Significance
Marine reserves that prohibit fishing are a critical tool for sustaining coral reef ecosystems, yet it remains unclear how human impacts in surrounding areas affect the capacity of marine reserves to deliver key conservation benefits. Our global study found that only marine reserves in areas of low human impact consistently sustained top predators. Fish biomass inside marine reserves declined along a gradient of human impacts in surrounding areas; however, reserves located where human impacts are moderate had the greatest difference in fish biomass compared with openly fished areas. Reserves in low human-impact areas are required for sustaining ecological functions like high-order predation, but reserves in high-impact areas can provide substantial conservation gains in fish biomass.
Keywords: marine reserves, fisheries, coral reefs, social-ecological, socioeconomic
Abstract
Coral reefs provide ecosystem goods and services for millions of people in the tropics, but reef conditions are declining worldwide. Effective solutions to the crisis facing coral reefs depend in part on understanding the context under which different types of conservation benefits can be maximized. Our global analysis of nearly 1,800 tropical reefs reveals how the intensity of human impacts in the surrounding seascape, measured as a function of human population size and accessibility to reefs (“gravity”), diminishes the effectiveness of marine reserves at sustaining reef fish biomass and the presence of top predators, even where compliance with reserve rules is high. Critically, fish biomass in high-compliance marine reserves located where human impacts were intensive tended to be less than a quarter that of reserves where human impacts were low. Similarly, the probability of encountering top predators on reefs with high human impacts was close to zero, even in high-compliance marine reserves. However, we find that the relative difference between openly fished sites and reserves (what we refer to as conservation gains) are highest for fish biomass (excluding predators) where human impacts are moderate and for top predators where human impacts are low. Our results illustrate critical ecological trade-offs in meeting key conservation objectives: reserves placed where there are moderate-to-high human impacts can provide substantial conservation gains for fish biomass, yet they are unlikely to support key ecosystem functions like higher-order predation, which is more prevalent in reserve locations with low human impacts.
The world’s coral reefs are rapidly degrading (1–3), which is diminishing ecological functioning and potentially affecting the well-being of the millions of people with reef-dependent livelihoods (4). Global climate change and local human impacts (such as fishing) are pervasive drivers of reef degradation (1, 5). In response to this “coral reef crisis,” governments around the world have developed a number of reef conservation initiatives (1, 6, 7). Our focus here is on the efficacy of management tools that limit or prohibit fishing. Management efforts that reduce fishing mortality should help to sustain reef ecosystems by increasing the abundance, mean body size, and diversity of fishes that perform critical ecological functions (8–10). In practice, however, outcomes from these reef-management tools have been mixed (5, 11–13).
A number of studies have examined the social, institutional, and environmental conditions that enable reef management to achieve key ecological outcomes, such as sustaining fish biomass (5, 14, 15), coral cover (16), or the presence of top predators (17). These studies often emphasize the role of: (i) types of key management strategies in use, such as marine reserves where fishing is prohibited, or areas where fishing gears and/or effort are restricted to reduce fishing mortality (8, 18); (ii) levels of compliance with management (12, 19, 20); (iii) the design characteristics of these management initiatives, for example the size and age of reserves, and whether they are placed in remote versus populated areas (11, 21); and (iv) the role of social drivers, such as markets, socioeconomic development, and human demography that shape people’s relationship with nature (14, 22).
In addition to examining when key ecological conditions are sustained, it is also crucial to understand the context under which conservation gains can be maximized (23, 24). By conservation gains, we are referring to the difference in a conservation outcome (e.g., the amount of fish biomass) when some form of management (i.e., a marine reserve or fishery restriction) is implemented relative to unmanaged areas. These conservation gains can be beneficial for both people and ecosystems. For example, increased fish biomass inside marine reserves is not only related to a range of ecosystem states and processes (18), but can also result in spillover of adults and larvae to surrounding areas, which can benefit fishers (25–27). The potential to achieve conservation gains may depend on the intensity of human impacts in the surrounding seascape (23, 24), yet these effects have never been quantified.
Here, we use data from 1,798 tropical coral reef sites in 41 nations, states, or territories (hereafter “nation/states”) in every major coral reef region of the world to quantify how expected conservation gains in two key ecological outcomes are mediated by the intensity of human impact, namely: (i) targeted reef fish biomass (i.e., species generally caught in fisheries) and (ii) the presence of top predators (Materials and Methods and SI Appendix, Table S1). To quantify human impact at each site, we draw from a long history of social science theory and practice to develop a metric referred to as “gravity” (Box 1). The concept of gravity (also called interactance) has been used in economics and geography to measure economic interactions, migration patterns, and trade flows since the late 1800s (28–30). We adapt this approach to examine potential interactions with reefs as a function of how large and far away the surrounding human population is (Box 1). At each site, we also determined the status of reef management, grouped into either: (i) openly fished, where sites are largely unmanaged and national or local regulations tend to be poorly complied with; (ii) restricted fishing, where there are actively enforced restrictions on the types of gears that can be used (e.g., bans on spear guns) or on access (e.g., marine tenure systems that restrict fishing by “outsiders”); or (iii) high-compliance marine reserves, where fishing is effectively prohibited (Materials and Methods). We hypothesized that our ecological indicators would decline with increasing gravity in fished areas, but that marine reserves areas would be less sensitive to gravity. To test our hypotheses, we used general and generalized linear mixed-effects models to predict target fish biomass and the presence of top predators, respectively, at each site based on gravity and management status, while accounting for other key environmental and social conditions thought to influence our ecological outcomes (14) (Materials and Methods). Based on our models, we calculated expected conservation gains along a gravity gradient as the difference between managed sites and openly fished sites.
Box 1
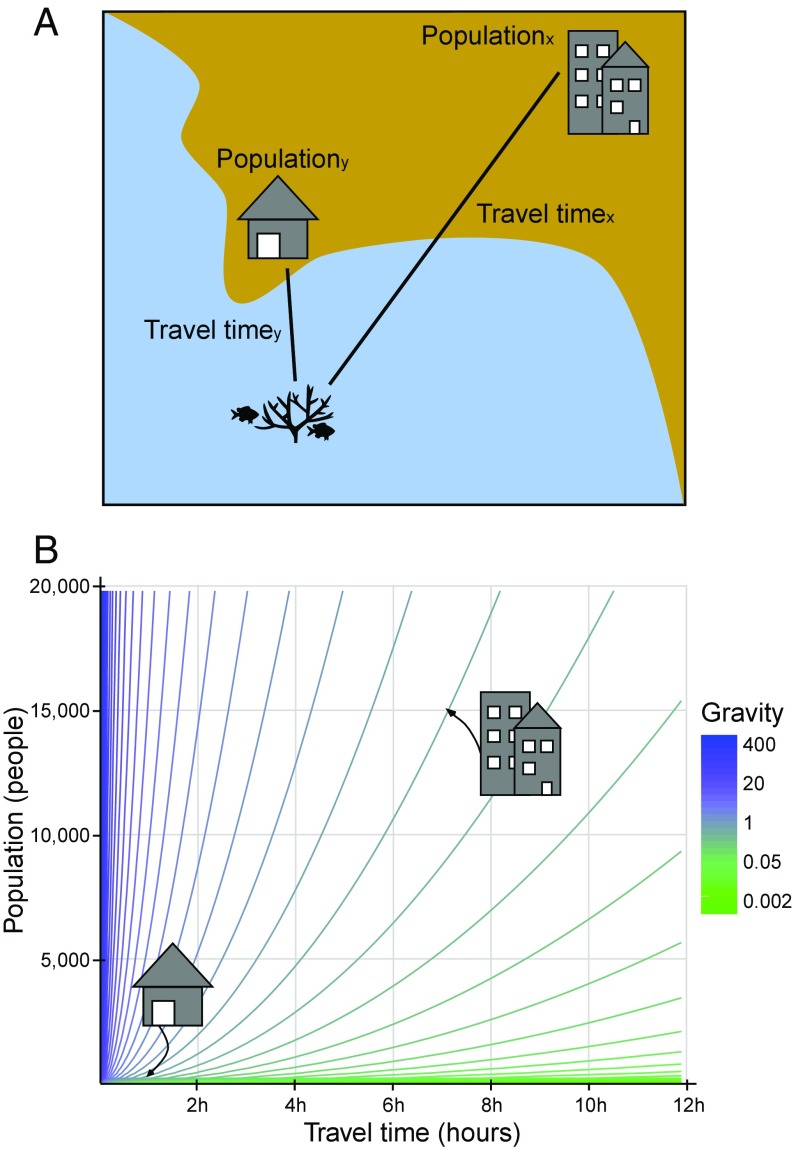
Drawing on an analogy from Newton’s Law of Gravitation, the gravity concept predicts that interactions between two places (e.g., cities) are positively related to their mass (i.e., population) and inversely related to the distance between them (31). The gravity concept is often considered one of the most successful and long-enduring empirical models in economics and geography (31), but has rarely been directly applied in a natural resource management setting and holds much promise in informing reef conservation and management. Application of the gravity concept in a reef governance context posits that human interactions with a reef are a function of the population of a place divided by the squared time it takes to travel to the reefs (we used travel time instead of linear distance to account for the differences incurred by traveling over different surfaces, such as water, roads, tracks) (14, 32) (see Fig. 1 and SI Appendix, Table S2). Here, we build upon previous work (14) by developing a new indicator that examines the cumulative human gravity of all populated places within a 500-km radius of a given reef, which aims to capture both market and subsistence pressures on reef fish biomass. We tested the predictive power of a series of gravity metrics with varying radii (50 km, 250 km, 500 km) and exponents of travel time (travel time, travel time2, travel time3) (Materials and Methods and SI Appendix, Table S3). A key limitation of our global gravity metric is that we are unable to capture local variations in efficiencies that may affect fishing mortality per capita, such as fishing fleet technology or infrastructure (e.g., road) quality.
Fig. 1.
Operationalizing gravity. (A) Applied to coral reefs, our heuristic of the gravity concept captures interactions between people and coral reef fish as a function of the population of a place divided by the squared time it takes to travel to the reefs (i.e., travel time). (B) Gravity isoclines along gradients of population size and travel time illustrate how gravity values could be similar for places that have large populations but are far from the reefs (e.g., populationx = 15,000 people, travel timex = 7 h, gravityx = 306) as to those with small populations that are close to the reef (e.g., populationy = 300 people, travel timey = 1 h, gravityy = 300). For ease of interpretation, we have illustrated travel time here in hours, but we use minutes in the main text.
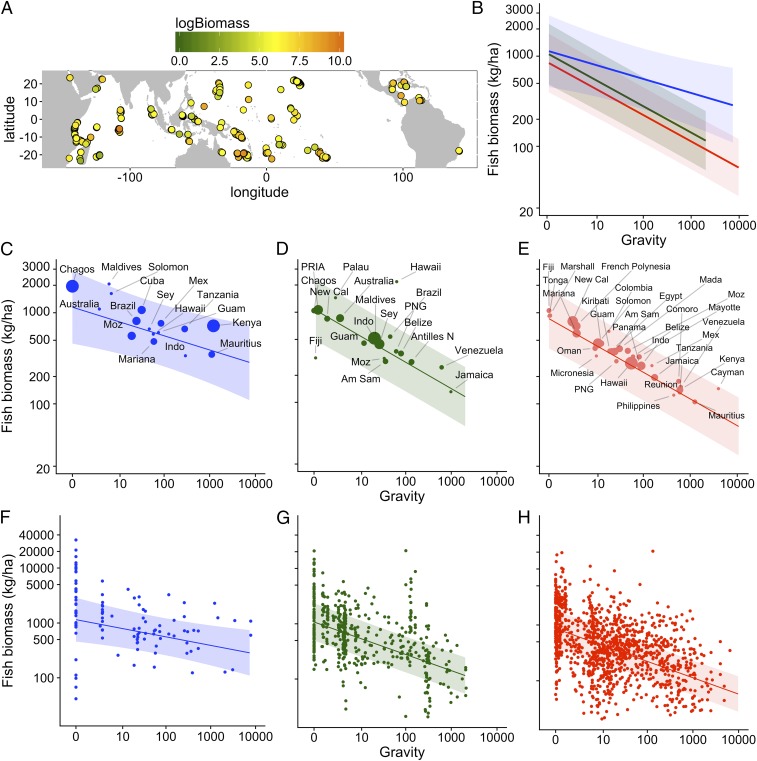
Our analysis reveals that human gravity was the strongest predictor of fish biomass (Fig. 2 and SI Appendix, Fig. S1). Fish biomass consistently declined along a human gravity gradient, a trend particularly evident at the nation/state scale (Fig. 2 C–E). However, this relationship can vary by management type (Fig. 2 and SI Appendix, Fig. S1). Specifically, we found that biomass in reserves demonstrated a flatter (but still negative) relationship with gravity compared with openly fished and restricted areas (Fig. 2B). Interestingly, this differential slope between reserves and fished areas (Fig. 2B) was due to a strong interaction between gravity and reserve age such that older reserves contributed more to biomass in high-gravity situations than in low-gravity ones (SI Appendix, Fig. S1). This is likely due to fish stocks at high-gravity sites being heavily depleted and requiring decades to recover, whereas low-gravity sites would likely require less time to reach unfished biomass levels (8). Thus, given average reserve age in our sample (15.5 y), biomass in reserves did not decline as rapidly with gravity compared with fished and restricted areas (Fig. 2B). In the highest-gravity locations, modeled fish biomass in marine reserves was approximately five times higher than in fished areas (270 kg/ha compared with 56 kg/ha) (Fig. 2B). At the reef site scale, there was considerable variability in reef fish biomass, particularly at low gravity (Fig. 2 F–H). For example, at the lowest-gravity locations, biomass levels in reserves spanned more than three orders-of-magnitude (Fig. 2F). Importantly, there was never extremely high biomass encountered in high-gravity locations. Our estimate of target fish biomass included top predators. As a supplemental analysis, we also examined target fish biomass with the biomass of top predators excluded, which displays a similar trend, but with lower fish biomass in reserves at low gravity compared with when top predators are included (SI Appendix, Fig. S2).
Fig. 2.
Model-predicted relationships between human gravity and reef fish biomass under different types of fisheries management. (A) Map of our study sites with color indicating the amount of fish biomass at each site. (B) Model-predicted relationships of how reef fish biomass declines as gravity increases by management type. Partial plots of the relationship between biomass and gravity under different types of management at the nation/state (C–E), and reef site (F–H) scale; openly fished (red), restricted (green), and high-compliance marine reserves (blue). Shaded areas represent 95% confidence intervals. Bubble size in C–E reflect the number of reef sites in each nation/state, scaled for each management type (such that the largest bubble in each panel represent the highest number of sites per nation/state for that type of management) (SI Appendix, Table S5). Nation/state name abbreviations for F–H are in SI Appendix, Table S5.
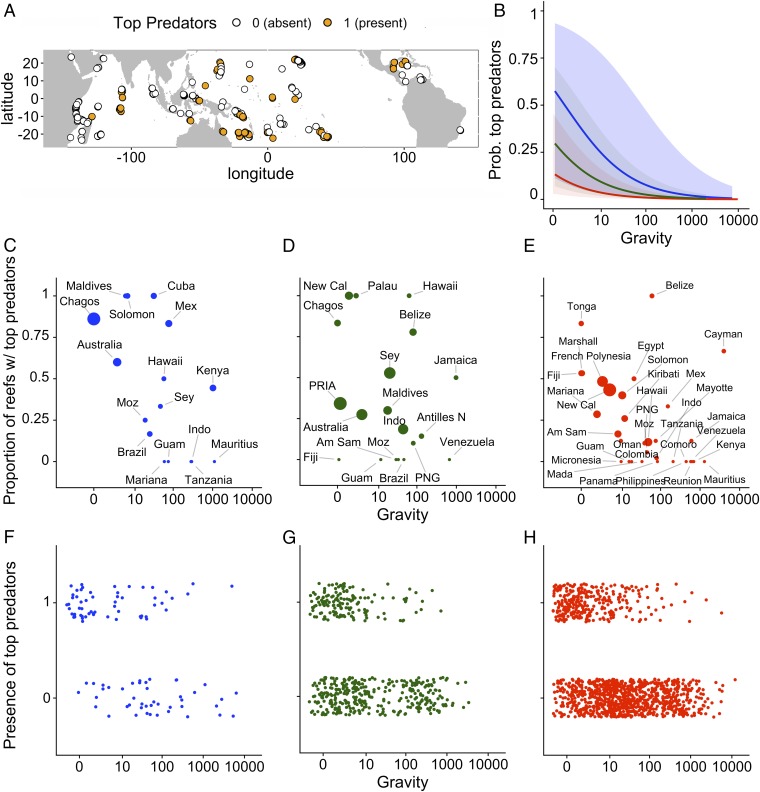
A key finding from our study is that top predators were encountered on only 28% of our reef sites, but as gravity increases, the probability of encountering top predator on tropical coral reefs dropped to almost zero (<0.005), regardless of management (Fig. 3). The probability of encountering top predators was strongly related to gravity and the type of management in place, as well as sampling methodology and area surveyed (Fig. 3 and SI Appendix, Fig. S1). At low gravity, the probability of encountering a top predator was highest in marine reserves (0.59) and lowest in fished areas (0.14), when controlling for sampling and other environmental and social drivers (Fig. 3 and SI Appendix, Fig. S1).
Fig. 3.
Model-predicted relationships between human gravity and the probability of encountering top predators under different types of fisheries management. (A) Map of our study sites indicating the presence of top predators. (B) Model-predicted relationships of how the probability of encountering predators declines as gravity increases. Shaded areas represent 95% confidence intervals. The presence of top predators along a gravity gradient under different types of management at the nation/state (C–E) and site (F–H) scale; openly fished (red), restricted (green), and high-compliance marine reserves (blue). Bubble size in C–E reflect the number of reef sites in each nation/state, scaled for each management type (such that the largest bubble in each panel represent the highest number of sites per nation/state for that type of management) (SI Appendix, Table S5). Nation/state name abbreviations for F–H in SI Appendix, Table S5.
Our study demonstrates the degree to which fish communities inside marine reserves can be affected by human impacts in the broader seascape (Figs. 2 and 3). Critically, high-compliance marine reserves in the lowest-gravity locations tended to support more than four times more fish biomass than the highest-gravity reserves (1,150 vs. 270 kg/ha, respectively) (Fig. 2B). Similarly, the modeled probability of encountering a top predator decreased by more than 100-fold from 0.59 in low-gravity reserves to 0.0046 in the highest-gravity reserves (Fig. 3B). Our study design meant that it was not possible to uncover the mechanisms responsible for this decline of ecological condition indicators within marine reserves along a gravity gradient, but this pattern of depletion is likely related to: (i) human impacts in the surrounding seascape (fishing, pollution, and so forth) affecting ecological processes (recruitment, feeding behavior, and so forth) within reserves (33, 34); (ii) almost every marine reserve is likely to have some degree of poaching, even where compliance is considered high (20, 35) and the cumulative impacts from occasional poaching events is probably higher in high-gravity situations; (iii) the life history of top predators, such as old age of reproduction and small clutch size for some (e.g., sharks), which makes then particularly susceptible to even mild levels of exploitation (36); and (iv) high-gravity marine reserves in our sample possibly being too young or too small to provide substantial conservation gains (11, 37). We conducted a supplementary analysis to further examine this latter potential explanation. Because of collinearity, we could not directly account for reserve size in our model, but conducted a supplemental analysis where we separated reserves into small (≤28 km2) and large (Materials and Methods and SI Appendix, Fig. S3). We found that the biomass and probability of encountering top predators was higher in large compared with small reserves, but surprisingly, we found a flatter slope for small compared with large reserves (SI Appendix, Fig. S3). However, there were no large high-compliance reserves in high-gravity areas in our sample, likely due to the social and political difficulties in establishing large reserves near people (38). Because there is little overlap between large and small reserves along the gravity gradient in our sample, we are unable to distinguish the effects of reserve size from those of gravity, but this is an important area for future research. Additionally, we modeled how the relationship between gravity and our ecological outcomes changed with reserve age, comparing outcomes using the average reserve age (15.5 y) to those from reserves nearly twice as old (29 y, which was the third quartile of our global distribution in reserve age). Older reserves were predicted to sustain an additional 180 kg/ha (+66%) of fish biomass at the highest levels of gravity compared with average age reserves. However, the effects of reserve age on the probability of encountering a top predator was less marked: the modeled probability of encountering a top predator in older reserves (29 y) was only 0.01, compared with <0.005 for average age (∼15 y) reserves, suggesting that small reserves common in high-gravity situations can support high levels of biomass, but are unlikely to sustain top predators, even when they are mature.
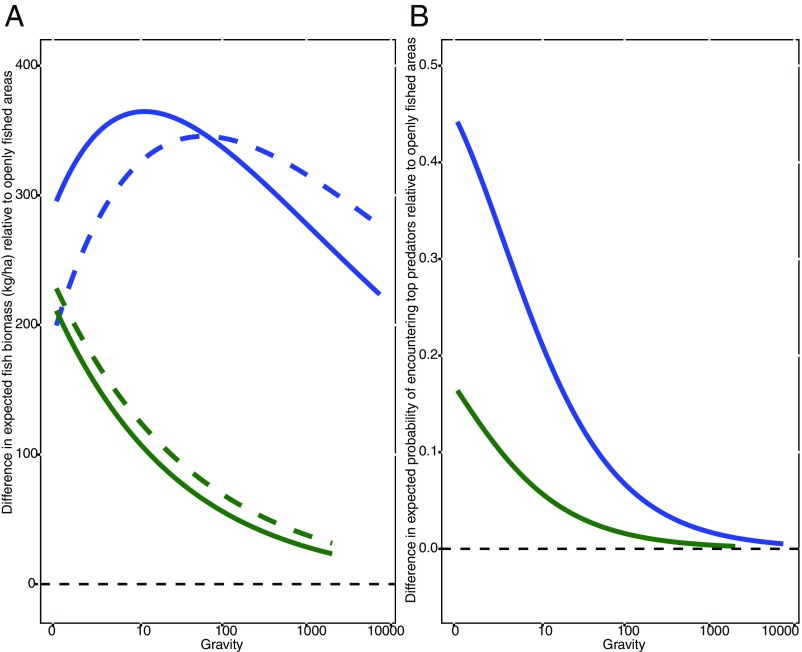
Although absolute fish biomass under all management categories declined with increasing gravity (Fig. 2B), the maximum expected conservation gains (i.e., the difference between openly fished and managed) differed by management type along the gravity gradient (Fig. 4A). Interestingly, the conservation gains for restricted fishing is highest in low-gravity situations, but rapidly declines as human impacts increase (Fig. 4A) (39). For marine reserves, biomass conservation gains demonstrated a hump-shaped pattern that peaked at very low gravity when predators were included in the biomass estimates (Fig. 4A, solid blue line). When top predators were excluded from biomass estimates, conservation gains peaked at intermediate gravity levels, and were higher in high gravity compared with low gravity (Fig. 4A, dotted blue line). Our results highlight how the expected differences between openly fished and marine reserves change along a gravity gradient, given a range of other social and environmental conditions that are controlled for within our model (SI Appendix, Fig. S1). Thus, differences in these trends are relative to average conditions, and individual reserves may demonstrate larger or smaller biomass build-up over time, which can vary by fish groups or families (e.g., ref. 40).
Fig. 4.
The conservation gains (i.e., the difference between openly fished sites and managed areas) for high-compliance marine reserves (blue line) and restricted fishing (green line) for (A) target fish biomass (solid lines include biomass of top predators, dotted lines exclude top predator biomass as per SI Appendix, Fig. S2), and (B) the probability of encountering top predators change along a gradient of gravity.
In an effort to minimize costs to users, many marine reserves, particularly the large ones, tend to be placed in remote locations that experience low human pressure (24, 41). However, critics of marine reserves in remote locations suggest that limited resources could be better spent protecting areas under higher threat that could potentially yield greater conservation gains (23, 24, 42). Our results make explicit the types of benefits—and the limitations—to placing reserves in high versus low human-impact locations. We found that for nontop predator reef fishes, substantial conservation gains can occur at even the highest-gravity locations but that optimal gains are obtained at moderate gravity (Fig. 4A). Our results also show that low-gravity marine reserves (and to a lesser extent low-gravity fisheries restrictions) are critical to support the presence of top predators (Fig. 3). However, the expected conservation gains for top predators declines rapidly with gravity in both marine reserves and restricted areas (Fig. 4B). Our results illustrate a critical ecological trade-off inherent in the placement of marine reserves: high-gravity reserves can have the substantial conservation gains for fish biomass, yet they are unlikely to support key ecosystem functions like predation, even with high levels of compliance. This highlights the importance of having clear objectives for conservation initiatives and recognizing the trade-offs involved (43, 44).
Our analysis does not allow us to uncover the mechanisms behind why we might observe the greatest differences in top predators between marine reserves and fished areas in low-gravity locations. A plausible explanation is that top predators, such as sharks, are particularly vulnerable to fishing (17) and are exposed to some fishing even in the most remote fished areas, driven by the extremely high price for shark fins [shark fins can fetch US$960/kg in wholesale markets (45), compared with only $43/kg for parrotfish in European supermarkets (46)]. Thus, even small amounts of fishing in remote openly fished areas may be depleting top predators, which creates a large difference between low-gravity–fished areas and marine reserves. This difference may diminish along the gravity because top predators tend to have large home ranges (37), and there were only small reserves in high-gravity locations (SI Appendix, Fig. S3), which may mean that existing high-gravity reserves are not likely big enough to support the large home ranges of many predators (37, 47).
Successful conservation also depends on a range of social considerations (48). For example, gear restrictions often have greater support from local fishers (49) and are usually implemented over greater reef areas than marine reserves. We show here that there are conservation gains produced by gear restrictions, although they are low relative to marine reserves (Fig. 4). Thus, in locations where a lack of support makes establishing marine reserves untenable, gear restrictions may still provide incremental gains toward achieving some conservation goals (8), particularly for specific fish groups and families (39).
As a supplemental analysis, we examined the conservation gains for biomass of nontarget species (SI Appendix, Figs. S1D and S4). This supplemental analysis addresses whether the effects of gravity on reef fish communities are from fishing or other impacts, such as sedimentation or pollution. We found very different patterns for nontarget species compared with target species, suggesting the relationship between target fish biomass and gravity (SI Appendix, Fig. S1) is primarily driven by fishing pressure.
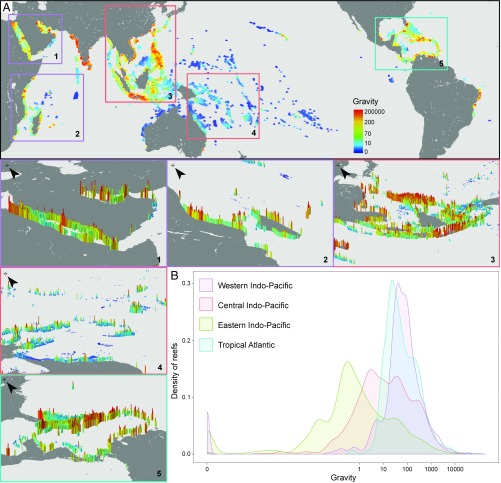
Overall, our results demonstrate that the capacity to not only sustain reef fish biomass and the presence of top predators, but also the potential to achieve conservation gains, may be highly dependent on the level of human impact in the surrounding seascape. It is therefore essential to consider the global context of present and future human gravity in coral reef governance. Consequently, we calculated gravity of human impacts for every reef cell globally using a 10- × 10-km grid across the world’s coral reefs (Fig. 5). Critically, the distribution of gravity varies substantially among regions, with the central and eastern Indo-Pacific demonstrating lower-gravity values. Even within a region, there can be substantial variability in gravity values. For example, the Central Indo-Pacific has highly contrasting gravity patterns, with Southeast Asian reefs (Fig. 5 A, 3) generally showing extremely high-gravity values while Australian and Melanesian reefs (Fig. 5 A, 4) are dominated by relatively low-gravity values.
Fig. 5.
Distribution of gravity on the world’s coral reefs. (A) Map of gravity calculated for every coral reef in the world ranging from blue (low gravity) to red (high gravity). The four coral reef realms (70) are delineated. Insets highlight gravity for key coral reef regions of the world: (1) Red Sea, (2) Western Indian Ocean, (3) Southeast Asia, (4) Great Barrier Reef of Australia and the South Pacific, (5) Caribbean. For visual effect, gravity values in Inset maps are also given vertical relief, with higher relief indicating higher gravity values. (B) Distribution of gravity values per coral reef realm.
The ways in which gravity will increase over time, and how the impacts of gravity on reef systems can be reduced, is of substantial concern for coral reef governance. The potential benefits of protecting locations that are currently remote could increase over time as human populations and the accessibility of reefs change (50). Demographic projections of high migration and fertility rates in some countries suggest substantial increases in coastal human populations in developing countries, where the majority of coral reefs are located (5, 51–53). Development projects that address high rates of fertility through improvements in women’s education, empowerment, and the expansion of family-planning opportunities have successfully reduced fertility rates (54, 55). Such initiatives, when partnered with resource management, have the potential to be beneficial to both people and reefs. Demographic changes, such as increased migration in coastal areas, are also expected to be coupled with coastal development and road building that will increase the accessibility of reefs. For example, previously uninhabited areas have become more accessible, as evidenced by China’s recent Belt and Roads Initiative and island-building enterprise in the South China Sea (56–58). Investments in sustainable planning of coastal development and road building could help to minimize unnecessary increases in reef accessibility. Importantly, stemming increases in gravity is only part of the potential solution space: it will also be important to dampen the mechanisms through which gravity operates, such that a given level of gravity can have a lesser impact on reef systems (1). People’s environmental behavior is fundamentally driven by their social norms, tastes, values, practices, and preferences (59), all of which can be altered by policies, media, and other campaigns in ways that could change the local relationship between gravity and reef degradation.
Gravity Future Directions
Our gravity index (Materials and Methods and Box 1) makes several key assumptions that could potentially be refined in further applications. First, our application of gravity held friction constant across each specific type of surface (i.e., all paved roads had the same friction value). Future applications of more localized studies could vary travel time to reflect the quality of road networks, topographic barriers to access (such as cliffs), and the availability of technology. Similarly, future applications could also aim to incorporate local information about fishing fleet efficiency. Second, our adaptation of the gravity model (31) is unidirectional, assuming a constant level of attraction from any reef (i.e., gravity varies based on human population size, but not on the quality or quantity of fish on a specific reef). Reefs with more fish, or higher fish value, could be more attractive and exert a higher pull for exploitation (60). Likewise, societal values and preferences can also make certain fish more or less attractive. Our adaptation of gravity was designed to examine the observed conditions of reefs as a function of potential interactions with markets and local settlements, so our modification of the concept for this application was appropriate. However, future applications wishing to predict where reefs may be most vulnerable might wish to consider incorporating fish biomass or composition (i.e., potential market price of reef fish) in the gravity equation. Third, our database was not designed to look at ecological changes in a single location over time. However, future applications could examine whether ecological recovery in reserves (8) depends on the level of gravity present. To this end, we provide a global dataset of gravity for every reef pixel globally (Materials and Methods).
We demonstrate that human impacts deplete reef fish stocks and how certain types of management can mediate but not eliminate these pressures. In an era of increasing change, the global network of marine reserves may not safeguard reef fish communities from human impacts adequately enough to ensure key ecological functions, such as predation, are sustained. Efforts must be made to both reduce and dampen key drivers of change (1, 61), while maintaining or improving the well-being of reef-dependent people. Importantly, we find evidence that both remote and human-surrounded reserves can produce different types of conservation gains. Ultimately, multiple forms of management are needed across the seascape to sustain coral reef fishes and the people who depend upon them.
Materials and Methods
Scales of Data.
Our data were organized at three spatial scales: reef site (n = 1,798), reef cluster (n = 734), and nation/state (n = 41).
Reef site is the smallest scale, which had an average of 2.4 surveys (transects), hereafter referred to as “reef.”
For reef cluster (which had an average of 2.4 ± 2.4 reef sites), we clustered reefs together that were within 4 km of each other, and used the centroid to estimate reef cluster-level social and environmental covariates. To define reef clusters, we first estimated the linear distance between all reef sites, then used a hierarchical analysis with the complete-linkage clustering technique based on the maximum distance between reefs. We set the cut-off at 4 km to select mutually exclusive sites where reefs cannot be more distant than 4 km. The choice of 4 km was informed by a 3-y study of the spatial movement patterns of artisanal coral reef fishers, corresponding to the highest density of fishing activities on reefs based on GPS-derived effort density maps of artisanal coral reef fishing activities (62). This clustering analysis was carried out using the R functions “hclust” and “cutree.”
A larger scale in our analysis was “nation/state” (nation, state, or territory, which had an average of 44 ± 59 reef clusters), which are jurisdictions that generally correspond to individual nations (but could also include states, territories, overseas regions), within which sites were nested for analysis.
Targeted fish biomass.
Reef fish biomass estimates were based on visual counts in 1,798 reef sites. All surveys used standard belt-transects, distance sampling, or point-counts, and were conducted between 2004 and 2013. Where data from multiple years were available from a single reef site, we included only data from the year closest to 2010. Within each survey area, reef-associated fishes were identified to species level, their abundance counted, and total length (TL) estimated, with the exception of one data provider who measured biomass at the family level. To make estimates of targeted biomass from these transect-level data comparable among studies, we did the following. (i) We retained families that were consistently studied, commonly targeted, and were above a minimum size cut-off. Thus, we retained counts of >10 cm diurnally active, noncryptic reef fish that are resident on the reef (14 families), excluding sharks and semipelagic species (SI Appendix, Table S1). We calculated total biomass of targeted fishes on each reef using standard published species-level length–weight relationship parameters or those available on FishBase (63). When length–weight relationship parameters were not available for a species, we used the parameters for a closely related species or genus. For comparison, we also calculated nontarget fish biomass (SI Appendix, Table S1). (ii) We directly accounted for depth and habitat as covariates in the model (see Environmental Drivers, below). (iii) We accounted for differences among census methods by including each census method (standard belt-transects, distance sampling, or point-counts) as a covariate in the model. (iv) We accounted for differences in sampling area by including total sampling area for each reef (m2) as a covariate in the model.
Top predators.
We examined the presence/absence of eight families of fish considered top predators (SI Appendix, Table S1). We considered presence/absence instead of biomass because biomass was heavily zero inflated.
Gravity.
We first developed a gravity index for each of our reef sites where we had in situ ecological data. We gathered data on both population estimates and a surrogate for distance: travel time.
Population estimates.
We gathered population estimates for each 1- by 1-km cell within a 500-km radius of each reef site using LandScan 2011 database. We chose a 500-km radius from the reef as a likely maximum distance fishing activities for reef fish are likely to occur.
Travel time calculation.
The following procedure was repeated for each populated cell within the 500-km radius. Travel time was computed using a cost–distance algorithm that computes the least “cost” (in minutes) of traveling between two locations on a regular raster grid. In our case, the two locations were the centroid of the reef site and the populated cell of interest. The cost (i.e., time) of traveling between the two locations was determined by using a raster grid of land cover and road networks with the cells containing values that represent the time required to travel across them (32) (SI Appendix, Table S2), we termed this raster grid a “friction-surface” (with the time required to travel across different types of surfaces analogous to different levels of friction). To develop the friction-surface, we used global datasets of road networks, land cover, and shorelines:
Road network data were extracted from the Vector Map Level 0 (VMap0) from the National Imagery and Mapping Agency’s (NIMA) Digital Chart of the World (DCW). We converted vector data from VMap0 to 1 km resolution raster.
Land cover data were extracted from the Global Land Cover 2000 (64).
To define the shorelines, we used the GSHHS (Global Self-consistent, Hierarchical, High-resolution Shoreline) database v2.2.2.
These three friction components (road networks, land cover, and shorelines) were combined into a single friction surface with a Behrmann map projection (an equal area projection). We calculated our cost-distance models in R using the accCost function of the “gdistance” package. The function uses Dijkstra’s algorithm to calculate least-cost distance between two cells on the grid taking into account obstacles and the local friction of the landscape (65). Travel time estimates over a particular surface could be affected by the infrastructure (e.g., road quality) and types of technology used (e.g., types of boats). These types of data were not available at a global scale but could be important modifications in more localized studies.
Gravity computation.
To compute gravity, we calculated the population of the cell and divided that by the squared travel time between the reef site and the cell. We summed the gravity values for each cell within 500 km of the reef site to get the “total gravity” within 500 km. We used the squared distance (or in our case, travel time), which is relatively common in geography and economics, although other exponents can be used (31) (Table S3).
We also developed a global gravity index for each 10- × 10-km grid of reef in the world (Box 1), which we provide as an open-access dataset. The procedure to calculate gravity was similar to above with the only difference being in the precision of the location; the former was a single data point (reef site), while the latter was a grid cell (reef cell). For the purpose of the analysis, gravity was log-transformed and standardized.
We also explored various exponents (1–3) and buffer sizes (50, 250, and 500 km) to build nine gravity metrics. The metric providing the best model, so with the lowest Akaike Information Criterion (AIC), was that with a squared exponent for travel time and a 500-km buffer (SI Appendix, Table S3).
Management.
For each observation, we determined the prevailing type of management, including the following. (i) Marine reserve (whether the site fell within the borders of a no-take marine reserve): we asked data providers to further classify whether the reserve had high or low levels of compliance. For this analysis, we removed sites that were categorized as low-compliance reserves (n = 233). (ii) Restricted fishing: whether there were active restrictions on gears (e.g., bans on the use of nets, spearguns, or traps) or fishing effort (which could have included areas inside marine protected areas that were not necessarily no take). Or (iii) openly fished: regularly fished without effective restrictions. To determine these classifications, we used the expert opinion of the data providers, and triangulated this with a global database of marine reserve boundaries (66). We also calculated size (median = 113.6 km2, mean = 217,516 km2, SD = 304,417) and age (median = 9, mean = 15.5 y, SD = 14.5) of the no-take portion of each reserve. Reserve size was strongly related to our metric of gravity and could not be directly included in the analysis. We conducted a supplemental analysis where we separated reserves into small (≤28 km2) and large (>65 km2) based on a natural break in the data to illustrate: (i) how biomass and the presence of top predators might differ between small and large reserves; and (ii) how large reserves are absent in our sample in high gravity.
Other Social Drivers.
To account for the influence of other social drivers that are thought to be related to the condition of reef fish biomass, we also included the following covariates in our model.
Local population growth.
We created a 100-km buffer around each site and used this to calculate human population within the buffer in 2000 and 2010 based on the Socioeconomic Data and Application Centre gridded population of the world database. Population growth was the proportional difference between the population in 2000 and 2010. We chose a 100-km buffer as a reasonable range at which many key human impacts from population (e.g., land-use and nutrients) might affect reefs (67).
Human development index.
Human development index (HDI) is a summary measure of human development encompassing a long and healthy life, being knowledgeable, and having a decent standard of living. In cases where HDI values were not available specific to the state (e.g., Florida and Hawaii), we used the national (e.g., United States) HDI value.
Population size.
For each nation/state, we determined the size of the human population. Data were derived mainly from the national census reports CIA fact book (https://www.cia.gov/library/publications/the-world-factbook/rankorder/2119rank.html), and Wikipedia (https://en.wikipedia.org/wiki/Main_Page). For the purpose of the analysis, population size was log-transformed.
Environmental Drivers.
Depth.
The depth of reef surveys was grouped into the following categories: <4 m, 4–10 m, >10 m to account for broad differences in reef fish community structure attributable to a number of interlinked depth-related factors. Categories were necessary to standardize methods used by data providers and were determined by preexisting categories used by several data providers.
Habitat.
We included the following habitat categories. (i) Slope: the reef slope habitat is typically on the ocean side of a reef, where the reef slopes down into deeper water. (ii) Crest: the reef crest habitat is the section that joins a reef slope to the reef flat. The zone is typified by high wave energy (i.e., where the waves break). It is also typified by a change in the angle of the reef from an inclined slope to a horizontal reef flat. (iii) Flat: the reef flat habitat is typically horizontal and extends back from the reef crest for tens to hundreds of meters. (iv) Lagoon/back reef: lagoonal reef habitats are where the continuous reef flat breaks up into more patchy reef environments sheltered from wave energy. These habitats can be behind barrier/fringing reefs or within atolls. Back reef habitats are similar broken habitats where the wave energy does not typically reach the reefs and thus forms a less continuous “lagoon style” reef habitat. Due to minimal representation among our sample, we excluded other less-prevalent habitat types, such as channels and banks. To verify the sites’ habitat information, we used the Millennium Coral Reef Mapping Project hierarchical data (68), Google Earth, and site depth information.
Productivity.
We examined ocean productivity for each of our sites in milligrams of C per square meter per day (mg C m−2 d−1) (www.science.oregonstate.edu/ocean.productivity/). Using the monthly data for years the 2005–2010 (in hdf format), we imported and converted those data into ArcGIS. We then calculated yearly average and finally an average for all these years. We used a 100-km buffer around each of our sites and examined the average productivity within that radius. Note that ocean productivity estimates are less accurate for nearshore environments, but we used the best available data. For the purpose of the analysis, productivity was log-transformed.
Climate stress.
We included an index of climate stress for corals, developed by Maina et al. (69), which incorporated 11 different environmental conditions, such as the mean and variability of sea-surface temperature.
Analyses.
We first looked for collinearity among our covariates using bivariate correlations and variance inflation factor estimates. This led to the exclusion of several covariates (not described above): (i) Biogeographic Realm (Tropical Atlantic, western Indo-Pacific, Central Indo-Pacific, or eastern Indo-Pacific); (ii) Gross Domestic Product (purchasing power parity); (iii) Rule of Law (World Bank governance index); (iv) Control of Corruption (World Bank governance index); (v) Voice and Accountability (World Bank governance index); (vi) Reef Fish Landings; (vii) Tourism arrivals relative to local population; (viii) Sedimentation; and (ix) Marine Reserve Size. Other covariates had correlation coefficients 0.7 or less and Variance Inflation Factor scores less than 5 (indicating multicollinearity was not a serious concern). Care must be taken in causal attribution of covariates that were significant in our model, but demonstrated collinearity with candidate covariates that were removed during the aforementioned process. Importantly, the covariate of interest in this study, gravity, was not strongly collinear with candidate covariates except reserve size (r = −0.8, t = 3.6, df = 104, P = 0.0004).
To quantify the relationships between gravity and target fish biomass, we developed a general linear-mixed model in R, using a log-normal distribution for biomass. To quantify the relationships between gravity and presence/absence of top predators, we developed a generalized linear-mixed model with a binomial family and a logit link function. For both models, we set reef cluster nested within nation/state as a random effect to account for the hierarchical nature of the data (i.e., reef sites nested in reef clusters, reef clusters nested in nation/states). We included an interaction between gravity and reserve age, as well as all of the other social and environmental drivers and the sampling method and total sampling area as covariates. We also tested interactions between gravity and management and used AIC to select the most parsimonious model. For fish biomass, the interaction between gravity and reserve age had AIC values >2 lower than the interaction between gravity and management (and a combination of both interactions). For the top predator models, both interactions were within 2 AIC values, so we chose the interaction with reserve age for consistency. All continuous covariates were standardized for the analysis, and reserve age was then normalized such that nonreserves were 0 and the oldest reserves were 1. In summary, our models thus predicted target fish biomass or probability of top predators being observed at the reef site scale with an interaction between gravity and reserve age, while accounting within the random factors for two bigger scales at which the data were collected (reef cluster, and nation/state) (SI Appendix), and key social and environmental characteristics expected to influence the biomass of reef fish (14). In addition to coefficient plots (SI Appendix, Fig. S1), we conducted a supplemental analysis of relative variable importance (SI Appendix, Table S4).
We ran the residuals from the models against size of the no-take areas of the marine reserves and no patterns were evident, suggesting it would explain no additional variance in the model. Trend lines and partial plots (averaged by site and nation/state) are presented in the figures (Figs. 2 B–H and 3H). We plotted the partial effect of the relationship between gravity and protection on targeted fish biomass and presence of top predators (Figs. 2 B–G and 3 B–G) by setting all other continuous covariates to 0 because they were all standardized and all categorical covariates to their most common category (i.e., 4–10 m for depth, slope for habitat, standard belt transect for census method). For age of reserves, we set this to 0 for fished and restricted areas, and to the average age of reserves (15.5 y) for reserves.
To examine the expected conservation gains of different management strategies, we calculated: (i) the difference between the response of openly fished areas (our counterfactual) and high-compliance marine reserves to gravity; and (ii) the difference between the response of openly fished areas and fisheries restricted areas to gravity. For ease of interpretation, we plotted conservation gains in kilograms per hectare (kg/ha; as opposed to log[kg/ha]) (Fig. 4A). A log-normal (linear) model was used to develop the slopes of the biomass (i) fished, (ii) marine reserve, and (iii) fisheries restricted areas, which results in the differences between (i) and (ii) and between (i) and (iii) being nonlinear on an arithmetic scale (Fig. 4A).
We plotted the diagnostic plots of the general linear-mixed model to check that the model assumptions were not violated. To check the fit of the generalized linear-mixed model, we used the confusion matrix (tabular representation of actual versus predicted values) to calculate the accuracy of the model, which came to 79.2%.
To examine homoscedasticity, we checked residuals against fitted values. We checked our models against a null model, which contained the model structure (i.e., random effects), but no covariates. We used the null model as a baseline against which we could ensure that our full model performed better than a model with no covariate information. In all cases our models outperformed our null models by more than 2 AIC values, indicating a more parsimonious model.
All analyses were undertaken using R (3.43) statistical package.
Data Access.
A gridded global gravity data layer is freely available at dx.doi.org/10.4225/28/5a0e7b1b3cc0e. The ecological data used in this report are owned by individual data providers. Although much of these data (e.g., NOAA CRED data, and Reef Life Surveys) are already open access, some of these data are governed by intellectual property arrangements and cannot be made open access. Because the data are individually owned, we have agreed upon and developed a structure and process for those wishing access to the data. Our process is one of engagement and collaboration with the data providers. Anyone interested can send a short (one-half to one page) proposal for use of the database that details the problem statement, research gap, research question(s), and proposed analyses to the Principle Investigator and database administrator Joshua.cinner@jcu.edu.au, who will send the proposal to the data providers. Individual data providers can agree to make their data available or not. They can also decide whether they would like to be considered as a potential coauthor if their data are used. The administrator will then send only the data that the providers have agreed to make available.
Supplementary Material
Acknowledgments
We thank J. Zamborian Mason and A. Fordyce for assistance with analyses and figures, and to numerous scientists who collected data used in the research. The Australian Research Council Centre of Excellence for Coral Reef Studies and The Pew Charitable Trusts funded working group meetings.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: A gridded global gravity data layer is freely available at dx.doi.org/10.4225/28/5a0e7b1b3cc0e.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708001115/-/DCSupplemental.
References
- 1.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 4.Teh LSL, Teh LCL, Sumaila UR. A global estimate of the number of coral reef fishers. PLoS One. 2013;8:e65397. doi: 10.1371/journal.pone.0065397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora C, et al. Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol. 2011;9:e1000606. doi: 10.1371/journal.pbio.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora C, Chittaro PM, Sale PF, Kritzer JP, Ludsin SA. Patterns and processes in reef fish diversity. Nature. 2003;421:933–936. doi: 10.1038/nature01393. [DOI] [PubMed] [Google Scholar]
- 7.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 8.MacNeil MA, et al. Recovery potential of the world’s coral reef fishes. Nature. 2015;520:341–344. doi: 10.1038/nature14358. [DOI] [PubMed] [Google Scholar]
- 9.Hopf JK, Jones GP, Williamson DH, Connolly SR. Synergistic effects of marine reserves and harvest controls on the abundance and catch dynamics of a coral reef fishery. Curr Biol. 2016;26:1543–1548. doi: 10.1016/j.cub.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Krueck NC, et al. Marine reserve targets to sustain and rebuild unregulated fisheries. PLoS Biol. 2017;15:e2000537. doi: 10.1371/journal.pbio.2000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgar GJ, et al. Global conservation outcomes depend on marine protected areas with five key features. Nature. 2014;506:216–220. doi: 10.1038/nature13022. [DOI] [PubMed] [Google Scholar]
- 12.McClanahan TR, Marnane MJ, Cinner JE, Kiene WE. A comparison of marine protected areas and alternative approaches to coral-reef management. Curr Biol. 2006;16:1408–1413. doi: 10.1016/j.cub.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 13.Gill DA, et al. Capacity shortfalls hinder the performance of marine protected areas globally. Nature. 2017;543:665–669. doi: 10.1038/nature21708. [DOI] [PubMed] [Google Scholar]
- 14.Cinner JE, et al. Bright spots among the world’s coral reefs. Nature. 2016;535:416–419. doi: 10.1038/nature18607. [DOI] [PubMed] [Google Scholar]
- 15.Williams ID, et al. PLOS ONE Staff Correction: Human, oceanographic and habitat drivers of central and western Pacific coral reef fish assemblages. PLoS One. 2015;10:e0129407. doi: 10.1371/journal.pone.0129407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozec YM, O’Farrell S, Bruggemann JH, Luckhurst BE, Mumby PJ. Tradeoffs between fisheries harvest and the resilience of coral reefs. Proc Natl Acad Sci USA. 2016;113:4536–4541. doi: 10.1073/pnas.1601529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulvy NK, Freckleton RP, Polunin NVC. Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol Lett. 2004;7:410–416. [Google Scholar]
- 18.McClanahan TR, et al. Critical thresholds and tangible targets for ecosystem-based management of coral reef fisheries. Proc Natl Acad Sci USA. 2011;108:17230–17233. doi: 10.1073/pnas.1106861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollnac R, et al. Marine reserves as linked social-ecological systems. Proc Natl Acad Sci USA. 2010;107:18262–18265. doi: 10.1073/pnas.0908266107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergseth BJ, Russ GR, Cinner JE. Measuring and monitoring compliance in no-take marine reserves. Fish Fish. 2015;16:240–258. [Google Scholar]
- 21.Graham NAJ, McClanahan TR. The last call for marine wilderness? Bioscience. 2013;63:397–402. [Google Scholar]
- 22.Cinner JE, et al. Linking social and ecological systems to sustain coral reef fisheries. Curr Biol. 2009;19:206–212. doi: 10.1016/j.cub.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 23.Pressey RL, Visconti P, Ferraro PJ. Making parks make a difference: Poor alignment of policy, planning and management with protected-area impact, and ways forward. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140280. doi: 10.1098/rstb.2014.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devillers R, et al. Reinventing residual reserves in the sea: Are we favouring ease of establishment over need for protection? Aquat Conserv. 2015;25:480–504. [Google Scholar]
- 25.Andrello M, et al. Global mismatch between fishing dependency and larval supply from marine reserves. Nat Commun. 2017;8:16039. doi: 10.1038/ncomms16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison HB, et al. Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr Biol. 2012;22:1023–1028. doi: 10.1016/j.cub.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Januchowski-Hartley FA, Graham NAJ, Cinner JE, Russ GR. Spillover of fish naïveté from marine reserves. Ecol Lett. 2013;16:191–197. doi: 10.1111/ele.12028. [DOI] [PubMed] [Google Scholar]
- 28.Ravenstein EG. The laws of migration. J R Stat Soc. 1889;52:241–305. [Google Scholar]
- 29.Dodd SC. The interactance hypothesis: A gravity model fitting physical masses and human groups. Am Sociol Rev. 1950;15:245–256. [Google Scholar]
- 30.Bergstrand JH. The gravity equation in international trade: Some microeconomic foundations and empirical evidence. Rev Econ Stat. 1985;67:474–481. [Google Scholar]
- 31.Anderson JE. The gravity model. Annu Rev Econ. 2011;3:133–160. [Google Scholar]
- 32.Maire E, et al. How accessible are coral reefs to people? A global assessment based on travel time. Ecol Lett. 2016;19:351–360. doi: 10.1111/ele.12577. [DOI] [PubMed] [Google Scholar]
- 33.Januchowski-Hartley FA, Graham NAJ, Cinner JE, Russ GR. Local fishing influences coral reef fish behavior inside protected areas of the Indo-Pacific. Biol Conserv. 2015;182:8–12. [Google Scholar]
- 34.Gil MA, Hein AM. Social interactions among grazing reef fish drive material flux in a coral reef ecosystem. Proc Natl Acad Sci USA. 2017;114:4703–4708. doi: 10.1073/pnas.1615652114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergseth BJ, Williamson DH, Russ GR, Sutton SG, Cinner JE. A social-ecological approach to assessing and managing poaching by recreational fishers. Front Ecol Environ. 2017;15:67–73. [Google Scholar]
- 36.Ward-Paige CA, et al. Large-scale absence of sharks on reefs in the greater-Caribbean: A footprint of human pressures. PLoS One. 2010;5:e11968. doi: 10.1371/journal.pone.0011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krueck Nils C, et al. Reserve sizes needed to protect coral reef fishes. Conserv Lett. 2017;0:1–9. [Google Scholar]
- 38.Christie P, et al. Why people matter in ocean governance: Incorporating human dimensions into large-scale marine protected areas. Mar Policy. 2017;84:273–284. [Google Scholar]
- 39.Campbell SJ, Edgar GJ, Stuart-Smith RD, Soler G, Bates AE. Fishing-gear restrictions and biomass gains for coral reef fishes in marine protected areas. Conserv Biol. 2018;32:401–410. doi: 10.1111/cobi.12996. [DOI] [PubMed] [Google Scholar]
- 40.McClanahan TR, Graham NAJ, Calnan JM, MacNeil MA. Toward pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecol Appl. 2007;17:1055–1067. doi: 10.1890/06-1450. [DOI] [PubMed] [Google Scholar]
- 41.O’Leary BC, et al. Addressing criticisms of large-scale marine protected areas. Bioscience. 2018;68:359–370. doi: 10.1093/biosci/biy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraro PJ, Pressey RL. Measuring the difference made by conservation initiatives: Protected areas and their environmental and social impacts Introduction. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140270. doi: 10.1098/rstb.2014.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beger M, et al. Integrating regional conservation priorities for multiple objectives into national policy. Nat Commun. 2015;6:8208. doi: 10.1038/ncomms9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boon PY, Beger M. The effect of contrasting threat mitigation objectives on spatial conservation priorities. Mar Policy. 2016;68:23–29. [Google Scholar]
- 45.Clark P. August 6, 2014 Shark fin sales in China take a dive. Financial Times. Available at https://www.ft.com/content/4be44336-1c9f-11e4-98d8-00144feabdc0. Accessed May 25, 2018.
- 46.Thyresson M, Nyström M, Crona B. Trading with resilience: Parrotfish trade and the exploitation of key-ecosystem processes in coral reefs. Coast Manage. 2011;39:396–411. [Google Scholar]
- 47.Green AL, et al. Designing marine reserves for fisheries management, biodiversity conservation, and climate change adaptation. Coast Manage. 2014;42:143–159. [Google Scholar]
- 48.Bennett NJ, et al. Conservation social science: Understanding and integrating human dimensions to improve conservation. Biol Conserv. 2017;205:93–108. [Google Scholar]
- 49.McClanahan TR, Abunge CA. Perceptions of fishing access restrictions and the disparity of benefits among stakeholder communities and nations of south-eastern Africa. Fish Fish. 2016;17:417–437. [Google Scholar]
- 50.Watson RA, et al. Marine foods sourced from farther as their use of global ocean primary production increases. Nat Commun. 2015;6:7365. doi: 10.1038/ncomms8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerland P, et al. World population stabilization unlikely this century. Science. 2014;346:234–237. doi: 10.1126/science.1257469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mora C. Revisiting the environmental and socioeconomic effects of population growth: A fundamental but fading issue in modern scientific, public, and political circles. Ecol Soc. 2014;19:38. [Google Scholar]
- 53.Mora C. Perpetual struggle for conservation in a crowded world and the needed paradigm shift for easing ultimate burdens. In: Mora C, editor. Ecology of Fishes on Coral Reefs. Cambridge Univ Press; Cambridge, UK: 2015. pp. 289–296. [Google Scholar]
- 54.Cottingham J, Germain A, Hunt P. Use of human rights to meet the unmet need for family planning. Lancet. 2012;380:172–180. doi: 10.1016/S0140-6736(12)60732-6. [DOI] [PubMed] [Google Scholar]
- 55.Sen A. The ends and means of sustainability. J Human Dev Capabil. 2013;14:6–20. [Google Scholar]
- 56.Mora C, Caldwell IR, Birkeland C, McManus JW. PLOS Biology Staff Dredging in the Spratly Islands: Gaining land but losing reefs. PLoS Biol. 2016;14:e1002497. doi: 10.1371/journal.pbio.1002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurance WF, Arrea IB. Roads to riches or ruin? Science. 2017;358:442–444. doi: 10.1126/science.aao0312. [DOI] [PubMed] [Google Scholar]
- 58.Alamgir M, et al. Economic, socio-political and environmental risks of road development in the tropics. Curr Biol. 2017;27:R1130–R1140. doi: 10.1016/j.cub.2017.08.067. [DOI] [PubMed] [Google Scholar]
- 59.Hicks CC, Crowder LB, Graham NAJ, Kittinger JN, Le Cornu E. Social drivers forewarn of marine regime shifts. Front Ecol Environ. 2016;14:253–261. [Google Scholar]
- 60.Berkes F, et al. Ecology. Globalization, roving bandits, and marine resources. Science. 2006;311:1557–1558. doi: 10.1126/science.1122804. [DOI] [PubMed] [Google Scholar]
- 61.Cinner JE, Kittinger JN. Linkages between social systems and coral reefs. In: Mora C, editor. Ecology of Fishes on Coral Reefs. Cambridge Univ Press; Cambridge, UK: 2015. pp. 215–220. [Google Scholar]
- 62.Daw T, et al. 2011 The Spatial Behaviour of Artisanal Fishers: Implications for Fisheries Management and Development (Fishers in Space). Available at https://www.researchgate.net/profile/Pascal_Thoya2/publication/321796381_The_spatial_behaviour_of_artisanal_fishers_Implications_for_fisheries_management_and_development_Fishers_in_Space_Final_Report/links/5a9ce57d0f7e9be379682636/The-spatial-behaviour-of-artisanal-fishers-Implications-for-fisheries-management-and-development-Fishers-in-Space-Final-Report.pdf?origin=publication_list. Accessed May 25, 2018.
- 63.Froese R, Pauly D. 2012 FishBase: World Wide Web Electronic Publication. Available at https://www.fishbase.de/home.htm. Accessed May 25, 2018.
- 64.Bartholomé E, et al. 2002 GLC 2000: Global land cover mapping for the year 2000: Project status November 2002. Available at https://www.researchgate.net/publication/235707577_GLC_2000_Global_Land_Cover_Mapping_for_the_Year_2000_Project_Status_November_2002. Accessed May 25, 2018.
- 65.Nelson A. Travel Time to Major Cities: A Global Map of Accessibility. Global Environment Monitoring Unit-Joint Research Centre of the European Commission; Ispra, Italy: 2008. [Google Scholar]
- 66.IUCN UNEP-WCMC 2016 The World Database on Protected Areas (WDPA) (UNEP-WCMC, Cambridge, UK). Available at https://www.protectedplanet.net/. Accessed May 25, 2018.
- 67.MacNeil MA, Connolly SR. Multi-scale patterns and processes in reef fish abundance. In: Mora C, editor. Ecology of Fishes on Coral Reefs. Cambridge Univ Press; Cambridge, UK: 2015. pp. 116–126. [Google Scholar]
- 68.Andréfouët S, et al. Global assessment of modern coral reef extent and diversity for regional science and management applications: A view from space. In: Suzuki Y, et al., editors. Tenth International Coral Reef Symposium. Japanese Coral Reef Society; Okinawa, Japan: 2006. pp. 1732–1745. [Google Scholar]
- 69.Maina J, McClanahan TR, Venus V, Ateweberhan M, Madin J. Global gradients of coral exposure to environmental stresses and implications for local management. PLoS One. 2011;6:e23064. doi: 10.1371/journal.pone.0023064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spalding MD, et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–583. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.