondroinductive signals, such as TGF-β1. Our findings substantially highlight the role of CaSR in the decision of the fate of MSCs as it pertains to bone formation.
Results and Discussion
BBHAp and MSC Differentiation in Vitro.
A nanoscale coating of the bone-like mineral phase (BBHAp) possessing a chemical composition, structure, and crystal size identical to biogenic apatite was rendered on a polyethylene terephthalate (PET) fibrous scaffold by immobilizing phosphorylated dentin matrix protein-1 (DMP1) in a highly defined conformation using a biomimetic approach (24) (Fig. 1 A and B). It is important to note that BBHAp is distinctly different in its physiochemical properties from synthetic HAp (25, 26), which has been explored extensively in bone repair (27), in that its crystallinity and chemical composition are similar to biogenic cancellous bone HAp (Fig. 1C); therefore, it has significantly higher solubility of over two orders of magnitude in comparison to synthetic HAp, which is highly crystalline due to the sintering process (26, 28).
Fig. 1.
Characterization of BBHAp. (A) SEM revealing a nanoscale homogeneous coating of apatite along the PET fibers (Inset). (B) Transmission electron microscopy confirming the presence of apatite with all of the characteristic diffraction rings of biogenic apatite (Inset). (C) Raman spectroscopy of BBHAp on PET fibers showing clear similarities between BBHAp and human cancellous bone.
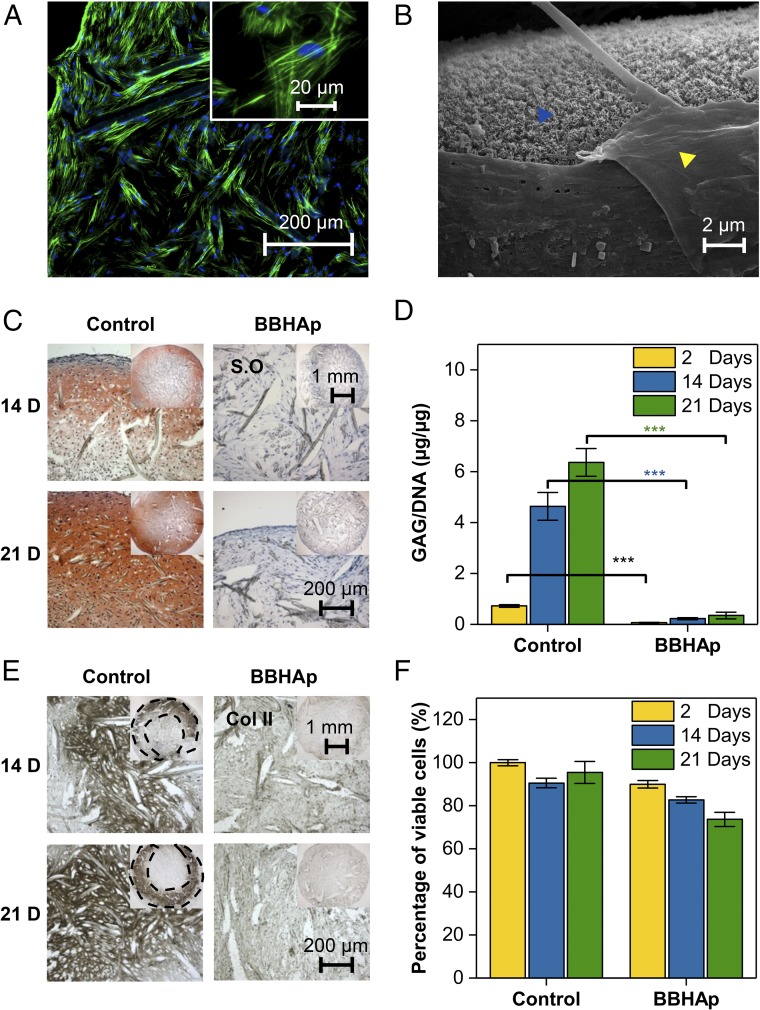
New bone deposition at a fracture site starts with a callus, a cartilaginous matrix deposited by differentiated MSCs. While the transformation of a cartilage template into bone via EO strictly occurs in vivo, the formation of the cartilage template, which represents a necessary first step in EO, can be recapitulated in vitro by differentiating MSCs into a chondrogenic phenotype. To recapitulate the unique biophysical environment of bone, we associated MSCs with confirmed multipotency (Fig. S1) with PET scaffolds coated with BBHAp and cultured the cells under conditions promoting chondrogenic differentiation in the presence of TGF-β1, a key inducer of chondrogenesis (29, 30). MSCs were able to attach to the BBHAp interface and produce matrix, as visualized by F-actin staining (Fig. 2A) and scanning electron microscopy (SEM) (Fig. 2B), respectively. Furthermore, BBHAp coating was stable during the 3 wk of in vitro culture (Fig. 2B). After 2 wk of chondrogenic differentiation, cells grown in absence of the BBHAp interface yielded tissue with clear cartilage characteristics, including expression of glycosaminoglycan (GAG) and collagen type II, while the tissue generated in the BBHAp microenvironment showed loose ECM with little to no GAG and collagen type II (Fig. 2 C–E). Typically, expression of collagen type X, which is related to the emergence of a hypertrophic phenotype in chondrocytes, is observed after the robust expression of collagen type II. However, the BBHAp microenvironment promoted the expression of collagen type X even in the absence of strong collagen type II expression (Fig. S2). Since fracture healing through EO involves two distinct steps (first, the development of cartilage matrix and, second, the differentiation of chondrocytes into a hypertrophic phenotype), cells were cultured for an additional week in chondrogenic medium lacking TGF-β1 but supplemented with l-thyroxin (31) to induce a hypertrophic chondrocyte phenotype in MSCs. After exposure to conditions inducing hypertrophy, cells cultured in control scaffolds were within large lacunae and embedded in an abundant matrix of GAG, collagen type II, and collagen type X, which is consistent with a hypertrophic chondrocyte phenotype (Fig. 2 C–E and Fig. S2). Quantitative GAG/DNA analysis also confirmed the qualitative Safranin-O staining (Fig. 2D). On the other hand, the cells cultured in the BBHAp microenvironment lacked these characteristics and exhibited moderately higher expression of collagen type X, especially in the extremities of scaffolds (Fig. S2). Furthermore, the BBHAp microenvironment did not alter the viability of the cells during in vitro culture, as assessed using a trypan blue exclusion assay on the dissociated cells from scaffolds after 2, 14, and 21 d (Fig. 2F). Since MSCs show similar attachment to control and BBHAp scaffolds and their viability was not altered significantly, all of the above-mentioned observations can be attributed purely to the presence of a bone-like microenvironment. As alterations in ECM composition are usually accompanied by changes in expression of collagenase, conditioned medium from both constructs were analyzed with gelatin zymography after 2 and 3 wk of in vitro culture, and it was found that even though the expression of pro–matrix metalloproteinase-2 (MMP-2) was comparable in both conditions, the expression of the active form was higher in the absence of BBHAp (Fig. S3). To ascertain if the observed inhibition of chondrogenesis in the BBHAp environment was due to calcium released from the BBHAp coating into the culture medium, we determined the amount of calcium released from the scaffold as a function of time (Fig. S4A). Theoretically the maximum concentration of calcium that could be achieved in the culture medium upon complete dissolution of the BBHAp coating is around 0.5 mM. Over a 21-d period, the cumulative release of calcium was determined to be around 0.12 mM. Considering that the concentration of calcium in culture medium is ∼1.8 mM, this represents a negligible contribution.
Fig. 2.
In vitro characterization of human MSC-seeded scaffolds in conditions promoting chondrogenic differentiation. (A) MSCs were able to adhere to the BBHAp interface. F-actin is shown in green, and nuclei are shown in blue. (B) SEM confirmed that the apatite mineral phase is retained in MSC-laden BBHAp scaffolds even after 21 d of in vitro culture. The yellow arrowhead points to a matrix produced by MSCs, and the blue arrowhead points to the BBHAp phase. (C) Tissue produced by MSCs in control constructs (PET) was rich in GAG as visualized by Safranin-O (S.O) staining, while tissue within BBHAp constructs showed no staining for GAGs. (D) Quantification of GAG/DNA in control and BBHAp scaffolds. (E) BBHAp inhibits the expression of collagen type II (Col II) by MSCs as visualized by immunohistochemistry. (F) Cell viability quantified by trypan blue exclusion assay on cells dissociated from scaffolds at different time points. Data are normalized to the number of viable cells retrieved from the control condition at day 2 of in vitro culture.
BBHAp Modulates Expression of CaSR and Caveolin-1.
CaSR was first identified in bovine PTG cells (8); in the chondrogenic cell line ATDC5 (32) subsequently; and then in several cell types, including primary GPCs and osteoblasts (19). It has been shown that extracellular calcium critically supports terminal differentiation of chondrocytes and normal growth plate development (33). Western blot analysis revealed hyperstimulation of CaSR in cells cultured on BBHAp versus control (Fig. 3A). This hyperstimulation of CaSR was accompanied by a pronounced increase in the expression of caveolin-1 (Cav-1) and phosphorylated Cav-1, a known CaSR-binding partner (34) (Fig. 3A), and this confirms the appreciated relationship between CaSR and Cav-1 (35, 36). The findings of the Western blot analysis were also confirmed by immunofluorescent staining for Cav-1 and CaSR (Fig. 3B). Therefore, Cav-1 may have a direct functional impact on CaSR by providing a microenvironment where signaling molecules necessary for CaSR activation are compartmentalized to assist intracellular signaling.
Fig. 3.
Molecular effects of hyperstimulation of extracellular CaSR on human MSCs. (A) Western blot analysis of total cell protein lysate at days 14 and 21 of in vitro culture. BBHAp notably induced the expression of CaSR, Cav-1, β-catenin (β-Cat), and pERK1/2, all with known roles in skeletal development. GAPDH was used as a loading control. Ctrl, control. (B) Immunofluorescent staining confirming the higher expression of CaSR and Cav-1 at day 14 of in vitro culture. (C) Flow cytometry analysis of MSCs constitutively expressing mCherry and EGFP+ under β-catenin promoter retrieved from BBHAp constructs, indicating an increase in β-catenin signaling in comparison to controls (*P < 0.05; ***P < 0.001). (Inset) Representative image of cells residing on fibers coated with BBHAp, coexpressing mCherry and EGFP. (Scale bar: 10 μm.) (D) Heat map showing 2D clustering of the 100 differentially regulated genes with a role in MSC adhesion, proliferation, and differentiation at day 2 of in vitro culture using log fold change (logFC) = 2 and P < 0.05. Expression intensity is represented by red and green, for high and low intensities, respectively. The genes of interest are indicated with arrows. (E) Hierarchical cluster analysis and heat map of the top 100 differentially regulated genes between conditions at two different time points. (F) K-mean clustering gene network analysis of genes shown in E plus CaSR and CTNBB1. The genes with no connection are not shown in the network. Blue cluster shows genes involved in the EO signaling pathway, while the genes in the green cluster belong to peptide ligand-binding SuperPath or immunoregulatory interactions between a lymphoid cell SuperPath and a nonlymphoid cell SuperPath. The network reveals that CaSR is connected to genes modulating the EO pathway (blue clusters) via the Wnt signaling cascade and GNG11 plays a central role in signal transduction. (G) Cfu assays for cfu-c, cfu-o, and cfu-f cells retrieved from the control and BBHAp scaffolds. MSCs once retrieved from the control and BBHAp environments are equally competent in forming colonies.
It has been shown that entire components of a mitogen-activated protein kinase (MAPK) pathway are concentrated in caveolae (37), and Cav-1 is essential for phosphorylation of extracellular signal-regulated kinase 1 and 2 (pERK1/2) in different cell types (38). Kifor et al. (39) have shown in human embryonic kidney cells transfected with CaSR and in bovine parathyroid cells that activation of CaSR leads to pERK1/2 via phosphotyrosine kinase or phosphatidylinositol-specific phospholipase C. It has been shown previously that activation of the MAPK signaling cascade inhibits chondrogenic differentiation of MSCs in vivo (40). We found that pERK1/2 was higher in BBHAp than in the control condition (Fig. 3A). Since MSCs exposed to different concentrations of calcium for 2 d show elevated expression of CaSR , Cav-1, and pERK1/2 in dose-dependent manner (Fig. S5A), our observations provide strong evidence that extracellular calcium has a direct impact on stimulation of CaSR and expression of Cav-1.
To ascertain if this hyperstimulation can be purely attributed to an increase in extracellular calcium, we determined the threshold for stimulation of CaSR by extracellular calcium and determined it occurs between 4 mM and 8 mM extracellular calcium. We exposed MSCs cultured on PET scaffolds to 8 mM extracellular calcium and found that even at this high concentration, chondrogenesis was still evident after 3 wk of in vitro culture, albeit to lesser extent (Fig. S4B). However, the hyperstimulation of CaSR observed in MSCs within the BBHAp environment could only be achieved at an extracellular calcium concentration of 24 mM (Fig. S5B), which was accompanied by significant cell death. It is well established that steep extracellular calcium gradients can drive apoptosis via overload of calcium in the mitochondria (41–43). Interestingly, it has been suggested that during skeletogenesis, calcium in a mineral form might modulate cell differentiation (44). Since MSCs on the BBHAp interface show no loss in viability as stated earlier, this provides further evidence that MSCs cultured on the BBHAp interface perceive a unique physicochemical (biophysical) environment consisting of a very high local concentration of calcium that cannot be realized and replicated through mere supplementation in the culture medium. Based on these observations, we conclude that the observed inhibition of chondrogenesis in the BBHAp environment is probably due to a synergistic effect of the localized high concentration of calcium perceived by the MSCs and the biophysical attributes of the BBHAp coating.
BBHAp Environment Enhances β-Catenin Signaling.
Canonical Wnt signaling plays a major role in skeletal development; therefore, we investigated the differential expression of β-catenin in the presence and absence of the BBHAp microenvironment. We found that β-catenin expression remained elevated at both 14 and 21 d in MSCs exposed to the BBHAp environment (Fig. 3A). To gain further insight into the activation of β-catenin signaling, we used MSCs transfected with a 7TGC vector (45) containing a T cell factor/lymphoid enhanced factor (TCF/LEF) promoter driving enhanced green fluorescent protein (EGFP) and an SV40 promoter driving mCherry using lentiviral shRNA to visualize and quantify the changes in β-catenin signaling. Flow cytometry analysis revealed that EGPF expression in MSCs associated with BBHAp scaffolds was significantly elevated over the time course of in vitro culture in comparison to controls (Fig. 3C). Since constant up-regulation of β-catenin signaling is known to inhibit condensation and SOX9-driven chondrogenesis (46, 47) and Wnt signaling is essential for regulation of MSC differentiation via the MAPK pathway (40), our findings provide strong evidence linking activation of CaSR with Wnt/β-catenin signaling in the inhibition of chondrogenic differentiation observed in the BBHAp microenvironment.
Hyperstimulation of CaSR Impacts Parathyroid Hormone 1 Receptor and Wnt Signaling.
To investigate the underlying mechanism for drastic differences in the fate of MSCs in the absence and presence of BBHAp, and to further evaluate the impact of CaSR in MSC differentiation pathways, we have analyzed the gene expression profile of MSCs in control and BBHAp constructs after 2 and 21 d of culture using an Affymetrix microarray analysis. This revealed that the BBHAp substantially alters mRNA expression of more than 530 genes with a minimum of twofold differential regulation and more than 130 genes with a minimum of threefold differential regulation. The 100 differentially regulated genes at day 2 of in vitro culture with a role in stem cell proliferation, adhesion, differentiation, and the EO pathway are shown in Fig. 3D. The BBHAp microenvironment impacted the expression of genes associated with parathyroid hormone 1 receptor (PTH1R), Wnt, phosphoinositide 3-kinase, and Hedgehog signaling pathways. Among the 21,400 genes analyzed, PTH1R was uniquely down-regulated by ∼12-fold in MSCs exposed to a bone-like microenvironment and was identified as the most significantly regulated upstream signaling molecule (Fig. 3D).
To date, the interplay between CaSR and PTH1R on bone formation has only been investigated in GPCs, where it has been shown that stimulation of GPCs with calcium inhibits the expression of PTH1R and parathyroid hormone-related protein (PTHrP) (14). Chang and coworkers (19, 48) have proposed that CaSR signaling counteracts PTHrP/PTH1R signaling by down-regulating PTH1R via inhibiting PTHrP expression independent of insulin-like growth factor 1 (IGF1)/IGF receptor (IGF1R) signaling in mouse GPCs. However, in our study, no differences in expression of PTHrP and PTH were observed between the conditions (Fig. 3D). Since (PTHrP)/PTH1R signaling is essential for EO and calcium homeostasis (49, 50) and it has been shown that up-regulation of PTH1R promotes chondrogenic differentiation of MSCs via up-regulation of SOX9 (51), the inhibition of chondrogenesis and down-regulation of SOX9 (approximately twofold) in the BBHAp microenvironment could be highly attributed to massive down-regulation of PTH1R. As our culture condition lacks any exogenous PTH or PTHrP, we conclude that the down-regulation of PTH1R is directly linked to stimulation of CaSR and this alludes to a nexus between these two signaling pathways.
One of the signaling cascades that is directly linked to PTH1R, and is also one of the key modulators of skeletal development, is the IGF signaling pathway (52–54). Our analysis revealed an approximately twofold up-regulation of IGF1 and IGF2 in MSCs associated with the BBHAp interface. Although, only moderate up-regulation of IGF1R (∼1.5-fold) was observed, considering that it has been shown that adenoviral expression of IGF1 in human MSCs completely blocked expression of collagen type II and inhibited the chondrogenic differentiation (55), the moderate increase in expression of the IGF signaling pathway molecules could be attributed to PTH1R.
Several in vivo and in vitro studies have proposed direct cross-talk between PTH1R and Wnt signaling (56, 57). A cooperative role for Wnt5a-induced noncanonical Wnt signaling and β-catenin signaling in the development of bone has been proposed recently (58, 59). We found that in addition to PTH1R, proteins involved in both canonical and noncanonical Wnt signaling were differentially regulated in the presence of BBHAp. Frizzled-related protein (FRZB), a negative regulator of canonical Wnt/β-catenin signaling (60), was strongly down-regulated (approximately fourfold), while Wnt5a, a classical modulator of noncanonical Wnt signaling that is implicated in various developmental processes, was considerably elevated (approximately fourfold) in the presence of BBHAp. This suggests that extracellular calcium might be a modulator of MSC phenotype via cross-talk between the CaSR and Wnt pathways.
Influence of Bone-Like Microenvironment on MSC Differentiation.
Our analysis revealed that genes involved in EO, such as aggrecan (ACAN), collagen type II (COL II; COL2A1), Indian hedgehog (IHH), SOX9, scinderin (SCIN), and alkaline phosphatase (ALPL), were up-regulated as expected in the control condition. While the genes associated with bone formation by osteoblasts, including iodothyronine deiodinase 2 (DIO2) (61), mesoderm-specific transcript (MEST) (62), cartilage intermediate layer protein (CILP) (63), Forkhead box O1 (FOXO1) (64), and NADPH oxidase 4 (NOX4) (65), were up-regulated in MSCs cultured in the BBHAp microenvironment, the hallmarks of osteogenic differentiation, such as DMP1, bone sialoprotein (IBSP), and osterix (SP7), did not show any differential regulation between the two conditions. Additionally, genes involved in calcium metabolism, such as spondin-1 (SPON1) and amphiregulin (AREG), were up-regulated, indicating the transport of extracellular calcium to intercellular space, which is in agreement with data reported before for MSCs seeded on a synthetic calcium phosphate scaffold (66).
To understand the impact of time on the expression of different genes in both samples, a hierarchal clustering of the top 100 differentially regulated genes between day 2 and day 21 was performed (Fig. 3E). While the SOX5 gene associated with EO was highly up-regulated in the control condition, the expression of PTH1R was remarkably increased in the BBHAp environment after 21 d. This increase in PTH1R expression levels is accompanied by an increase in the expression of osteogenic differentiation genes, such as bone morphogenetic protein 4 (BMP4), DMP1, IBSP, and ALPL, in BBHAp constructs, and this could therefore be attributed to the emergence of an osteogenic phenotype in MSCs.
To see if the regulated genes are linked through signaling pathways involved in bone formation, a network analysis was performed through a STRING web tool (67) among CASR, CTNBB1 (β-catenin), and the top 100 differentially regulated genes identified in the hierarchal cluster analysis shown in Fig. 3E. A confidence level of 0.7 was used, k-mean clustering with two numbers of clusters was performed, and the genes with no connection were removed (Fig. 3F). In Fig. 3F, the blue cluster shows genes involved in the EO signaling pathway, while the genes in the green cluster belong to peptide ligand-binding SuperPath or immunoregulatory interactions between a lymphoid cell SuperPath and a nonlymphoid cell SuperPath. This analysis revealed that CaSR signal transduction modulated by guanine nucleotide-binding protein (G protein) subunit gamma 11 (GNG11), and CaSR hyperstimulation impacts the expression of genes involved in the EO signaling pathway via genes belonging to the Wnt signaling cascade. Our analysis shows a direct interplay between these signaling pathways in MSCs and chondrogenesis. Based on the data at hand, we propose that stimulation of CaSR by the BBHAp interface has a two-prong effect. In the early stages of in vitro culture, it functions as an inhibitor of chondrogenesis via down-regulation of PTH1R, and in the latter stages of in vitro culture, it functions as a promoter of osteogenesis by up-regulation of PTH1R (Fig. 3 E and F).
MSC Colony-Forming Capacity Remains Unchanged in the Presence of BBHAp.
The BM has long been thought to have a regulatory role in the maintenance of MSC function. MSCs residing in the BM are recruited to different sites in the body in case of pathologies or injuries, where they differentiate into tissue-specific/tissue-centric linages. To investigate the impact of long-term exposure of MSCs to the bone-like microenvironment on its differentiation potential, we carried out a colony-forming unit (cfu) assay on MSCs after their withdrawal from control and BBHAp conditions. MSCs were isolated from scaffolds after 3 wk of in vitro culture and tested for their capacity to form fibroblastic (cfu-f), chondrogenic (cfu-c), and osteogenic (cfu-o) colonies. While cells retrieved from both scaffolds after 21 d were able to form similar amounts of cfu-o and cfu-c colonies, cells cultured in the BBHAp condition showed slightly higher clonogenicity (16.4% higher cfu-f) (Fig. 3G). This suggests that the bone mineral phase in the MSC niche might have a role, along with soluble signals, in the maintenance of the MSC phenotype (68).
Stimulating PTH1R Using Human PTH Fragment (1–34) Partially Rescues Chondrogenesis in the BBHAp Environment.
To further elucidate the role of PTH1R in the loss of the chondrogenic phenotype in BBHAp constructs, a PTH1R rescue experiment was carried out using a human PTH fragment [PTH (1–34)], as it has been shown that PTH (1–34) can stimulate PTH1R (69). The BBHAp and control constructs were stimulated with PTH (1–34) (10 nM) only for the initial 2 d of in vitro culture while keeping the concentration of other soluble factors unchanged. Our Western blot analysis on total cell lysate after 2 d (Fig. 4A) confirmed the Affymetrix gene array analysis (Fig. 3D) by showing down-regulation of PTH1R in MSCs cultured in the BBHAp scaffold. Furthermore, the Western blot data illustrated that supplementation with PTH (1–34) indeed up-regulates expression of PTH1R in MSCs residing in the BBHAp environment (Fig. 4A). Additionally, the fate of the tissue was assessed by histologically staining for GAGs (Fig. 4B), and the content of GAG was quantified after 14 and 21 d (Fig. S6). Although BBHAp constructs treated with PTH (1–34) (Fig. 4, BBHAp+PTH) did not show evident signs of chondrogenic differentiation after 14 d of in vitro culture (Fig. 4B, Top), after an additional week, the regions in the periphery of the BBHAp scaffold showed clear signs of chondrogenesis (Fig. 4B, Middle), with chondrocytes residing in lacunae and possessing similar morphology to the ones seen in untreated and PTH-stimulated control scaffolds (Fig. 4B, Bottom), suggesting that stimulation of MSCs using PTH (1–34) can partially rescue the chondroinhibitory effects imposed by hyperstimulation of CaSR. In contrast, no chondrogenesis was observed in the BBHAp constructs as stated earlier (Fig. 2). Quantitative analysis of GAG/DNA also confirmed the Safranin-O staining and showed a statistically significant increase in GAG/DNA in the BBHAp group stimulated with PTH versus unstimulated BBHAp constructs at both 2 and 3 wk (Fig. S6). As expected, control groups stimulated with PTH (1–34) (Fig. 4 and Fig. S6) showed an increase in the expression of GAGs, which is consistent with literature reports on the effect of PTH (1–34) on chondrogenesis (51, 70).
Fig. 4.
Effect of stimulation of PTH1R using PTH (1–34) on human MSC-seeded scaffolds under conditions promoting chondrogenic differentiation. (A) Western blot analysis of total cell protein lysate following stimulation of PTH1R for 2 d showing that PTH (1–34) rescued the expression of PTH1R significantly in the BBHAp group. GAPDH was used as a loading control (Ctrl). (B) Safranin-O staining of cryosectioned slides from conditions with and without PTH (1–34) supplementation at 14 d (Top) and 21 d (Middle). Tissues within BBHAp constructs stimulated with PTH (BBHAp+PTH) showed expression of GAGs at day 21 of in vitro culture in the periphery region (red-orange regions) as assessed by positive staining by Safranin-O, while tissue within BBHAp constructs showed no staining for GAGs. Both control and control scaffolds stimulated with PTH (Control+PTH) were rich in GAG. (Bottom) Higher magnification images of cells residing within the dashed black boxes (Middle) are shown in this row.
In Vivo Fate of MSCs in the BBHAp Environment.
We inquired if an in vivo environment can rescue the fate of MSCs after prolonged in vitro exposure to the BBHAp interface. Therefore, control and BBHAp constructs generated in vitro were implanted s.c. (ectopic site) in nude mice and harvested after 2, 4, and 8 wk. After 2 and 4 wk, control constructs developed into cartilaginous tissues rich in GAG containing chondrocytes embedded in large lacunae in the outer rim, while the middle portion of the constructs showed signs of early bone formation resembling a bony collar and strong expression of noncollagenous proteins (Fig. 5 A and B). However, in stark contrast, in BBHAp constructs, no GAG production was observed at both time points (Fig. 5 A and B). Eight weeks after implantation, the cartilage matrix in control samples was significantly remodeled and bone ossicles appeared in the central and outer region, which is consistent with the EO process. In sharp contrast, even after 8 wk, BBHAp constructs showed no indications of cartilaginous matrix; instead, bone ossicles were observed in both the central and outer regions of constructs after 8 wk (Fig. 5 A and B and histology from second replicate in Fig. S7), which is a clear indication of an IMO process. These findings were also confirmed using cells from a second donor (Fig. S8). Furthermore, after 4 wk of implantation, control and BBHAp constructs were probed against COL II, tartrate-resistant acid phosphatase (TRAP), and MMP-9 as main indicators of the EO pathway (Fig. 5 D–F). In control conditions, strong expression of COL II and MMP-9 and additional positive staining for TRAP around the cartilaginous areas, indicating the presence of multinucleated cells of the osteoclastic lineage, were observed (Fig. 5 D–F). Quantitative microtomography (µCT) analysis of explants confirmed deposition of peripheral and central mineralized matrix in the control construct as early as 4 wk after implantation. This finding was also confirmed in the second replicate (Fig. S9 A and B). After 8 wk, the differences between the quantity and stage of maturation of the mineralized matrix in both conditions were clearly evident in µCT analysis (Fig. 5 G and H). Furthermore, the bone mineral density in the control sample was significantly greater than that in the BBHAp constructs (Fig. 5I). In general, the quantity of mineralized tissue generated in control samples was higher at both time points in comparison to BBHAp as it was also confirmed by analyzing H&E and Safranin-O staining of multiple sections (Fig. S9C). Since in situ hybridization for Arthrobacter luteus (Alu) repeats revealed the presence of human cells in control and BBHAp constructs at all time points (Fig. 5C), it is clear that transplanted human cells participated in the formation of tissues in both conditions. Furthermore, as human MSCs were also found in bony areas in BBHAp constructs (Fig. 5C, 8 wk) and SEM analysis of thin tissue sections after 8 wk confirmed that the BBHAp coating was still present, albeit partially degraded (Fig. 5J), the bone formation in the BBHAp environment via IMO can be attributed to the presence of the bone-like microenvironment. Additionally, after 8 wk of in vivo implantation, both samples showed an abundant number of CD45+ cells; however, the quantity of CD45+ cells was lower in the BBHAp condition (Fig. 5K). Since we have found that CaSR hyperstimulation increases VCAM1 and ICAM1 expression, this provides a possible link between the expression profiles of these molecules and the lower recruitment of CD45+ cells and suggests that the bone mineral phase might have a role in modulating immunosuppression by MSCs. To rule out any role for the scaffolds by themselves in the observed outcome, we have investigated the ability of both scaffolds, in the absence of MSCs or in the presence of undifferentiated MSCs, to induce bone formation. Control and BBHAp-coated scaffolds without cells or with MSCs cultured only for 2 d were implanted in vivo, and analyses of the tissues isolated after 4, 8, and 12 wk revealed that the constructs were invaded only by inflammatory cells in all instances and there was no sign of cartilaginous or bone tissue development (Fig. S10). This finding highlights the contribution of MSCs and the cooperative relationship between cells and physicochemical aspects of the scaffold in bone formation.
Fig. 5.
Bone-like mineral phase inhibits EO in vivo. Safranin-O (S.O) (A) and H&E staining (B) of control and BBHAp constructs after 2, 4, and 8 wk following ectopic implantation in nude mice. Control samples showed GAG-rich cartilaginous matrix, which remodels into bone through EO after 8 wk. While the cartilaginous matric was remarkably absent in the BBHAp constructs at all time points, the bony ossicles were formed after 8 wk via IMO. The magnified area is denoted with a dashed black rectangle in the images. (C) In situ hybridization for visualization of human nuclei using an Alu probe. The region within the section that was probed using Alu is denoted by the dashed black rectangles in B. The presence of Alu+ nuclei (arrows) confirmed that the formation of bone in both conditions involves the participation of transplanted human MSCs. Explanted BBHAp constructs after 4 wk lack expression of characteristics of bone formation via EO, such as collagen type II (Col II) (D), MMP-9 (E), and TRAP (F). Coll II and MMP-9 are visualized as brown-colored regions positive for horseradish peroxidase activity. TRAP-positive areas in the control section are indicated by an arrow. (G) Three-dimensional reconstructed μCT images after 8 wk of in vivo implantation confirms the presence of mineralized tissue in both conditions. (H) Degree of maturation in mineralized tissue is visualized by an X-ray attenuation heat map. (I) Bone mineral density in both conditions calculated based on X-ray attenuation. ***P < 0.001. (J) SEM confirming the presence of BBHAp coating even after 8 wk of implantation. Magnified image of the region within the rectangular box on the Top is shown in the Bottom. (K) Fluorescent microscopy images of the area represented by the dashed circle in B (8-wk time point), which corresponds to bone deposits after staining for mouse CD45+ cells, showing the presence of immune cells in close proximity to ectopic bone. Magnified image of the region within the rectangular box on the Top is shown in the Bottom. (L) Visual representation of a proposed mechanism showing the counteractive effects of the PTH1R and IGF1 signaling pathways on hyperstimulation of CaSR between days 2 and 21 in vitro preceding the formation of bone via IMO in BBHAp constructs upon implantation.
In sum, the fact that the BBHAp microenvironment blocked chondrogenic differentiation of MSCs in vitro and EO in vivo and promoted bone formation through IMO via stimulation of CaSR could be attributed to various signaling cascades. For instance, it has been shown that FOXO1 expression in MSCs is associated with up-regulation of osteoblastic genes and is necessary for skeletogenesis (64). FOXO1 up-regulation in the early stages of in vitro culture could also play a role in IMO-derived bone formation in BBHAp-associated cells. However, considering the fact that the role of PTH and PTH1R signaling in osteoblast differentiation is well appreciated (71) and the expression of PTH1R was confirmed in osteoprogenitors and preosteoblasts (72), the temporal modulation of PTH1R expression via CaSR during in vitro culture (Fig. 5L) could orchestrate the observed sequence of events.
CaSR Knockdown Results in Suppression of MSC Proliferation and Differentiation.
To further understand the impact of CaSR on MSC differentiation, we have employed the GIPZ lentiviral shRNA targeting CaSR in MSCs [CaSR knockdown (KD)] (Fig. 6 A and B). Our transduction studies demonstrated that KD of CaSR by 75% leads to a slight increase in expression of Cav-1 (Fig. 6 B and C) and suppression of MSC proliferation potential (Fig. 6D). Furthermore, MSC chondrogenic differentiation potential was also hampered in vitro (Fig. 6 E and F). After 3 wk of in vitro culture, both control and BBHAp constructs lacked expression of proteoglycans and collagen type II as confirmed by Safranin-O and immunohistochemistry staining, respectively (Fig. 6 E and F). Constructs were subsequently implanted in an ectopic site in nude mice after 3 wk of in vitro culture (Fig. 6G). After 4 and 8 wk of in vivo implantation, the generated tissues were analyzed and no sign of cartilage or bone formation was observed in the constructs generated by CaSR KD cells in both the control and BBHAp conditions as evident by histological and TRAP staining (Fig. 6 H–J). Our data suggest that CaSR is necessary for bone formation and its KD negatively impacts MSC proliferation and differentiation potential in vitro and in vivo.
Fig. 6.
Effect of CaSR KD on the fate and bone-forming capacity of MSCs. (A) MSCs transduced with GIPZ lentiviral shRNA targeting CaSR showing TurboGFP expression 2 d after initial transduction and before puromycin selection. Arrow points to a TurboGFP positive cell. (B) Western blot analysis of total cell lysate for CaSR and Cav-1 in wild type (WT), nonsilencing control (NS), and CaSR KD MSCs after stimulation with 24 mM calcium chloride. GAPDH was used as a loading control. (C) Quantification of Western blot showing an ∼75% decrease in CaSR expression and a slight increase in Cav-1 expression in CaSR KD cells (values are normalized to WT). (D) Frequency of cell cycle per time unit was calculated for NS and KD cells and normalized to WT cells. (Inset) Suppression of growth rate in KD cells. Safranin-O (S.O) staining (E) and immunohistochemistry for collagen type II (Col II) (F) of KD cells seeded in control and BBHAp constructs after 3 wk of in vitro culture revealed that CaSR KD inhibits MSC chondrogenic differentiation independent of the substrate. (G) Representative fluorescent microscopy image of scaffold seeded with CaSR KD cells before implantation clearly showing the expression of TurboGFP. (H–J) In vivo implantation of CaSR KD constructs in an ectopic site of the nude mice after 4 and 8 wk revealed that the potential of MSCs for bone formation via EO and IMO is completely hampered by CaSR KD, indicating that CaSR signaling in MSCs is essential for bone formation. Both control and BBHAp constructs lack expression of GAG after 4 and 8 wk (H), and, furthermore, no characteristic of bone or cartilage tissue is observed in H&E-stained sections (I). (J) Negative TRAP staining indicates an absence of cells of osteoclastic lineage.
Our observations suggest that a biomimetic bone-like interface inhibits chondrogenic differentiation of MSCs and formation of bone through cartilage intermediate matrix via hyperstimulation of CaSR. Our study unravels that CaSR is not only necessary for MSC proliferation and differentiation but also dictates the choice of bone formation pathway via temporal modulation of PTH1R. Furthermore, our observations shed light on the contribution of MSCs in the process of fracture healing. Since our experimental conditions are a reliable mimic of bone mineral environment and the EO pathway was abrogated in MSCs within the BBHAp environment despite them being exposed to chondroinductive signals, this suggests that biophysical aspects of calcium phosphate are important modulators of MSC fate. In fact, a recent study using synthetic HAp has shown that the presence of calcium phosphate can inhibit miR-138 and trigger osteogenesis via activation of the SMAD and RAS families of genes (66). This study also provides valuable mechanistic insights into our earlier finding that injection of calcium alginate in the subperiosteal space in the in vivo bioreactor model yields bone formation without involving a cartilaginous matrix (73). In sum, these observations highlight the need to mimic the bone microenvironment, with respect to both its biophysical and biochemical attributes, when investigating MSCs in bone repair. In addition to adult marrow MSCs, in recent years, a new population of cells called mesodermal progenitors, which bear many of the hallmarks of MSCs, has been differentiated from human embryonic stem cells (74) and has also been identified during various stages of mouse ontogeny in sites that harbor hematopoietic stem cells (75). Although immediate mesodermal progenitors that can lead to expandable multipotent MSCs have not been isolated to date, mesodermal progenitors are believed to be the counterparts of MSCs during development, and one cannot rule out a role for such cells in EO and bone repair; therefore, their functional status in a bone-like microenvironment needs be investigated in future studies. In conclusion, the key observations of this study provide valuable insights into the molecular mechanisms of bone development in the presence of bone-like microenvironments.
Materials and Methods
MSCs were isolated from BM aspirates collected from healthy donors (n = 5, average age = 30 y) under informed consent in accordance with the guidelines of the local ethical committee (University Hospital Basel; reference no. 78/07).
Details of preparation of biomimetic bone-like HAp coating on fibrous mesh, Raman spectroscopy, SEM, transmission electron microscopy, in vitro cell culture and in vivo implantation, multilineage differentiation assay, GAG/DNA quantification, calcium quantification assay, Western blot, zymography, histology, immunohistochemistry and immunofluorescent staining, Affymetrix gene array, flow cytometry, cfu assay, in situ hybridization for Alu repeats, lentiviral transfection, and micro-CT analysis can be found in Supporting Information.
Supplementary Material
Acknowledgments
We thank Dr. Daniel Vonwil for the µCT measurements; Dr. Wouter Habraken for Raman analysis; Dr. Ralf Thomann for assistance with SEM; Francine Wolf for her assistance with the ALU staining; and Esther Kohler, Vincent Ahmadi, Kate Kutsenok, and Laurent Starck for technical assistance. This work was funded by a grant from Helmholtz Zentrum Geesthacht through the Helmholtz Virtual Institute on Multifunctional Biomaterials for Medicine, the excellence initiative of the German Federal and State governments (EXC 294), and a Swiss National Foundation-Sinergia Grant (CRSII3_136179).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805159115/-/DCSupplemental.
References
- 1.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013;5:a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaplis AC. Embryonic development of bone and regulation of intramembranous and endochondral bone formation. Princ Bone Biol. 2008;1:53–84. [Google Scholar]
- 4.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 5.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 6.Pfeilschifter J, Mundy GR. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci USA. 1987;84:2024–2028. doi: 10.1073/pnas.84.7.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown EM, Chattopadhyay N, Yano S. Calcium-sensing receptors in bone cells. J Musculoskelet Neuronal Interact. 2004;4:412–413. [PubMed] [Google Scholar]
- 8.Brown EM, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 9.Brown EM, Pollak M, Hebert SC. The extracellular calcium-sensing receptor: Its role in health and disease. Annu Rev Med. 1998;49:15–29. doi: 10.1146/annurev.med.49.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 11.Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: A review. Crit Rev Clin Lab Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- 12.Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: A molecular perspective. Endocr Rev. 2011;32:3–30. doi: 10.1210/er.2009-0043. [DOI] [PubMed] [Google Scholar]
- 13.Chang W, Rodriguez L, Chen TH, Tu C, Shoback D. Extracellular Ca2+-sensing in cartilage. J Musculoskelet Neuronal Interact. 2004;4:410–411. [PubMed] [Google Scholar]
- 14.Rodriguez L, Cheng Z, Chen TH, Tu C, Chang W. Extracellular calcium and parathyroid hormone-related peptide signaling modulate the pace of growth plate chondrocyte differentiation. Endocrinology. 2005;146:4597–4608. doi: 10.1210/en.2005-0437. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay N, et al. Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology. 2004;145:3451–3462. doi: 10.1210/en.2003-1127. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka S, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast differentiation and mineralization. Horm Metab Res. 2010;42:627–631. doi: 10.1055/s-0030-1255091. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak MM, et al. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA. 2004;101:5140–5145. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown EM. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract Res Clin Endocrinol Metab. 2013;27:333–343. doi: 10.1016/j.beem.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Chang W, Tu C, Chen T-H, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang W, et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology. 1999;140:5883–5893. doi: 10.1210/endo.140.12.7190. [DOI] [PubMed] [Google Scholar]
- 21.Theman TA, Collins MT. The role of the calcium-sensing receptor in bone biology and pathophysiology. Curr Pharm Biotechnol. 2009;10:289–301. doi: 10.2174/138920109787847538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cianferotti L, Gomes AR, Fabbri S, Tanini A, Brandi ML. The calcium-sensing receptor in bone metabolism: From bench to bedside and back. Osteoporos Int. 2015;26:2055–2071. doi: 10.1007/s00198-015-3203-1. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak-Ewell MM, et al. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res. 2011;26:2935–2947. doi: 10.1002/jbmr.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarem M, et al. Disordered conformation with low Pii helix in phosphoproteins orchestrates biomimetic apatite formation. Adv Mater. 2017;29:1701629. doi: 10.1002/adma.201701629. [DOI] [PubMed] [Google Scholar]
- 25.Budz JA, LoRe M, Nancollas GH. Hydroxyapatite and carbonated apatite as models for the dissolution behavior of human dental enamel. Adv Dent Res. 1987;1:314–321. doi: 10.1177/08959374870010022301. [DOI] [PubMed] [Google Scholar]
- 26.Wopenka B, Pasteris JD. A mineralogical perspective on the apatite in bone. Mater Sci Eng C. 2005;25:131–143. [Google Scholar]
- 27.Samavedi S, Whittington AR, Goldstein AS. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013;9:8037–8045. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Chen ZF, Huang BX, Pan HB, Darvell BW. Solubility of bovine-derived hydroxyapatite by solid titration, pH 3.5-5. Cryst Growth Des. 2009;9:2816–2820. [Google Scholar]
- 29.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 30.Watabe T, Miyazono K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 31.Scotti C, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang W, et al. Calcium sensing in cultured chondrogenic RCJ3.1C5.18 cells. Endocrinology. 1999;140:1911–1919. doi: 10.1210/endo.140.4.6639. [DOI] [PubMed] [Google Scholar]
- 33.Riccardi D, Brennan SC, Chang W. The extracellular calcium-sensing receptor, CaSR, in fetal development. Best Pract Res Clin Endocrinol Metab. 2013;27:443–453. doi: 10.1016/j.beem.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kifor O, Diaz R, Butters R, Kifor I, Brown EM. The calcium-sensing receptor is localized in caveolin-rich plasma membrane domains of bovine parathyroid cells. J Biol Chem. 1998;273:21708–21713. doi: 10.1074/jbc.273.34.21708. [DOI] [PubMed] [Google Scholar]
- 35.Razani B, Woodman SE, Lisanti MP. Caveolae: From cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 36.Kifor O, et al. Decreased expression of caveolin-1 and altered regulation of mitogen-activated protein kinase in cultured bovine parathyroid cells and human parathyroid adenomas. J Clin Endocrinol Metab. 2003;88:4455–4464. doi: 10.1210/jc.2002-021427. [DOI] [PubMed] [Google Scholar]
- 37.Liu P, Ying Y, Anderson RGW. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA. 1997;94:13666–13670. doi: 10.1073/pnas.94.25.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetterkind S, Poythress RH, Lin QQ, Morgan KG. Hierarchical scaffolding of an ERK1/2 activation pathway. Cell Commun Signal. 2013;11:65. doi: 10.1186/1478-811X-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kifor O, et al. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol. 2001;280:F291–F302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- 40.Matsushita T, et al. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol Cell Biol. 2009;29:5843–5857. doi: 10.1128/MCB.01549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demaurex N, Distelhorst C. Cell biology. Apoptosis–The calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- 42.Farber JL. The role of calcium in cell death. Life Sci. 1981;29:1289–1295. doi: 10.1016/0024-3205(81)90670-6. [DOI] [PubMed] [Google Scholar]
- 43.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 44.Jacenko O, Tuan RS. Chondrogenic potential of chick embryonic calvaria: I. Low calcium permits cartilage differentiation. Dev Dyn. 1995;202:13–26. doi: 10.1002/aja.1002020103. [DOI] [PubMed] [Google Scholar]
- 45.Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS One. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Santa Maria C, et al. Interplay between CaSR and PTH1R signaling in skeletal development and osteoanabolism. Semin Cell Dev Biol. 2016;49:11–23. doi: 10.1016/j.semcdb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mannstadt M, Jüppner H, Gardella TJ. Receptors for PTH and PTHrP: Their biological importance and functional properties. Am J Physiol. 1999;277:F665–F675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- 50.Chung UI, Lanske B, Lee K, Li E, Kronenberg H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci USA. 1998;95:13030–13035. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Kumagai K, Saito T. Effect of parathyroid hormone on early chondrogenic differentiation from mesenchymal stem cells. J Orthop Surg Res. 2014;9:68. doi: 10.1186/s13018-014-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guntur AR, Rosen CJ. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013;2:437. doi: 10.1038/bonekey.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 54.Wang Y, Bikle DD, Chang W. Autocrine and paracrine actions of IGF-I signaling in skeletal development. Bone Res. 2013;1:249–259. doi: 10.4248/BR201303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamura K, et al. Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol. 2005;33:865–872. doi: 10.1016/j.exphem.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 57.Romero G, et al. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-catenin signaling and osteoclastogenesis. J Biol Chem. 2010;285:14756–14763. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okamoto M, et al. Noncanonical Wnt5a enhances Wnt/β-catenin signaling during osteoblastogenesis. Sci Rep. 2014;4:4493. doi: 10.1038/srep04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baron R, Rawadi G. Targeting the Wnt/β-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 61.Bassett JHD, et al. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc Natl Acad Sci USA. 2010;107:7604–7609. doi: 10.1073/pnas.0911346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lefebvre L, et al. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- 63.Staines KA, Zhu D, Farquharson C, MacRae VE. Identification of novel regulators of osteoblast matrix mineralization by time series transcriptional profiling. J Bone Miner Metab. 2014;32:240–251. doi: 10.1007/s00774-013-0493-2. [DOI] [PubMed] [Google Scholar]
- 64.Teixeira CC, et al. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J Biol Chem. 2010;285:31055–31065. doi: 10.1074/jbc.M109.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015;24:1150–1163. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viti F, et al. Osteogenic differentiation of MSC through calcium signaling activation: Transcriptomics and functional analysis. PLoS One. 2016;11:e0148173. doi: 10.1371/journal.pone.0148173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szklarczyk D, et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones DL, Wagers AJ. No place like home: Anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 69.Rickard DJ, et al. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39:1361–1372. doi: 10.1016/j.bone.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, et al. Runx1 is critical for PTH-induced onset of mesenchymal progenitor cell chondrogenic differentiation. PLoS One. 2013;8:e74255. doi: 10.1371/journal.pone.0074255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal. 2009;21:1245–1254. doi: 10.1016/j.cellsig.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee K, Deeds JD, Segre GV. Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology. 1995;136:453–463. doi: 10.1210/endo.136.2.7835276. [DOI] [PubMed] [Google Scholar]
- 73.Stevens MM, et al. In vivo engineering of organs: The bone bioreactor. Proc Natl Acad Sci USA. 2005;102:11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vodyanik MA, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendes SC, Robin C, Dzierzak E. Mesenchymal progenitor cells localize within hematopoietic sites throughout ontogeny. Development. 2005;132:1127–1136. doi: 10.1242/dev.01615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.