Significance
Humans are a polymorphic species with a broad geographical distribution. Diversity in growth and development plays an important role in biological adaptation and can be addressed through studies of life-history variation across different populations, particularly in hunter-gatherer societies. This paper reports the results from our study on fertility and mortality in the Baka pygmies based on individuals of known age. The Baka are characterized by low infant and juvenile mortalities, slow growth, and high fertility at an early age. However, the arrival of cheap alcohol has drastically reduced fertility early in life, which seriously compromises this population’s survival. We provide empirical evidence of the effects of alcohol consumption on the fertility rate of a hunter-gatherer society.
Keywords: Baka pygmies, hunter-gatherers, fertility, mortality, alcohol
Abstract
To understand the diversity of human growth and development from an evolutionary point of view, there is an urgent need to characterize the life-history variables of vanishing forager societies. The small body size of the Baka pygmies is the outcome of a low growth rate during infancy. While the ages at sexual maturity, menarche, and first delivery are similar to those in other populations, fertility aspects are unknown. In the Le Bosquet district in Cameroon, thanks to systematic birth records kept from 1980 onwards, we were able to assign ages to individuals with certainty. This study, based on chronological records and on data collected from 2007 to 2017, presents life-history variables related to fertility and mortality among the Baka pygmies: total fertility rate, age-specific fertility rate, completed family size, reproductive span, age at menopause, and infant and juvenile mortality. The Baka present low infant and juvenile mortality, and their fertility pattern differs from that of other forager societies in the higher age-specific fertility rates found in the two lower age classes. Future studies will need to assess whether this particular pattern and the short interbirth interval are related to highly cooperative childrearing, which in the Baka is associated with slow growth. The fertility rate has fallen drastically since 2011, and this matches the arrival of cheap alcohol in the community. Our data provide a first-hand record of the impact of alcohol on fertility in a hunter-gatherer society which appears to be seriously compromising the survival of the Baka.
Our species, Homo sapiens, is the most polymorphic species of primate and is distinct from all others in the wide range of contrasting environmental conditions it inhabits. Although culture in the broad sense is largely responsible for the broad geographical distribution of our species, biological plasticity has certainly played a fundamental role in our adaptation (1). Biological adaptation to environmental conditions depends on the allocation of an organism’s energy to growth, maintenance, reproduction (including raising offspring to independence), and avoiding death. The timing of key developmental events that determine the pace at which these factors play out within the lives of humans is called “life history” and is described by a suite of life-history variables (LHVs) that include age at first reproduction, number of offspring, interbirth interval (IBI), age at menopause, infant mortality, and survival to maturity (2).
LHVs are well known for several modern human groups (3), and their variability through time can be tracked over several decades. However, a broad knowledge of the variability of life history in our species requires studies of LHVs in many human societies. Along this line of reasoning, as argued by Hill and Hurtado (4), the adaptive functional significance of human life history can be understood better by studying forager societies (5). Although variation in life history has been studied across several forager societies, these studies have shown that significant differences exist between societies living under dissimilar environmental conditions (4, 6–8). This has led to the suggestion that no single society can be taken as representative of all hunter-gatherers (4) and, more importantly, that we need broad-ranging studies of LHVs in more societies to understand the variation we observe across hunter-gatherer societies.
Particular attention has been paid to populations with a small adult body size (6, 7). From an evolutionary developmental perspective, adult size is the final product of growth. Energy acquired throughout life must be allocated to growth, on the one hand, and reproduction, on the other (9). A small adult body size therefore implies a specific growth pattern with low energy allocated to it but enough to ensure an adult size capable of producing the necessary energy for reproduction. Migliano et al. (10) have suggested that small body size in the Aeta results from precocious reproduction due to a high mortality rate; in other words, small body size might be a by-product of growth ceasing at an early age to divert energy away from growth and toward reproductive activity. However, this observation cannot be extrapolated to all forager societies with a small body size. For instance, among the Baka pygmies in Cameroon (SI Appendix, Text S1), a low rate of growth from birth to 2.5 y of age accounts for the pygmy phenotype, and this process is not followed by any change in the timing of sexual maturation (i.e., age at menarche and age at first reproduction) (11). However, it is not known whether fertility parameters such as the total fertility rate (TFR), age-specific fertility rate (ASFR), and age at menopause are affected by the particular growth pattern that produces a small adult size. Knowledge of these parameters is fundamental to understand life-history variations since a small adult body size produces less energy to be allocated to reproduction by lowering the fertility rate or by advancing the age at menopause. The aim of this study is thus to characterize fertility in the Baka.
The relationship between fertility and mortality is often used to build models for demographic transitions and is essential to understand population dynamics (12). Any change in fertility is associated with a change in mortality. At the population level, a reduction in the fertility rate is expected after a drop in the infant mortality rate; conversely, a rise in fertility can be observed after high mortality due to natural disasters (13). At the family level, the average birth interval is substantially shorter following an infant death than when an infant survives (14). Mortality in infants and mothers is complementary to data on fertility aspects.
During our 10-y study, we witnessed the arrival of cheap alcohol which became widely available to the community. We observed that changes in human fertility occurred at that point. The relationship between these two phenomena is discussed.
Overview: Moange Le Bosquet
Most studies on life-history variation as an aspect of the demographics of forager societies have been hampered by the lack of chronological data for individuals. This is a fundamental problem because our understanding of population dynamics and evolution revolves around processes that occur at known chronological stages. Except for Early and Headland’s work (7) on the Agta, in which the ages of 271 of the 857 individuals (31.6%) were known (117 individuals to within a year, 80 to within a month, and 74 exactly to the day; see table 4.1 in ref. 7), authors have had to develop various methods to estimate the missing chronological information (15). From data provided by many scholars, Weiss (16) built model life tables to infer, from a limited number of variables, many demographic/life-history parameters, such as life expectancy or the ASFR for each 5-y age cohort. Until recently, these tables were widely used to characterize all forager societies. The lack of chronological data available to benchmark his models means his data should be viewed as preliminary and used cautiously.
Because we were aware of the limitations imposed by the lack of chronological dates, we carried out field studies at Moange Le Bosquet, a Baka pygmy village in southeastern Cameroon where birth records had been kept for many years. Le Bosquet was founded in 1973 by Sister Marie-Albéric of the Congregation of Holy Spirit. Given the complex socioeconomic relationships that the Baka have with their Bantu-speaking farming neighbors (17), Sister Marie-Albéric invited those Baka living in camps attached to nearby Bantu villages to move to a new locality to found a village where, for the first time, the chief would be a Baka. One of the first buildings erected by the Catholic mission was a medical center run by nuns with medical training, who provided health care for the community, kept birth records, and carried out regular censuses to integrate the Baka into the organizational structure of the civilian community in Cameroon (e.g., helping them obtain identity cards and land titles). By the end of 1973, about 700 Baka were living at the village of Le Bosquet.
Le Bosquet comprises an aggregate of Baka camps. As people come in from different camps attached to nearby Bantu villages, they form different neighborhoods, each keeping the name of the original village, possessing its own meeting hut, and organized socially around a family chief. Le Bosquet extends over approximately 5 km from east to west along the road from Lomié to Messok. The neighborhoods near the Catholic mission are very close to one another, while others are separated by large tracts of forest and look like the camps attached to Bantu villages.
Although the Baka are inhabitants of Le Bosquet, their way of life has remained seminomadic. Many individuals and family groups arrive or leave from one year to the next with no particular pattern: Some families leave and return a few years later, while other families leave after staying at Le Bosquet for a time and never return. During each field season we met some Baka who spent several months of the year away from the village, moving to camps in the middle of the forest where they feel “at home.” Hunting and gathering are the main activities in the forest camps. When at Le Bosquet, the main activity for women is gathering, on top of other chores including housekeeping, childcare, and fishing; the men’s activities include agricultural work for farmer neighbors, carrying logs cut by clandestine timber companies, hunting, and sometimes gathering (18). Many Baka also cultivate their own banana and/or manioc crops. According to several censuses carried out by the nuns and our own records kept over 11 y, the number of Baka individuals at Le Bosquet has remained more or less constant at around 800 individuals.
Birth records with both parents identified are available from 1980 to 1983 and from December 1987 until the present (SI Appendix, Table S1). Demographic records had previously been kept in the 1960s and 1970s by Father Dhellemmes (19), who lived and traveled in the region. Dhellemmes built up an enormous database from individual index cards identifying people by family and clan links, including a large number of Baka living in the wider area of southeastern Cameroon. The nuns used these index cards to provide identity cards for the first inhabitants at Le Bosquet, a strategy that allowed us to determine exact ages for a subset of people approximately 50+ y of age.
Ages are not known for all the people living at Le Bosquet. The nuns constantly encourage the Baka to declare births. Because of the fluid residential pattern mentioned above, many families live at Le Bosquet for only a short period, while others may have lived at a forest camp at the time of birth and forgotten to formally declare the baby later as part of the census exercise. To further complicate census efforts, if a newborn dies during the first year of life and another baby follows within a short time (≤2 y), the new baby is considered to be the former baby who has “left for a while and returned a little later,” so the parents believe it is enough to declare the first birth. A consequence of this cultural belief is that the number of deliveries, both live and dead, is not the same as the total number of offspring for a couple during their entire reproductive period. Births may also not be declared because of the wishes of one or both parents: For instance, one father stopped declaring births after his fourth girl and opted in the future to declare a new birth only if it was a boy.
With around 800 Baka inhabitants, Le Bosquet is not a Baka settlement, because these settlements are made up of only 45 people on average (20–22). We are aware that the social and economic situation at Le Bosquet is probably not the same as that described for the camps; however, it should be remembered that the main characteristic of Baka society is the heterogeneity of its camp environments and its patterns of economic and social organization (23). While the social and economic aspects of Baka society have been investigated many times (24–26), variations in key reproductive aspects of their life history have never been explored. Thanks to the birth records available at Le Bosquet, we were able to describe growth and LHVs based on an absolute chronology in a hunter-gatherer society. Although our analysis centers on the subpopulation at Le Bosquet (only individuals with a known date of birth are included), we believe the results presented here are representative of the entire population at Le Bosquet, that they reflect a transition to a period increasingly affected by external influences, and that they provide a basis for studies of other pygmy groups that are genetically close to the Baka (e.g., the Aka from the Central Africa Republic).
Results
Timing of Reproduction and Family Size.
Conjugal relationships do not occur immediately after menarche: There is a gap while young adult Baka enjoy different kinds of friendly relationships. In the past, the choice of partner could have been directed by clan membership (avoiding a partner from the same clan), but almost all clans in and around Le Bosquet have abandoned this practice. Some constraints do exist in the case of widowhood, since widows and widowers must choose a new partner among members of the deceased partner’s family; the widow or widower can make a different choice only if the deceased partner’s family refuses to provide a new partner. There are no cultural factors regulating delayed or precocious conception: The first birth therefore reflects fecundity. In the Baka at Le Bosquet, the earliest first births occurred at 16 y of age, and the average age at first birth was at 18 y. The age at latest birth varies widely (from 25 to 51 y) and averages 39.9 y. The average age at menopause is estimated to be around 42 y.
The average completed family size for postmenopausal women (n = 15, SI Appendix, Table S2) in this sample is 7.3 living offspring, varying from 4 to 10 children per woman. Nine of 16 women declared they had lost one baby in its infancy (at or before 1 y of age), while three women had each lost two infants. Four women said they had experienced a miscarriage. The difference between the average age at first and last birth suggests a reproductive span of 22 y, although the average reproductive period for the 15 women is 20.2 y. An average family size of 7.3 people therefore generates an average IBI of 3 y or 2.77 y, the latter figure being very close to the modal value of 2.5 and 2.75 y obtained in a previous study on the Baka (11). Comparisons with other hunter-gatherer societies (3, 4, 6, 7, 27–35) show that results for the Baka are similar to those suggested for the Aché, the Agta, and the Yanomamo, although the Baka have the shortest IBI (Table 1).
Table 1.
Reproductive aspects in hunter-gatherer societies
| Hunter-gatherer population | H F | H M | CFS | IBI, y | Age at first birth, y | Age at last birth, y | RS, y | IM | PS F, % | PS M, % |
| Ache Forest (4, 29) | 149* | 158* | 8.15 | 3.1 | 19.5 | 42.1 | 22.6 | 11 | 68* (61) | 79* (71) |
| Hiwi (29, 30) | 145 | 154 | 5.13 | 3.76 | 20.5 | 37.8 | 45 | 51 | ||
| Ju/’hoansi (6) | 150 | 161 | 4.7 | 4.12 | 18.8 | 34.4 | 15.6 | 20.2 | 60 | 56 |
| Yanomamo (3, 27, 28) | 142 | 152 | 7.8 | 3.3 | 18.4 | 37.9 | 19.5 | 19 | 70 (48†) | 70 (48†) |
| Agta (7) | 143 | 153 | 7.6 | 2.82–2.85 | 19.5 | early 40s | 24 | 29 | 42 | 42 |
| Aka (3, 50) | 145 | 153 | 3.5 | 55 | 55 | |||||
| Efé (3, 31, 32) | 136 | 144 | 2.7 | 3.1 | 78 | |||||
| Pumé savanna (33–35) | 151.5 | 163.2 | 3.1 | 15.5 | ||||||
| Pumé river (33) | 2.87 | |||||||||
| Hadza (3, 29) | 150 | 160 | 19 | 54 (58) | 54 (55) | |||||
| Baka | 147 | 154 | 7.3 | 2.77 | 18 | 39.9 | 22 | 5.2 | 66–92 | 66–92 |
CFS, completed family size; F, female; H, height (cm); IM, infant mortality (%); M, male; PS, proportion of survival to maturity; RS, reproductive span (the difference between mother’s age at last and first births). For the Baka, the difference between mother’s age at last and first births gives an RS of 22 y, but from the full reproductive history of 15 women RS is 20.2 y.
From Reserve (3).
See ref. 3.
TFR and ASFR.
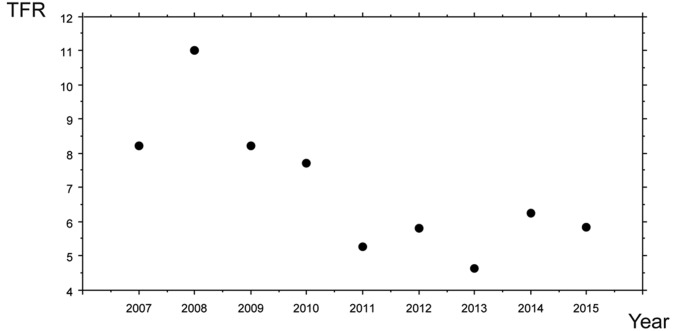
The TFR by year is shown in Fig. 1 and Table 2. The average TFR is very close to the average completed family size of 7.3 individuals. Clearly, two periods can be distinguished, one from 2007–2010, when the TFR values are high (>7) and another from 2011–2015 when the TFR decreases markedly (P = 0.008). The TFR values are not associated with any variation in the number of women or in the number of births (P > 0.05). The TFR for the Baka during the first period (2007–2010), which varies from 7.71 to 11.03 (x̄ = 8.8), is close to that for the Aché forest and reserve populations (x̄ = 8.08 and 8.53, respectively) and Yanomamo (x̄ = 8.1) but is a little higher than for the Agta (x̄ = 7.04) (Table 3). During the second period (2011–2015), the TFR for the Baka is much lower (x̄ = 5.53), higher than for the Ju/’hoansi (x̄ = 4.69) but much lower than for all other hunter-gatherer populations reported here.
Fig. 1.
TFR in the Baka from 2007–2015. High values during the first years of the study are followed by a drop in the TFR. The year 2011 marks a significant change in the TFR in the Baka.
Table 2.
TFR in the Baka
| Year | TFR | Log | No. women | No. births |
| 2007 | 8.21 | 0.9143 | 95 | 17 |
| 2008 | 11.03 | 1.0426 | 113 | 37 |
| 2009 | 8.23 | 0.9156 | 163 | 40 |
| 2010 | 7.71 | 0.8873 | 167 | 37 |
| 2011 | 5.26 | 0.7213 | 157 | 26 |
| 2012 | 5.82 | 0.7648 | 180 | 33 |
| 2013 | 4.62 | 0.6651 | 155 | 21 |
| 2014 | 6.25 | 0.7959 | 189 | 35 |
| 2015 | 5.83 | 0.7658 | 177 | 30 |
| Average | 7 | 0.8303 |
Table 3.
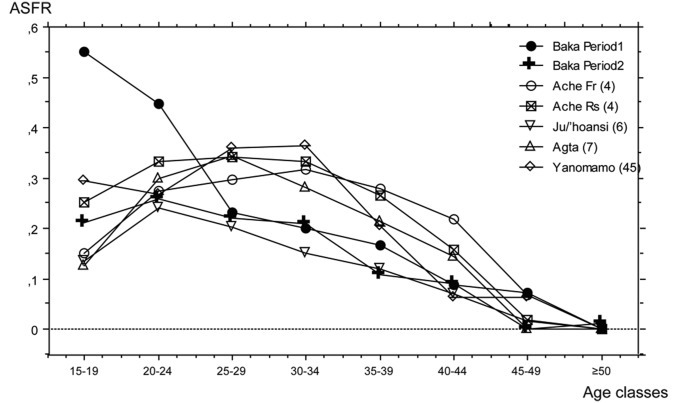
Comparison of TFR and ASFR in the Baka and other hunter-gatherer societies
| Age, y | Baka | Aché (4) | Ju/’hoansi (6) | Agta (7) | Yanomamo (28) | ||
| Period 1 | Period 2 | Forest | Reserve | ||||
| 15–19 | 0.550 | 0.212 | 0.151 | 0.253 | 0.135 | 0.126 | 0.295 |
| 20–24 | 0.448 | 0.259 | 0.275 | 0.333 | 0.242 | 0.299 | 0.267 |
| 25–29 | 0.233 | 0.220 | 0.298 | 0.341 | 0.203 | 0.344 | 0.361 |
| 30–34 | 0.200 | 0.210 | 0.318 | 0.333 | 0.152 | 0.282 | 0.364 |
| 35–39 | 0.168 | 0.109 | 0.279 | 0.265 | 0.119 | 0.213 | 0.205 |
| 40–44 | 0.088 | 0.090 | 0.219 | 0.157 | 0.071 | 0.144 | 0.063 |
| 45–49 | 0.073 | 0.000 | 0.069 | 0.019 | 0.016 | 0 | 0.063 |
| >50 | 0.000 | 0.012 | 0.000 | 0.000 | 0 | 0 | |
| TFR | 8.797 | 5.558 | 8.081 | 8.529 | 4.69 | 7.04 | 8.098 |
The Baka present the same pattern in the ASFR in all years (2007–2015), with the highest value occurring in the second age class (20–24 y). In general, the highest values occur in the first two age classes, declining throughout a woman’s reproductive life (Fig. 2, Table 4, and SI Appendix, Text S2). Longitudinal ASFR data reinforce the identification of two different periods, as suggested by the TFR data. Births were recorded for mothers 45–49 y of age during the first period (2007–2010) but not during the second period (2011–2015). Furthermore, ASFR values in the two younger age classes are much higher in the first period than in the second (P < 0.05). The proportion of births per woman declines during the second period but only in the two youngest age classes (x̄ = 0.55 and 0.45, respectively, for the first period; x̄ = 0.21 and 0.26, respectively, for the second period).
Fig. 2.
ASFR over 9 y among the Baka (one color for each year). From 2007–2010 (period 1, dashed lines) the lower age classes have much higher values than the next classes; these values dropped from 2011–2015 (period 2, solid lines), suggesting a major change in the reproduction capacities of young adults.
Table 4.
ASFR by year
| Age, y | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | X 1°P | X 2°P |
| 15–19 | 1.00 | 0.53 | 0.39 | 0.28 | 0.12 | 0.24 | 0.19 | 0.21 | 0.30 | 0.55 | 0.21 |
| 4/4 | 8/15 | 7/18 | 8/29 | 3/26 | 9/37 | 5/26 | 6/28 | 8/27 | |||
| 20–24 | 0.26 | 0.65 | 0.47 | 0.42 | 0.30 | 0.26 | 0.22 | 0.27 | 0.24 | 0.45 | 0.26 |
| 6/23 | 11/17 | 15/32 | 10/24 | 7/23 | 7/27 | 4/18 | 8/30 | 7/29 | |||
| 25–29 | 0.23 | 0.32 | 0.17 | 0.22 | 0.22 | 0.25 | 0.18 | 0.29 | 0.16 | 0.23 | 0.22 |
| 5/22 | 7/22 | 6/35 | 8/37 | 8/36 | 8/32 | 5/28 | 9/31 | 5/31 | |||
| 30–34 | 0.08 | 0.25 | 0.30 | 0.17 | 0.29 | 0.22 | 0.12 | 0.23 | 0.19 | 0.20 | 0.21 |
| 1/13 | 5/20 | 6/20 | 4/23 | 6/21 | 6/27 | 3/26 | 7/30 | 5/26 | |||
| 35–39 | 0.00 | 0.27 | 0.18 | 0.22 | 0.06 | 0.07 | 0.11 | 0.20 | 0.11 | 0.17 | 0.11 |
| 0/11 | 4/15 | 4/22 | 4/18 | 1/16 | 1/14 | 2/19 | 4/20 | 2/19 | |||
| 40–44 | 0.00 | 0.10 | 0.08 | 0.17 | 0.06 | 0.12 | 0.11 | 0.05 | 0.11 | 0.09 | 0.09 |
| 0/8 | 1/10 | 1/12 | 2/12 | 1/16 | 2/17 | 2/18 | 1/22 | 2/18 | |||
| 45–49 | 0.08 | 0.09 | 0.05 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 |
| 1/13 | 1/11 | 1/19 | 1/14 | 0/8 | 0/12 | 0/10 | 0/8 | 0/10 | |||
| >50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.01 |
| 0/1 | 0/3 | 0/5 | 0/10 | 0/11 | 0/14 | 0/10 | 0/20 | 1/17 |
Ratios in italics show number of births/number of women. X 1°P, average for the first period; X 2°P, average for the second period.
Compared with other hunter-gatherer societies reported here, the Baka have the highest ASFR values in the first two age classes, although the values for the second period are closer to those reported for other populations (Fig. 3 and Table 3). For the third age class the low values for the Baka are close to those observed for the Ju/’hoansi. In other hunter-gatherer societies, the ASFR increases from the first years of reproductive activity and reaches the highest values in the third age class (25–29 y) in the Yanomamo and the Aché living in the reserve and in the fourth age class (30–34 y) in the Aché living in the forest. Weiss (16) suggested that the highest values in the third or fourth age classes are “virtually universal among human females” (ref. 16, p. 31), and he followed this pattern to establish his models of anthropological fertility rates. However, the Ju/’hoansi, like the Baka, have the highest value in the second age class (age 20–24 y). The Baka thus have a very characteristic ASFR pattern that clearly distinguishes them from other hunter-gatherer societies, with a high rate of reproduction from age 15–29 y and a lower rate in the older age classes (Fig. 3).
Fig. 3.
Pattern of the ASFR in the Baka compared with other hunter-gatherer societies. In contrast to ASFR variations in other forager societies, for which the curve is bell-shaped, the highest values for the Baka are found in the lower age classes, producing a particular pattern of reproduction. Fr, forest; Rs, reserve.
ASFR enables estimations of the mean annual probability of birth in the last two reproductive age classes (40–49 y). In forager societies, this ranges from 0.044 (Ju/’hoansi) to 0.158 (Aché). In the Baka it falls between those two populations, with a probability of 0.081 during the first period (2007–2010) and 0.045 for the second period (2011–2015).
Infant and Juvenile Mortality.
Table 5 shows the number of births, the number of infant deaths during the first year of life, the number of infants who survive the first year of life, and the number of infants who moved away from Le Bosquet from 2007–2015 (and whose survival after leaving Le Bosquet is unknown). The infant mortality rate from 2007–2015 is extremely low, as only six infants died before the first year of life out of 229 births (this number was obtained by subtracting the number of infants reported as “out” from the total number of births). This corresponds to an infant mortality rate of 2.62%. It is possible that infants who died within a few days after birth were not declared. In the reproductive history of the 44 women used to obtain parity-specific infant mortality (see below) we observed that four infants who died a few days after birth were not declared, which corresponds to 50% of cases. Even with this correction, the estimated infant mortality rate, at 5.24%, remains extremely low compared with infant mortality in other hunter-gatherer societies (Table 1). Marlowe (36) reported a mean infant mortality rate of 23.29% for 12 hunter-gatherer groups living in a warm climate. It is worth noting that no infant death is recorded from 2007–2010: The first evidence of infant mortality appears in 2011 (Table 5). There is no difference between the two periods in weight during the first 2 mo of life (SI Appendix, Table S3).
Table 5.
Mortality in Le Bosquet
| Data | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
| No. births | 17 | 38 | 38 | 37 | 25 | 33 | 22 | 35 | 30 |
| No. infant deaths | 1 | 2 | 3 | ||||||
| No. child deaths | 1 | 2 | 3 | 2 | 1 | ||||
| No. out | 3 | 10 | 6 | 6 | 4 | 3 | 5 | 3 | 6 |
No. out: number of individuals not met again and whose status is not known.
Among the 44 mothers with a known full reproductive history used to obtain infant mortality per woman by parity (Table 6), 13 miscarriages were recorded. Two mothers had two successive miscarriages. The abortion indicated in Table 6 was produced deliberately because the woman became pregnant when her last child was still “too small,” which for the Baka means less than 1.5 y old. Abortions recorded for other women or at other periods and thus not included in this analysis were performed for the same reason. Parity-specific infant mortality per woman obtained for the Pumé (35) shows that Pumé mothers can have five children by 25 y of age. None of the Baka mothers 15–25 y of age had five infants, and only one mother 25 y of age had four infants. This difference with the Pumé can be related to the age at first pregnancy, the average being 15.5 y for the Pumé (35) but 18 y for the Baka (11). Parity-specific infant mortality per woman was obtained in June 2010 and in June 2014. Two main differences can be observed: The number of miscarriages was higher in 2010 than in 2014, while infant mortality in first-time mothers was 0% in 2010 but reached 50% in 2014. In 2010, 9 of 12 mothers with a first live infant were 19 y of age or less. In 2014, however, the three mothers with one live infant were 21–23 y of age, whereas two of the mothers with one dead infant were 18 y old, and one was 21 y of age. Infant mortality in mothers with more than one infant was much lower than in mothers with only one infant, which probably suggests that high infant mortality is a new phenomenon.
Table 6.
Infant mortality per woman by parity
| Infant deaths | Parity | |||
| 1 (%) | 2 (%) | 3 (%) | 4 (%) | |
| 2010 (n = 32) | ||||
| 0 | 12 (100) | 8 (80) | 7 (78) | 1 |
| 1 | 2 (20) | 2 (22) | ||
| 2+ | ||||
| Miscarriage | 3 | 3 | 4 | |
| Abortion | ||||
| 2014 (n = 24) | ||||
| 0 | 3 (50) | 12 (92) | 4 (80) | |
| 1 | 3 (50) | 1 (8) | 1 (20) | |
| 2+ | ||||
| Miscarriage | 3 | |||
| Abortion | 1 | |||
The average IBI for mothers 15–25 y of age in 2010 was 2.85 y (n = 19), but in three cases the IBI may have been affected by particular factors: Two high IBIs occurred when the mothers suffered miscarriages, and the other high IBI happened when the woman was not married for a while. When these particular IBIs are excluded, the average IBI for 2010 is 2.36 y. For mothers 15–25 y of age in 2014, the average IBI is 2.77 y (n = 11). Only one IBI was very long, almost certainly due to two miscarriages. When this is excluded, the average IBI is 2.56 y. When these two IBIs are compared, no significant difference is found (P = 0.384).
Mortality was estimated for Baka children born from 1996–2001 and reaching 15 y of age in 2011 and later. The high mobility of the Baka prevented an accurate estimation. As shown in Table 7, 60 children (30 girls and 30 boys) out of 212 births (28%) were no longer living at Le Bosquet, and it was not possible to find out whether they had lived to age 15 y. If these children are considered dead, juvenile mortality in the Baka varies from 29 to 41% with a mean for the six years of 34% (66% probability of survival to maturity). If only known alive and dead infants are considered (excluding those classified as out), juvenile mortality drops to 8% (92% probability of survival to maturity). The values are the same for males and females (Table 7). Among the hunter-gatherer groups compared with the Baka, only the Ache present a high probability of survival (68% in female, 79% in male) (Table 1). Walker et al. (3) reported values of around 80% for the Tsimane and Turkana. However, the lower value of 66% for the Baka is certainly an underestimation of the probability of survival in this group because it assumes that all the young adults who left Le Bosquet are dead. A 92% probability of survival to maturity in the Baka is much higher than in any other hunter-gatherer group and must be considered with caution. Table 5 also provides some information on juvenile mortality. It is surprising that the number of children that died is as high among those who were born in 2011–2015 as it is in the 2007–2010 cohort.
Table 7.
Juvenile mortality in the Baka
| % reaching age 15 y | |||||||
| Sex | Year | No. births recorded | No. reaching age 15 y | Dead | Out | Minimum | Maximum |
| Males | 1996 | 15 | 9 | 2 | 4 | 60 | 82 |
| 1997 | 8 | 7 | 1 | 88 | 100 | ||
| 1998 | 17 | 9 | 8 | 53 | 100 | ||
| 1999 | 19 | 14 | 5 | 74 | 100 | ||
| 2000 | 19 | 10 | 2 | 7 | 53 | 83 | |
| 2001 | 28 | 21 | 2 | 5 | 75 | 91 | |
| 106 | 70 | 6 | 30 | 66 | 92 | ||
| Females | 1996 | 26 | 17 | 1 | 8 | 65 | 94 |
| 1997 | 17 | 10 | 1 | 6 | 59 | 91 | |
| 1998 | 10 | 7 | 3 | 70 | 100 | ||
| 1999 | 20 | 12 | 3 | 5 | 60 | 80 | |
| 2000 | 20 | 16 | 1 | 3 | 80 | 94 | |
| 2001 | 13 | 8 | 5 | 62 | 100 | ||
| 106 | 70 | 6 | 30 | 66 | 92 | ||
| Total | 212 | 140 | 12 | 60 | 66 | 92 | |
Only four cases of death of young Baka mothers were recorded. If a mother dies during the first year of an infant’s life, the infant is abandoned by the family and dies (SI Appendix, Fig. S1).
Discussion
The first pregnancy marks an abrupt change in the life of any mammal, since the energy used for growth until this point is allocated from then on to reproduction (9). Given the intersection of ecology and demography in any population, natural selection can presumably act on the timing of this important life-history event, resulting in either an earlier or a later age at first reproduction. Any organism will favor growth to reach its maximum size, which is closely linked to energy production: A larger size will produce more energy for reproduction and survival. However, each time unit of delay before the onset of reproduction decreases the organism’s chances of surviving to reproductive age (37). The average age at first birth thus appears to be the best compromise that a population can achieve between allocating surplus energy to growth or to reproduction.
The hunter-gatherer populations compared in this study have a small average adult size and an average age at first reproduction of 18–19 y, which is similar to that of European populations. Thus, the small body size with the limited amount of surplus energy it provides for reproduction does not appear to be related to the age at first delivery in these populations. Results for the Pumé point to the same conclusion. The Pumé are relatively tall compared with other hunter-gatherer populations referred to here (Table 1), and the first birth occurs at a much younger age (15.5 y) (34). This seems to be coupled with rapid juvenile growth, which produces greater gains in stature in youth than in adolescence to achieve a body size close to that of neighboring populations. As adult stature may not be associated with age at first birth (35), short stature is not necessarily linked with early maturation. Variations in adult size (height and weight) probably play some role in the age at maturation among individuals from the same population (4), but extrapolating this relationship to entire populations seems hazardous.
However, Kramer (38), like Migliano et al. (10) for the Aeta, sees faster growth as an adaptive response in high-mortality environments where early reproduction is advantageous. This links up with the suggestion made by Walker et al. (3) that a high survival rate is associated with slower and later development. The Baka show a very low infant mortality rate and a high, even extremely high, probability of survival to maturity (Table 1). The environmental conditions of the Baka thus do not appear to be challenging in terms of mortality and offer appropriate conditions for a slow growth pattern. This agrees with the pattern of growth described for the Baka, who reach maturity (menarche, first reproduction) at a similar age as nonforager populations (11). The short stature of the Baka is not a consequence of early cessation of growth, as has been suggested for the Aeta, but results from a slower rate of growth during infancy (11), which has a genetic foundation (39–41). Therefore, high mortality rates (infant, juvenile, or adult) may influence the timing of development (38, 42) but do not seem to be linked with adult body size.
Walker et al. (3) propose that Pygmies (as well as Negritos and Hiwi) have a faster growth rate and that this is related to high juvenile mortality. Our results suggest, on the contrary, a low infant mortality and a high proportion of survival to maturity which agree with slow growth in the Baka (11). This difference in results certainly comes from the methodology and data samples, as these authors recognize (3). Based on cross-sectional data, Walker et al. assume a linear growth rate from 3–10 y of age, suggesting that this is one of the best measures for comparisons across societies, although the assumption of linear growth in humans is much criticized (43, 44). Furthermore, sample size is limited to a few individuals with considerable uncertainty as to their estimated age, and the 0.78 probability value for survival to maturity for the Eastern Pygmies is disregarded (32). High-quality data (longitudinal data based on many individuals whose ages are known from birth to adulthood) are needed for an appropriate assessment of life-history variation in human populations.
Low infant and juvenile mortality rates in the Baka contrast with fertility aspects, as shown by the ASFR and the short IBI. The pattern of shifting ASFR shown by the Baka, with high values in the lower age classes and lower values in the higher age classes, suggests that the main reproductive period for Baka women is between 15 and 29 y of age. The age of menopause at around 42 y is close to the ages reported by Goodman et al. (43.9 y) (45) and by Early and Headland for the Agta (early 40s) (7) (Table 1). Therefore, the age of reproductive senescence in the Baka is not a constraint that induces a high rate of reproduction in the lower age classes. Furthermore, the Baka have an average IBI of approximately 33 mo (2.77 y), which is shorter than the IBI for the Aché, Batek, Inuit, Ju/’hoansi, and Pumé from savannah areas (4, 6, 11, 33, 46, 47) but not the Batak (48), Agta (7), and the Pumé from the river (33). It is well known that cooperative childrearing benefits mothers by redistributing the energy cost of raising offspring, which allows shorter birth intervals, higher maternal fertility, or increased infant survival (49). In the geographically and genetically closely related Aka pygmies, Meehan (50, 51) has quantified the parental and alloparental assistance a mother receives during the period of bride service (approximately 1 y). Fathers and grandmothers provide the second and third highest frequency of direct care to infants, but unlike the father’s presence, which increases the mother’s energy expenditure by increasing her work, a grandmother’s presence is a substitute for maternal care and allows the mother to invest energy in other activities, including those that increase maternal reproductive success. Another Pygmy group, the Efé from the Ituri forest, have a child-rearing system in which infants receive a remarkably high level of parental and alloparental assistance (52). Childcare is mainly provided by nonreproductive women and is characterized primarily by intermittent play and physical contact, whereas mothers invest in more demanding forms of care and economic tasks. Interestingly, mothers are also relieved of competing childcare demands and can engage in subsistence activities from which children and others benefit (52); the help received by mothers seems to translate into infant survival and not into fertility. It is possible that the Baka share a high level of parental and alloparental assistance in childrearing with the Aka and the Efé. The high rate of reproduction in the lower age classes, the short IBI, and the low infant mortality rate might be favored by the help that other adult individuals are able to provide to young mothers by caring for newborns and infants or sharing resources. However, unlike the Aka and the Efé, Baka mothers can probably engage more time and energy in increasing reproductive success, which would explain why the Baka have the lowest IBI (Table 1). If parental and alloparental investment in the Baka is confirmed by empirical data, it is worth noting that such a childrearing system would not be exclusively associated with fast growth, as has been suggested for the Pumé (34) and speculated for the Aka and Efé (3).
It is worth noting that if the mother dies when the infant is younger than 1 or 1.5 y of age, no one replaces the mother in caring for the child, not even members of the family, including the child’s father. Even if a close member of the family is breastfeeding, she does not invest in the survival of the orphaned baby. As a result, this infant dies a few months after its mother (SI Appendix, Fig. S1).
From 2007 to 2010 (period 1), the ASFRs in the lower age classes were higher than those observed in any of the other hunter-gatherer societies, but they dropped from 2011–2015 (period 2), resulting in a 37% reduction in the TFR. This drop in fertility in the lower age classes during period 2 is also observed in the results concerning infant mortality per woman by parity (Table 6), because the mothers with one infant in period 1 were 19 y of age or less, whereas in period 2 five of the six mothers were at least 21 y of age. Several studies on forager societies made at intervals over a long period have enabled us to observe various changes in LHVs that can be related to new living conditions (4, 7). Hill and Hurtado (4) reported a lower age at menarche and at first reproduction for the Aché when they settled in a reserve, developing a horticulturalist economy and reducing hunting treks in the forest. They also showed that ASFR increased in the lower age classes but decreased later, producing a minimal increase in the TFR in the reserve. Many factors can lead to a change in the fertility rate of a population. Physiological causes can alter fertility as a result of reproductive failure or reduced fecundity. Various social or environmental constraints, such as migration, war, work stress, epidemics, and starvation, can reduce access to mating or increase the energy cost of childbearing.
A change in the frequency of miscarriages or in the IBI may lead to a change in the fertility rate. However, even if they are associated with fertility, they do not explain the underlying cause of the change in fertility. The number of miscarriages was higher in period 1 (2007–2010) than in period 2 (2011–2015) (Table 6). Tables 6 and 7 show that infant mortality was higher in period 2. Higher infant mortality is associated with a shorter IBI (14). However, in the Baka, neither the IBI nor infant weight in first 2 mo of life differed between the two periods. The number of miscarriages, the IBI, and weight in early infancy are not associated with the drop in fertility during period 2. It would be expected that the drop in fertility during period 2 would be associated with a drop in mortality (12, 14), but infant and juvenile mortality seems, on the contrary, to have increased during this period compared with the previous one.
From the foundation of Le Bosquet in 1977 until our first field study in 2007, and during the 11 y of our regular visits to Le Bosquet, there is no record of epidemics, mass mortality, or starvation (a word that does not exist in the Baka language). There was no demographic change: The population of Baka at Le Bosquet has remained at around 800 individuals since the 1970s. The Baka have no access to clinics, and vaccination programs are random. Basic health care and education are provided by the health center and the school established by the nuns in the 1970s. A drop in fertility is sometimes associated with parental investment in child skills, and investment in education is a particularly favored option in transitional conditions. Records of school attendance in Le Bosquet are scattered over the years, but they show 131 enrolments in 1989–1990, 125 in 2010–2011, and 129 in 2015–2016. Analysis of school attendance has not revealed any change in parental investment from period 1 to period 2 (SI Appendix, Text S3).
One change in the lives of the Baka relates to logging, both legal and illegal. Both activities obey the same dynamic, advancing along forest tracks to fell only well identified trees and moving on when all of these have been cut. Legal and illegal logging companies employ local villagers, preferably the Baka since they know the forest very well and can penetrate deep into the interior. The villagers do not move on with the employers: They are not welcome in neighboring villages because they would deprive local people of work. Legal timber felling ended in the Le Bosquet area at the end of 2006 and moved away in 2007, and illegal logging with chainsaws that could be dismantled for easy transport was present at Le Bosquet during 2010 and 2011. In 2012, illegal logging moved away to the east. The impact of legal and illegal logging therefore has been irregular and limited in time.
One major event occurred in the last month of 2010, when cheap alcohol arrived in the community. Local wine and beer were already consumed by the Baka: A bottle of palm wine cost 500 Central African francs (CFA) (€0.75; $0.87), and beer cost around 800 CFA (€1.20; $1.40). Salaried employment is very uncommon among the Baka, but when available the daily wage for working in a plantation is 300 CFA (€0.45; $0.52). The high cost of these alcoholic beverages thus prevented widespread consumption on a daily basis. In 2010, a bar was set up within the confines of Le Bosquet with cheap alcohol sold in plastic bags as the main commodity, costing 50 or 100 CFA (€0.07–0.15; $0.08–0.17) per bag, a lower price than that of any other consumer product and corresponding to the lowest value coins in the country. Although very little money circulates among the Baka, occasional work in plantations, for illegal loggers and for nuns or researchers produces some monetary income for the Baka community, which they mainly use to buy alcohol (53). From the end of 2010, consumption of these newly introduced alcoholic drinks, called “nofia” locally and “whisky” in general, became widespread among individuals of all ages: Even infants were picking up the bags from the floor, opening them, and sucking on them. Although this is anecdotal evidence, as we have no data on the frequency of such behavior, we observed it several times (ref. 53, minute 25:22). During each field season, the first comments from our Baka collaborators were always about people who had died in Le Bosquet since our last visit. Never previously mentioned as such, alcohol poisoning has become one of the main causes of death since 2011. The chemical composition of nofia has never been studied in detail, but it is a mixture of ethanol and methanol whose main purpose is to make people drunk as quickly as possible. Government authorities have recognized that these drinks are causing major disorders of the nervous system, cancers, and death, even when consumed in small quantities. Their production has been banned, but traders are still allowed to sell them until current stocks are exhausted (54–56).
The impacts of alcohol in encounters between different cultures have been widely analyzed (e.g., ref. 57). The effects of drinking alcohol are determined largely by the rate at which it and its main by-products are metabolized after consumption. It is well established that the main metabolic pathway for ethanol involves a variety of enzymes and that their presence and proportions vary among populations (58). It is also well established that the consumption of alcohol has a direct impact on reproduction by increasing the risk of infertility in women or altering semen quantity or quality (59–61), even in people with a low level of alcohol intake (e.g., ref. 62). At Le Bosquet, the consumption of cheap alcohol that began in the last month of 2010 has continued until now. It is not a one-off phenomenon whose effects would dissipate over time: Its arrival changed the behavior of people at Le Bosquet because buying alcohol has become one of the main motivations for taking on any kind of work to earn cash. Alcohol has also shifted the center of activities in Le Bosquet. For years, the Catholic Mission was the main place where people converged to discuss matters concerning the village. Since 2010, the main place has become the bar, where people meet at any time and talk while drinking. It is difficult not to infer a direct link between the arrival and mass consumption of cheap alcohol and the drop in the TFR in the Baka.
During a meeting with Baka women, we asked them if they had noticed any change in fertility over time and, if so, whether they had any idea about its cause. They said that there probably was a change in fertility due to living close to the road. This implied a need to give more care to children, mainly by giving more attention to clean clothes: Having more children implies having more resources, which they do not have, to pay for clean clothes for themselves and the children. Asked about how they could control the number of children, they said they could reduce the number of times they have intercourse. We then asked about the alcohol. After a great many exclamations, they said that the arrival of alcohol had affected everybody, even the children who would rush to buy some when they obtained a coin. About the possible link between fertility reduction and alcohol, they said that alcohol was now very cheap and consumption was massive. They said that men coming back from the field spend a few coins on alcohol before going home and arriving there drunk, and that the women refused to have sexual relations when the men are in this condition. This situation persists during the entire period of wage labor, which can last from a few days to several weeks. The women then added that this situation was mainly affecting young mothers who, even if intercourse is easier now than before, took longer to become pregnant. They thought that young mothers were probably more affected than older ones because they had been drinking alcohol since they were young.
It is very interesting to note that these Baka women have realized that fertility in younger women has become more problematic. The lower age classes are the most important in the reproductive cycle of the Baka and make the largest contribution to the TFR. Reproduction in the lower age classes is the most distinctive aspect in the Baka and probably reflects a particular adaptation that we cannot elucidate at the moment. Surprisingly, these are the age classes most affected by the drop in fertility. There is no record of any major and enduring change in Le Bosquet, except for the arrival of cheap alcohol at the end of 2010, which coincides with the drop in fertility in the Baka. Alcohol consumption could thus be the main reason for the drop in fertility in the younger age classes. Our results provide clear, first-hand evidence of the damaging effects on fertility produced by alcohol in a hunter-gatherer society. Downward trends in TFR such as those seen in the Baka will have the long-term impact of reducing the number of Baka individuals.
Materials and Methods
The data presented in this article were obtained over 11 y from 2007–2017. At least one field study was conducted each year in May–June, and a second field study in October was added from 2011–2015. Informed consent was obtained from all participants and from both parents of any participants aged under 18. This study obtained approval of the Centre National de la Recherche Scientifique, Agence National de la Recherche (France) and Institut de Recherche et Développement and was carried out as part of the international agreement between the L’Institut de Recherche pour le Développement (IRD) and the Ministry of Scientific Research and Technology of Cameroon. Only individuals whose age was known or could be estimated accurately were included in the study. Thanks to Dhellemmes’ records, the age of some women more than 25 y old is known to within a year. By combining census and birth records with our questionnaires, we were able to establish the first birth and the total number of infants for each woman very accurately. The modal IBI in the Baka is 2.5 y (11), and we worked on this assumption for children for whom we had no birth record. Similarly, since 18 y is the average age for the first delivery (11), this was the age assumed for the mothers at first birth. For instance, if we knew that the first record of the birth of a sibling was for the third infant, we assumed that the first infant was born 5 y earlier (two IBIs of 2.5 y each) and that the mother was 18 y old at the first birth. We are aware that this procedure introduces some bias, but when it was applied to mothers and infants whose real chronology was known, the differences were never greater than 2 y. The bias therefore has a minimal impact, if any, on the distribution into 5-y age classes. Many analyses were performed in this study, and the sample size varies from one to another (SI Appendix, Table S2).
Timing of Reproduction and Family Size.
To obtain these variables, only 16 women whose birth records were available were included in the analysis. These were women born between 1952 and 1970. The majority had already begun their reproductive life when systematic recordkeeping started at Le Bosquet in 1988, except for two whose first baby was born during the earlier period when records had been kept. The birth dates were recorded, and full personal histories detailing the numbers of live births, stillbirths, and miscarriages were established by questionnaires. Among the women whose reproductive history is fully recorded (n = 16), one woman had only one child, which is very rare among the Baka; she was not included in the calculation of the average completed family size. The reproductive period was obtained by subtracting the mother’s age at the first birth from her age at the last birth. For these women, the last birth had occurred several years previously, which implied that their reproductive life was over. An estimated age at menopause is suggested, which we obtained by averaging the ages at last birth of the 15 women. The full family history is also known for many other women, but it was not certain that their reproductive life was already over.
TFR and ASFR.
The TFR and ASFR were obtained every year from 2007–2015. For each year, the number of births was taken from the records in the medical center, and we used questionnaires to establish the number of women present at Le Bosquet (Table 2).
It is important to emphasize that the ASFR does not mean the same thing to all authors (SI Appendix, Text S4). In our study, as in Hill and Hurtado (4) and Howell (6), the ASFR is the number of women in a 5-y age group who gave birth during a given year, divided by the total number of women in that age group; the TFR corresponds to the sum of all ASFRs multiplied by 5.
Since two periods can be distinguished, one from 2007–2010, and the other from 2011–2015, the ASFR and the TFR were compared using the Mann–Whitney u test (SPSS) to establish the significance of any changes. The same test was used to observe whether the TFR values are related to changes in the number of women or in the number of births.
The ASFR and TFR for the Baka were compared with those for other hunter-gatherer populations with a low average adult stature: the Agta, Yanomamo, Ju/’hoansi, and Aché. The ASFR values for the Yanomamo are those reported by Melancon (28) and used by Hill and Hurtado (4); for the TFR we used Neel and Weiss’ (27) results, which we multiplied by 2.05. Hill and Hurtado (ref. 4, p. 255) provided the basis for choosing Melancon’s study for the ASFR by pointing out problems in previous studies. They do not reject a variation in ASFR among Yanomamo populations (see variation in the Baka), but none has been accurately observed to date.
Infant and Juvenile Mortality.
The infant mortality rate (the percentage of children who die within the first year of life) and the juvenile (or child) mortality rate (the percentage of children who die within the first 15 y of life) were obtained for the Baka. Their seminomadic lifestyle makes it difficult to measure mortality: Families move at any time of the year, and whether an infant or child has died can be established only when the family is found again at a later date. To estimate the infant mortality rate, we recorded, for each year from 2007–2015, the number of births, the number of infant deaths during the first year of life, the number of infants who survived beyond the first year of life (we know this because we met them), and the number of infants who moved away from Le Bosquet and whom we never met again.
Deliveries take place in private at home, so it was not possible to obtain birth weights, but many mothers go to the health center a few days later to declare the birth and have the baby weighed. Infant weight is monitored by the nuns for several months. We used these measurements to compare weight during the first 2 mo of life for infants born between 2008 and 2010 and between 2012 and 2014 to observe any differences in birth weight between period 1 (2007–2010) and period 2 (2011–2015). The Mann–Whitney u test (SPSS) was used to compare the significance of results.
To estimate infant mortality per woman by parity (33), we used the individual reproductive histories of 44 mothers between 15 and 25 y of age whose age at birth was recorded or well established because their deliveries were recorded. Some children were not declared at birth, but we were able to place them thanks to siblings whose age was known. Since fertility seems to change from 2011, suggesting the probability of two distinct periods, infant mortality per woman by parity was obtained in May 2010 for mothers born between June 1985 and May 1995 (n = 32) and in May 2014 for mothers born between June 1989 and May 1999 (n = 24). Mothers 15–21 y of age in 2010 are those who were 19–25 y of age in 2014. Miscarriages and abortions were also recorded. IBIs were obtained for both periods when exact chronological data for successive deliveries were available.
We estimated juvenile mortality for children who were born from 1996–2001 and had reached 15 y of age. Again, mobility prevented us from obtaining exact values because several families had moved away, and we did not know if their children were alive. When we never met the children and could not find out whether they were still alive, they are referred to as “out.”
Deaths of young mothers can affect infant survival. We report here what happens with infants when the mother dies, but the number of such cases is very small among the Baka at Le Bosquet.
Supplementary Material
Acknowledgments
We thank X. Garde, B. Bordage, and all the personnel of IRD Yaounde for logistical support; the nuns at the Le Bosquet mission for their kind hospitality; S. Pavard, S. Gallois, and two anonymous reviewers for helpful comments; A. Froment and L. Maget, my buddies in the field; and especially P. Kalo, J. B. Etoa, and other Baka collaborators for their help, assistance, and friendship. This study was supported by L. Di Giorgio (Dental Film), the CNRS, the IRD, Wenner–Gren Foundation Grant 7810, National Geographic Grant 8863-10, and the Agence Nationale de la Recherche Blanc SVSE7-2011 GrowinAP programme.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719637115/-/DCSupplemental.
References
- 1.Mace R, Jordan F, Holden C. Testing evolutionary hypotheses about human biological adaptation using cross-cultural comparison. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:85–94. doi: 10.1016/s1095-6433(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith BH, Tompkins RL. Toward a life history of the Hominidae. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 3.Walker R, et al. Growth rates and life histories in twenty-two small-scale societies. Am J Hum Biol. 2006;18:295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- 4.Hill K, Hurtado AM. Aché Life History: The Ecology and Demography of a Foraging People. Aldine de Gruyter; New Haven, CT: 1996. [Google Scholar]
- 5.Marlowe F. The Hadza, Hunter-Gatherers of Tanzania. Univ of California Press; Berkeley, CA: 2010. [Google Scholar]
- 6.Howell N. Demography of the Dobe! Kung. Aldine; London: 1979. [Google Scholar]
- 7.Early JD, Headland TN. Population Dynamics of a Philippine Rain Forest People: The San Ildefonso Agta. Univ Press Florida; Gainesville, FL: 1998. [Google Scholar]
- 8.Blurton Jones N. Demography and Evolutionary Ecology of Hadza Hunter-Gatherers. Cambridge Univ Press; Cambridge, UK: 2016. [Google Scholar]
- 9.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 10.Migliano AB, Vinicius L, Lahr MM. Life history trade-offs explain the evolution of human pygmies. Proc Natl Acad Sci USA. 2007;104:20216–20219. doi: 10.1073/pnas.0708024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozzi FV, Koudou Y, Froment A, Le Bouc Y, Botton J. Growth pattern from birth to adulthood in African pygmies of known age. Nat Commun. 2015;6:7672. doi: 10.1038/ncomms8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low BS, Simon CP, Anderson KG. An evolutionary ecological perspective on demographic transitions: Modeling multiple currencies. Am J Hum Biol. 2002;14:149–167. doi: 10.1002/ajhb.10043. [DOI] [PubMed] [Google Scholar]
- 13.Nobles J, Frankenberg E, Thomas D. The effects of mortality on fertility: Population dynamics after a natural disaster. Demography. 2015;52:15–38. doi: 10.1007/s13524-014-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preston SH. Causes and consequences of mortality declines in less developed countries during the twentieth century. In: Easterlin RA, editor. Population and Economic Change in Developing Countries. Univ of Chicago Press; Chicago: 1980. pp. 289–360. [Google Scholar]
- 15.Diekmann Y, et al. Accurate age estimation in small-scale societies. Proc Natl Acad Sci USA. 2017;114:8205–8210. doi: 10.1073/pnas.1619583114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss KM. A method for approximating age-specific fertility in the construction of life tables for anthropological populations. Hum Biol. 1973;45:195–210. [PubMed] [Google Scholar]
- 17.Robillard M, Bahuchet S. Les Pygmées et les autres: Terminologie, catégorisation et politique. J Africanistes. 2012;82:15–51. [Google Scholar]
- 18.Gallois S, Duda R. Beyond productivity: The socio-cultural role of fishing among the Baka of southeastern Cameroon. Revue d’ethnoécologie. 2016 doi: 10.4000/ethnoecologie.2818. [DOI] [Google Scholar]
- 19.Dhellemes RP. Le père des Pygmées. Flammarion; Paris: 1986. [Google Scholar]
- 20.Sato H. Notes on the distribution and settlement pattern of hunter-gatherers in northwestern Congo. Afr Study Monogr. 1992;13:203–216. [Google Scholar]
- 21.Hayashi K. Hunting and gathering activities of the Baka in southeastern Cameroon: The actual condition and contemporary significance. Hum Sci. 2000;14:27–38. [Google Scholar]
- 22.Kitanishi K. Cultivation by the Baka hunter-gatherers in the tropical rain forest of Central Africa. Afr Study Monogr. 2003;28:143–157. [Google Scholar]
- 23.Althabe G. Changements sociaux chez les Pygm’eesBaka de l’est Cameroun. Cah Etud Afr. 1965;5:561–592. [Google Scholar]
- 24.Bundo D. Social relationship embodied in singing and dancing performances among the Baka. Afr Study Monogr. 2001;26(Suppl):85–101. [Google Scholar]
- 25.Hayashi K. Hunting activities in forest camps among the Baka hunter-gatherers of southeastern Cameroon. Afr Study Monogr. 2008;29:73–92. [Google Scholar]
- 26.Yasuoka H. The variety of forest vegetations in south-eastern Cameroon, with special reference to the availability of wild yam for the forest hunter-gatherers. Afr Study Monogr. 2009;30:89–119. [Google Scholar]
- 27.Neel JV, Weiss KM. The genetic structure of a tribal population, the Yanomama Indians. XII. Biodemographic studies. Am J Phys Anthropol. 1975;42:25–51. doi: 10.1002/ajpa.1330420105. [DOI] [PubMed] [Google Scholar]
- 28.Melancon T. 1982. Marriage and reproduction among the Yanomamo Indians of Venezela. PhD dissertation (Pennsylvania State University, State College, PA)
- 29.Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- 30.Hurtado AM, Hill K. Early dry season subsistence ecology of Cuiva (Hiwi) foragers of Venezuela. Hum Ecol. 1987;15:163–187. [Google Scholar]
- 31.Bailey R, Devore I. Research on the Efe and Lese populations of the Ituri forest, Zaire. Am J Phys Anthropol. 1989;78:459–471. [Google Scholar]
- 32.Bailey RC, Aunger RV. Sexuality, infertility, and sexually transmitted disease among farmers and foragers in Central Africa. In: Abramson PR, Pinkerton SD, editors. Sexual Nature/Sexual Culture. Univ of Chicago Press; Chicago: 1995. pp. 195–222. [Google Scholar]
- 33.Kramer KL, Greaves RD. Changing patterns of infant mortality and fertility among Pumé foragers and horticulturalists. Am Anthropol. 2007;109:713–726. [Google Scholar]
- 34.Kramer KL, Greaves RD, Ellison PT. Early reproductive maturity among Pumé foragers: Implications of a pooled energy model to fast life histories. Am J Hum Biol. 2009;21:430–437. doi: 10.1002/ajhb.20930. [DOI] [PubMed] [Google Scholar]
- 35.Kramer KL, Greaves RD. Synchrony between growth and reproductive patterns in human females: Early investment in growth among Pumé foragers. Am J Phys Anthropol. 2010;141:235–244. doi: 10.1002/ajpa.21139. [DOI] [PubMed] [Google Scholar]
- 36.Marlowe FW. Hunter-gatherers and human evolution. Evol Anthropol. 2005;14:54–67. doi: 10.1016/j.jhevol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Charnov E. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford Univ Press; New York: 1993. p. 23. [Google Scholar]
- 38.Kramer KL. Early sexual maturity among Pumé foragers of Venezuela: Fitness implications of teen motherhood. Am J Phys Anthropol. 2008;136:338–350. doi: 10.1002/ajpa.20817. [DOI] [PubMed] [Google Scholar]
- 39.Becker N, et al. Indirect evidence for the genetic determination of short stature in African pygmies. Am J Phys Anthropol. 2011;145:390–401. doi: 10.1002/ajpa.21512. [DOI] [PubMed] [Google Scholar]
- 40.Becker NS, et al. The role of GHR and IGF1 genes in the genetic determination of African pygmies’ short stature. Eur J Hum Genet. 2013;21:653–658. doi: 10.1038/ejhg.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry GH, et al. Adaptive, convergent origins of the pygmy phenotype in African rainforest hunter-gatherers. Proc Natl Acad Sci USA. 2014;111:E3596–E3603. doi: 10.1073/pnas.1402875111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geronimus AT. Damned if you do: Culture, identity, privilege, and teenage childbearing in the United States. Soc Sci Med. 2003;57:881–893. doi: 10.1016/s0277-9536(02)00456-2. [DOI] [PubMed] [Google Scholar]
- 43.Molinari L, Gasser T. The human growth curve: Distance, velocity and acceleration. In: Hauspie RC, Cameron N, Molinari L, editors. Methods in Human Growth Research. Cambridge Univ Press; Cambridge, UK: 2004. pp. 27–54. [Google Scholar]
- 44.Johnson W. Analytical strategies in human growth research. Am J Hum Biol. 2015;27:69–83. doi: 10.1002/ajhb.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodman MJ, Estioko-Griffin A, Griffin PB, Grove JS. Menarche, pregnancy, birth spacing and menopause among the Agta women foragers of Cagayan province, Luzon, the Philippines. Ann Hum Biol. 1985;12:169–177. doi: 10.1080/03014468500007661. [DOI] [PubMed] [Google Scholar]
- 46.Evans I. The Negritos of Malaya. Cambridge Univ Press; Cambridge, UK: 1937. [Google Scholar]
- 47.Condon RG. Inuit Youth: Growth and Change in the Canadian Arctic. Princeton Univ Press; Princeton, NJ: 1987. [Google Scholar]
- 48.Eder JF. On the Road to Tribal Extinction. Univ of California Press; Berkeley, CA: 1987. p. 32. [Google Scholar]
- 49.Kramer KL. Maya Children: Helpers at the Farm. Harvard Univ Press; Cambridge, MA: 2005. [Google Scholar]
- 50.Meehan CL. The effects of residential locality on parental and alloparental investment among the Aka foragers of the central African Republic. Hum Nat. 2005;16:58–80. doi: 10.1007/s12110-005-1007-2. [DOI] [PubMed] [Google Scholar]
- 51.Meehan CL, Quinlan R, Malcom CD. Cooperative breeding and maternal energy expenditure among Aka foragers. Am J Hum Biol. 2013;25:42–57. doi: 10.1002/ajhb.22336. [DOI] [PubMed] [Google Scholar]
- 52.Ivey P. Cooperative reproduction in Ituri forest hunter-gatherers: Who cares for Efe infants? Curr Anthropol. 2000;41:856–866. [Google Scholar]
- 53.Maget L. 2013 The Baka Pygmies, the Turning Point (CNRS-IRD Production). Available at videotheque.cnrs.fr/visio=4069. Accessed May 2018.
- 54. Anonymous (October 9, 2014) C’est du Poison, mais vous pouvez en consommer. Le Petit Écolier — Éduquer pour changer les mentalités. Available at lepetitecolier.mondoblog.org/2014/10/09/cest-du-poison-pouvez-en-consommer/. Accessed May 2018.
- 55. Bahane N, F (September 21, 2016) Cameroun—Interdiction du whisky en sachet: Pour quoi la résistance. Cameroon Tribune. Available at https://actucameroun.com/2016/09/21/cameroun-interdiction-whisky-sachet-resistance/. Accessed May 2018.
- 56. Abdelkader H (September 20, 2016) Cameroun—Vente de whisky en sachet: Le gouvernement recule face aux fabricants. Camerpost. Available at https://actucameroun.com/2016/09/20/cameroun-vente-de-whisky-sachet-gouvernement-recule-face-aux-fabricants/. Accessed May 2018.
- 57.McPherson K, Wakefield P. The perfect colonizer: Understanding alcoholism and its treatments in native America through humanistic inquiry. J Stud Res. 2015;4:174–179. [Google Scholar]
- 58.Hurley TD, Edenberg HJ. Genes encoding enzymes involved in ethanol metabolism. Alcohol Res. 2012;34:339–344. doi: 10.35946/arcr.v34.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gude D. Alcohol and fertility. J Hum Reprod Sci. 2012;5:226–228. doi: 10.4103/0974-1208.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eggert J, Theobald H, Engfeldt P. Effects of alcohol consumption on female fertility during an 18-year period. Fertil Steril. 2004;81:379–383. doi: 10.1016/j.fertnstert.2003.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Waldron M, Heath AC, Bucholz KK, Madden PA, Martin NG. Alcohol dependence and reproductive onset: Findings in two Australian twin cohorts. Alcohol Clin Exp Res. 2008;32:1865–1874. doi: 10.1111/j.1530-0277.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen TK, et al. Does moderate alcohol consumption affect fertility? Follow up study among couples planning first pregnancy. BMJ. 1998;317:505–510. doi: 10.1136/bmj.317.7157.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.