Significance
Transporters from the multidrug and toxic compound extrusion (MATE) superfamily protect the cell from cytotoxic molecules through an efflux mechanism that is dependent on ion electrochemical gradients. This study examined the role of specific residues in supporting conformational changes associated with ion and drug binding in NorM, an archetype of MATE transporters. The results show that a network of conserved residues in the N-terminal domain is critical for Na+- and H+-driven conformational changes, whereas residues in the C-terminal domain mediate drug binding. Informed by a correlation of conformational dynamics with transport activity, we propose a model that describes how conserved residues mediate ion-coupled structural changes underlying drug efflux.
Keywords: MATE, NorM, EPR, DEER, transport mechanism
Abstract
Secondary active transporters belonging to the multidrug and toxic compound extrusion (MATE) family harness the potential energy of electrochemical ion gradients to export a broad spectrum of cytotoxic compounds, thus contributing to multidrug resistance. The current mechanistic understanding of ion-coupled substrate transport has been informed by a limited set of MATE transporter crystal structures from multiple organisms that capture a 12-transmembrane helix topology adopting similar outward-facing conformations. Although these structures mapped conserved residues important for function, the mechanistic role of these residues in shaping the conformational cycle has not been investigated. Here, we use double-electron electron resonance (DEER) spectroscopy to explore ligand-dependent conformational changes of NorM from Vibrio cholerae (NorM-Vc), a MATE transporter proposed to be coupled to both Na+ and H+ gradients. Distance measurements between spin labels on the periplasmic side of NorM-Vc identified unique structural intermediates induced by binding of Na+, H+, or the substrate doxorubicin. The Na+- and H+-dependent intermediates were associated with distinct conformations of TM1. Site-directed mutagenesis of conserved residues revealed that Na+- and H+-driven conformational changes are facilitated by a network of polar residues in the N-terminal domain cavity, whereas conserved carboxylates buried in the C-terminal domain are critical for stabilizing the drug-bound state. Interpreted in conjunction with doxorubicin binding of mutant NorM-Vc and cell toxicity assays, these results establish the role of ion-coupled conformational dynamics in the functional cycle and implicate H+ in the doxorubicin release mechanism.
Expression of membrane transporters capable of exporting a broad range of toxic yet chemically diverse compounds is one of multiple mechanisms of multidrug resistance (1). Five distinct transporter families that enhance survivability against biologically active drugs (2–5) have been identified in organisms across the evolutionary spectrum. The multidrug and toxic compound extrusion (MATE) family confers resistance to a vast array of therapeutics such as aminoglycoside antibiotics, fluoroquinolones, and anticancer agents (6–8). Human orthologs expressed in the kidney not only remove organic cations but also transport pharmacologically diverse compounds including metformin (9–12). Therefore, a fundamental knowledge of the molecular mechanism of MATE transporter function is of pressing clinical relevance.
Phylogenetic analysis of the MATE family has so far identified three distinct transporter subfamilies (NorM, DinF, and eukaryote) that typically couple substrate efflux to either Na+ or H+ electrochemical gradients (13, 14). Crystal structures of five members representing the NorM and DinF subfamilies obtained in both detergent micelles and lipid mimetic environments have revealed a unique topology of 12-transmembrane (TM) helices arranged with twofold pseudosymmetry into separate N- and C-terminal domains (NTD and CTD, respectively) (15–19). In most cases, the overall architecture of MATE transporters retains general features observed in structures of Na+-dependent NorM from Vibrio cholerae (NorM-Vc). Two moderate-resolution NorM-Vc crystal structures have been determined in the presence or absence of Rb+, an electron-dense Na+ congener, identifying a putative Na+ binding site in the CTD and the location of residues indispensable to function (15). However, these two structures adopt similar conformations characterized by a large V-shaped opening formed at the periplasmic interface of TM1/TM2 of the NTD with TM7/TM8 of the CTD, which extends into the middle of the bilayer. In accordance with the classical description of an alternating access mechanism (20, 21), these structures have been interpreted as outward-facing conformations.
The lack of atomic-resolution models in multiple conformations, including substrate-bound states, has contributed to an incomplete picture of the transport cycle of NorM-Vc. Furthermore, the current library of other MATE structures obtained in the presence of various compounds argues against a consensus transport mechanism for the family. Indeed, the identification of distinct multidrug or inhibitor binding sites from both Na+- and H+-coupled MATE transporters supports diverging mechanisms of ion/substrate coupling, even within the same subfamily (16, 17, 19, 22, 23). In general, these mechanisms either invoke allosteric coupling of ion and substrate binding in nonoverlapping binding sites [Na+-dependent NorM from Neisseria gonorrhaeae (NorM-Ng)] (17) or postulate direct competition through a mutually exclusive ion/substrate binding site (H+-dependent DinF-BH from Bacillus halodurans) (16). Compounding the apparent mechanistic diversity is the recent observation of coupling ion promiscuity in the precolibactin transporter ClbM in which ethidium transport was supported by protons or a number of alkali cations (24).
Common to all proposed antiport mechanisms is the involvement of conserved acidic residues in the binding and coupling of ligands. An Asp residue (D36 in NorM-Vc) located on the periplasmic side of TM1 in the NTD that is strictly conserved in the NorM and DinF subfamily (14) has been suggested to either participate directly in substrate binding (16, 17) or to facilitate conformational changes in TM1 associated with substrate extrusion (19). In the NorM subfamily, a conserved pair of acidic residues in the CTD located near the center of the bilayer projects into the protein core and participates in Rb+ coordination (15, 25). These residues, E255 and D371 in NorM-Vc, are critical for drug resistance (15) and were implicated in ion flux in a recent transport model of NorM-Vc that envisions substrate extrusion driven by both Na+ and H+ gradients (26). However, how these and other conserved residues are involved in ion-coupled conformational dynamics of the transporter remains unclear.
To illuminate this aspect of NorM-Vc transport mechanism, we carried out double-electron electron resonance (DEER) spectroscopy (27–30) to monitor ion- and substrate-dependent conformational changes on the periplasmic side of NorM-Vc. We uncover the presence of a unique Na+ and H+ binding site in the NTD that coordinates the functional dynamics of TM1. Furthermore, formation of the substrate-bound state is H+ dependent and involves conserved acidic residues in the CTD. By correlating drug resistance assays with conformational dynamics in backgrounds of conserved residue mutants, we propose a model for the contribution of Na+ and H+ to the conformational cycle.
Results
Sodium- and DXR-Dependent Conformational Dynamics of NorM-Vc.
Since current models of transport envision an important role for TM1, we sought to define the structural rearrangements of this helix in NorM-Vc solubilized in β-dodecyl maltoside (DDM) detergent micelles. For this purpose, pairs of sulfhydryl-reactive nitroxide spin labels were incorporated at defined periplasmic positions on helices and the short TM7/TM8 loop (PL7–8) via engineered cysteines into a construct devoid of endogenous cysteines (Cysless, Methods). The spin label pairs chosen for DEER analysis were selected to probe distances from TM1 (V35C, A45C) to other positions within the NTD or positions within the CTD across the large periplasmic cleft as shown in Fig. 1. Expression of most double-cysteine mutants conferred robust resistance to toxicity of the chemotherapeutic drug doxorubicin (DXR) relative to the Cysless construct, although A45C/A267C (TM1/TM7) and A45C/V274C (TM1/TM8) were substantially more susceptible at the tested drug concentration (SI Appendix, Fig. S1). The apparent functional attenuation of these mutants may be related to disulfide-mediated trapping during the conformational cycle as observed for similar pairs in NorM-Ng (31). Nevertheless, the measured activities for these mutants were significantly above (P < 0.0001) the vector control and thus included in the dataset.
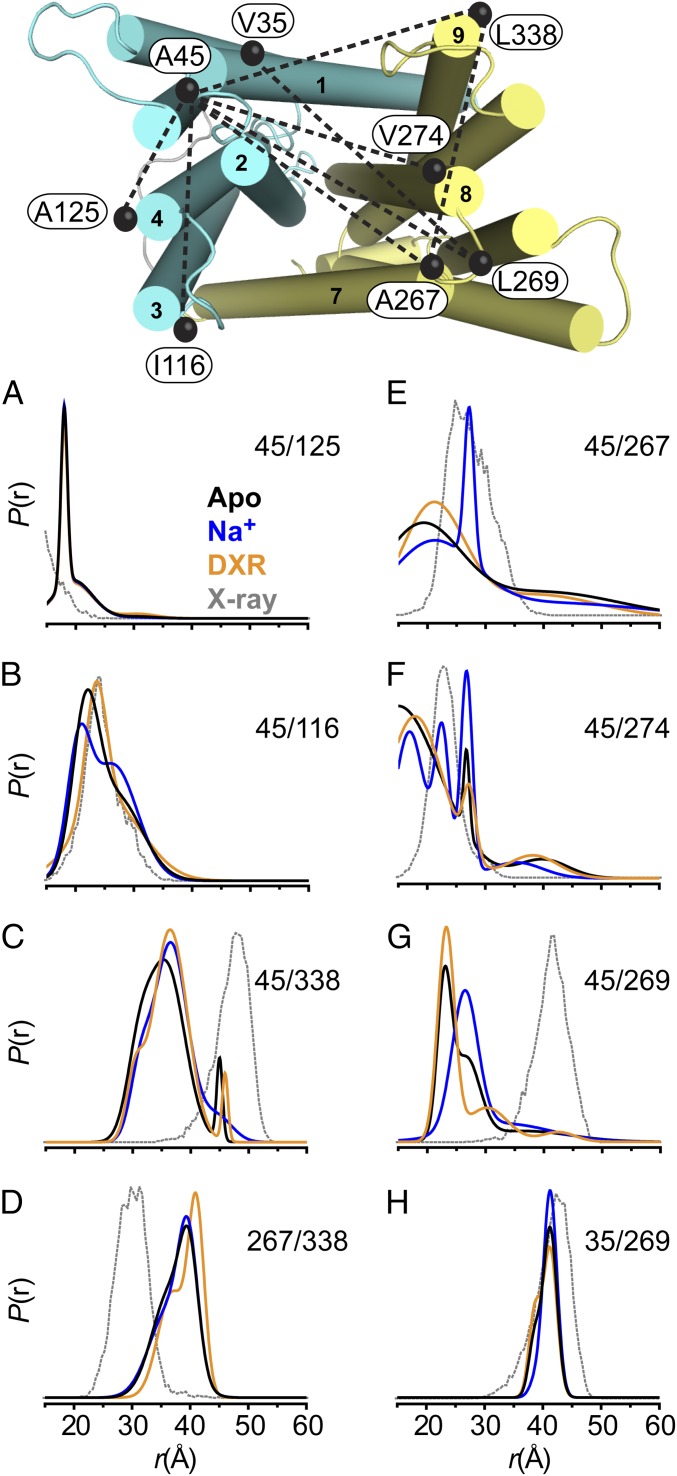
Fig. 1.
Ligand-dependent conformational dynamics on the periplasmic side of NorM-Vc. Spin label pairs designed to measure distances within the NTD (cyan), CTD (yellow), or between the domains from TM1 are shown as black spheres connected with a dashed line. (A–H) The distance distributions depicting the probability of a distance P(r) versus the distance r are shown for three biochemical conditions: Apo (black), Na+ (blue), and DXR (orange). The predicted distance distribution based on the X-ray structure (PDB ID code 3MKU) is shown in gray.
The DEER analysis uncovered structural differences relative to the available atomic-scale models and identified Na+/DXR-dependent conformational changes (Fig. 1). Deviations were observed between the experimentally derived distance distributions [P(r)] and predicted P(r) from modeling spin labels onto the crystal structure (32), which suggest differences between the conformations of NorM-Vc adopted in solution and the crystallographic model obtained in nonyl-glucoside (Fig. 1, compare black with gray curves). This conspicuous result was illustrated prominently for distances measured within the CTD (Fig. 1D) and between TM1 and the CTD (Fig. 1 E–H). For example, the largest distance probability under all biochemical conditions for the A45C/L269C pair is shorter than the prediction (Fig. 1G). Spin–spin interaction was observed in the EPR spectrum for A45C/A267C and A45C/V274C, consistent with the major short distance population (r ≤ 20 Å) obtained from the DEER analysis (Fig. 1 E and F and SI Appendix, Fig. S2). In the absence of an exhaustive dataset, we speculate that these differences may arise from subtle repacking of transmembrane helices or altered helix orientations relative to the crystallographic model. Nevertheless, the collective pattern of measured distances implies that the large cavity in the crystal structure may be collapsed in solution, a result consonant with atomistic molecular-dynamics (MD) simulations of NorM-Vc (33, 34) and NorM-Ng (35) showing collapse of the characteristic V-shape vestibule in a lipid bilayer.
DEER analysis performed in the absence of ion or drug (i.e., the Apo state) revealed multicomponent P(r) characteristic of dynamic backbone fluctuations that could be modulated by either Na+ or DXR (Fig. 1 and SI Appendix, Fig. S2). Furthermore, Na+/DXR-dependent shifts in P(r) were larger for spin label pairs sampling distances across the periplasmic cleft than for pairs within the same domain, consistent with relative movement between domains. The presence of Na+ selectively increased the population of longer distance components while suppressing shorter distances for most spin label pairs, which suggest stabilization of an outward-facing state. This change in conformation is best illustrated by A45C/L269C where binding of Na+ selectively increased the population of the longer distance component relative to an Apo distance distribution consisting of two equally populated components. This transition was not supported by K+ and was characterized by an ∼ 0.4 mM with saturation at ∼5 mM Na+ (SI Appendix, Fig. S3), indicating that this conformation is favored under normal physiological conditions in the absence of substrate.
In contrast, DXR binding selectively suppressed the longer distance component observed in the Apo state of A45C/L269C and increased the population of shorter distances. Similar opposite changes in P(r) across the cleft relative to Na+ suggests that the ion and substrate stabilize distinct structural intermediates. Relatively minor changes in P(r) compared with the Apo state were observed upon binding of DXR for most other pairs (Fig. 1 and SI Appendix, Fig. S2).
Protonation Induces a Distinct Structural Intermediate.
The imposition of a proton motive force has been observed to potentiate NorM-Vc–mediated ethidium efflux in proteoliposomes, implying that H+ are involved in the antiport mechanism (26). Therefore, we investigated whether low pH conditions alter the conformation of NorM-Vc, using the library of spin label pairs probing distances across the periplasmic cleft, and compared the results to the Na+-induced intermediate. As shown in Fig. 2A, we observed changes in P(r) at pH 4 manifested by unique distance components relative to the Apo (pH 7.5) and Na+ conditions. For V35C/L269C (TM1/PL7–8), A45C/A267C (TM1/TM7), and A45C/V274C (TM1/TM8), protonation induced a decrease in average distance between probes, a result opposite to that observed for Na+. In contrast, protonation increased the distance between A45C/L269C (TM1/PL7–8), although not to the same extent as Na+.
Fig. 2.
Global and local conformational changes induced by H+. (A) DEER analysis of spin label pairs sampling distances between TM1 and the CTD. (B) EPR spectra of spin labels along TM1 demonstrates line shape changes consistent with Na+- and H+-dependent conformational changes in TM1. TM2 has been removed for clarity in this panel.
Given that side chains of V35 and A45 on TM1 point in opposite directions in the crystal structure, simple translation of TM1 alone is unlikely to account for the distinct patterns of ion-dependent changes in P(r) for the V35C/L269C and A45C/L269C pairs. To explore the nature of the underlying movement, single spin labels were incorporated along a short periplasmic stretch of TM1 and analyzed for ion-dependent changes in their mobilities as reported by the continuous-wave EPR spectral line shape. The exquisite sensitivity of spin labels to variations in the local environment has been shown previously to correlate with global structural rearrangements in transporters (36, 37). The distinct EPR line shapes for each labeled position were consistent with a helix demonstrating an asymmetric pattern of contacts (Fig. 2B). Importantly spin labels at residue T37C and A40C report opposing H+- and Na+-dependent changes in mobility, rationalizing the opposite pattern of distance changes for V35C/L269C and A45C/L269C. While the finding that TM1 is a sensor of ion binding is consistent with crystallographic analysis of a transporter from Pyrococcus furiosus (Pf-MATE) that captures different conformations of TM1 putatively associated with protonation (19), distinct structural changes induced by Na+ and H+ have not been previously observed and suggest that both ions may be involved in the transport mechanism. The limited set of line shape changes can be consistent with either TM1 bending as seen in Pf-MATE, or a small clockwise (H+) and counterclockwise (Na+) rotation of the helix.
A Unique NTD Ion Binding Site Modulates Conformational Dynamics.
We exploited the sensitivity of P(r) to ligand binding to define the role of conserved residues in the structural dynamics underlying transport. Specifically, background mutations were introduced into a parent construct (P), A45C/L269C, to monitor changes in the ion/drug-dependent conformational equilibrium. A combination of sequence and structural alignments of MATE transporters (SI Appendix, Fig. S4) was used to identify target residues for mutagenesis. The alignments underscored a conserved network of polar residues within the NTD cavity, which included the critical TM1 Asp (D36), as shown in Fig. 3. Therefore, these positions were interrogated for modulation of conformational dynamics as reported by the A45C/L269C pair. In some cases, mutation of targeted residues to Ala substantially compromised expression of folded protein (SI Appendix, Fig. S5) and therefore not included in the analysis.
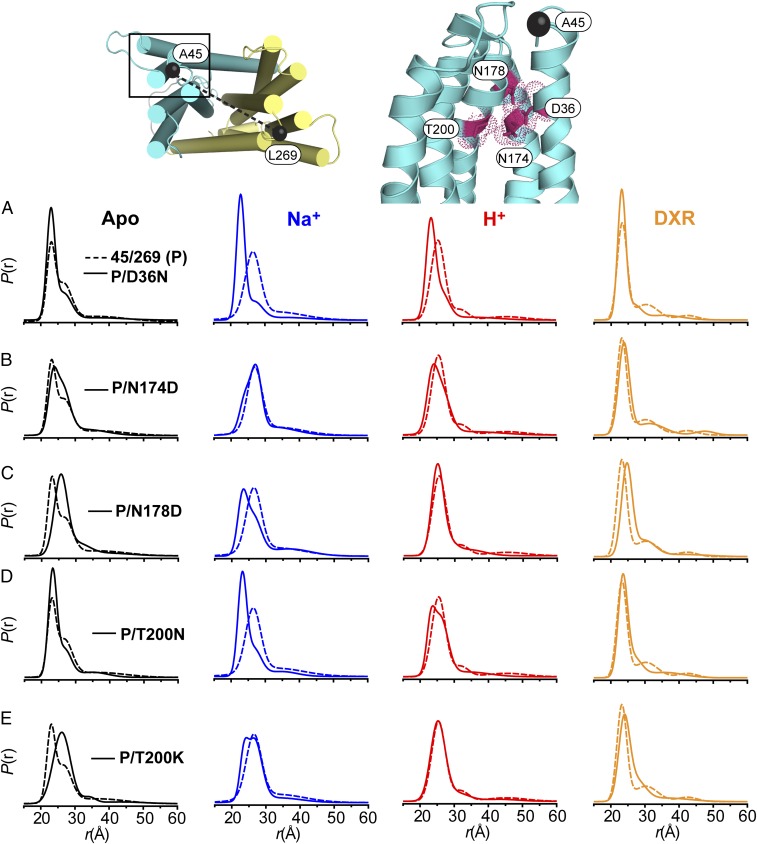
Fig. 3.
Conformational dynamics of NTD background mutants. Polar residues lining a cavity within the NTD of NorM-Vc targeted for mutagenesis are shown as sticks outlined by a space-filling representation. TM2 has been omitted for clarity. The location of spin labels for the A45C/L269C pair is shown as black spheres connected by a dashed line. (A–E) P(r) of each mutant (solid line) is compared with the P(r) of the parent construct (P, dashed line) for each biochemical condition.
Significant changes were observed in the P(r) of the A45C/L269C reporter upon mutagenesis of conserved residues (Fig. 3 and SI Appendix, Fig. S6). These changes were well outside the calculated 95% confidence bands for each distribution (SI Appendix, Fig. S7). In the Apo state, the D36N mutation increased the population of the short distance component while reducing the longer distance component (Fig. 3A). Furthermore, this mutation did not support the Na+- or H+-dependent transition to longer distances. Impaired conformational transition from the Apo to Na+ states was observed also for T200N, although this mutant retained a response to H+ (Fig. 3D).
N174D and N178D induced a right shift toward longer distances in P(r) of the Apo state (Fig. 3 B and C), but unlike D36N and T200N, these two mutations did not disrupt Na+-driven conformational changes. Although N174D demonstrated an Na+-dependent shift toward longer distances as observed in the parent construct (Fig. 3B), the presence of Na+ decreased the distance between spin labels for N178D (Fig. 3C). A reduction in sensitivity to H+ was observed for both mutants, which was likely due to the shift toward longer distances in the Apo state. Indeed, P(r) for N174D and N178D in the presence of H+ was nearly superimposable with the respective Apo condition (SI Appendix, Fig. S6E).
Collectively, the dependence of ion-driven conformational changes on the functional groups of conserved residues in the NTD suggests that Na+ and H+ form specific interactions with these side chains that determine the nature of TM1 movement. In support of this interpretation, the T200K mutation, which mimics an ion-bound state by introducing a permanent positive charge (under normal physiological conditions) within the cavity, induced a shift toward longer distances in the Apo state (Fig. 3E). Importantly, the DXR-driven decrease in average distance between spin labels relative to the Apo state was observed in these NTD mutants (even though the DXR states are not necessarily equivalent to the parent A45C/L269C reporter), suggesting that DXR binding was not significantly affected (Fig. 3 and SI Appendix, Fig. S6E), a conclusion corroborated by the comparable DXR binding affinity of these mutants (Table 1).
Table 1.
DXR binding affinity to NorM-Vc mutants under distinct conditions
| Construct | Mutant location | Condition | KD ± SD,* μM | N† |
| pH 7.5 | 1.66 (0.26) | 6 | ||
| Cysless | N/A | pH 7.5, 10 mM NaCl | 3.01 (0.22) | 6 |
| pH 4.0 | 22.21 (2.83) | 5 | ||
| A45C/L269C (P)‡ | N/A | pH 7.5 | 2.52 (0.23) | 6 |
| P/D36N‡ | NTD, TM1 | pH 7.5 | 2.64 (0.08) | 2 |
| P/N174D‡ | NTD, TM5 | pH 7.5 | 1.66 (0.09) | 3 |
| P/N178D‡ | NTD, TM5 | pH 7.5 | 2.34 (0.19) | 3 |
| P/T200K‡ | NTD, TM6 | pH 7.5 | 3.92 (0.43) | 3 |
| P/T200N‡ | NTD, TM6 | pH 7.5 | 2.29 (0.09) | 2 |
| P/E255Q‡ | CTD, TM7 | pH 7.5 | 14.80 (1.22) | 3 |
| P/D371N‡ | CTD, TM10 | pH 7.5 | 14.07 (1.69) | 3 |
| P/E255Q/D371N‡ | CTD, TM7/TM10 | pH 7.5 | 17.08 (2.02) | 3 |
For n ≥ 3, average ± SD; for n = 2, average ± spread.
Total number of experiments; where n > 3, replicates include multiple protein preparations.
Experiments performed with spin labeled protein.
DXR Binding Is Proton Dependent and Mediated by Conserved CTD Residues.
The spectroscopic analysis establishes that the population of structurally distinct Na+- and H+-dependent intermediates is controlled in part by residues in the NTD cavity. To investigate the interplay between Na+ and H+ in driving the antiport cycle, we examined the ion dependence of DXR binding to NorM-Vc. NorM-Vc/DXR complex formation resulted in an increase in DXR fluorescence anisotropy that was pH dependent (Fig. 4A). DXR-binding isotherms generated in the presence of 10 mM Na+ (pH 7.5) reported a subtle right shift in the curve relative to isotherms obtained in the absence of Na+ corresponding to an approximate twofold lower binding affinity (Fig. 4B and Table 1). In contrast, binding curves generated at pH 4 (no Na+) reported a substantial decrease (>10-fold) in affinity (Fig. 4B and Table 1). Consistent with these experiments, EPR and DEER analysis indicated that the drug-bound conformation is stable in the presence of Na+, but not H+ (SI Appendix, Fig. S8).
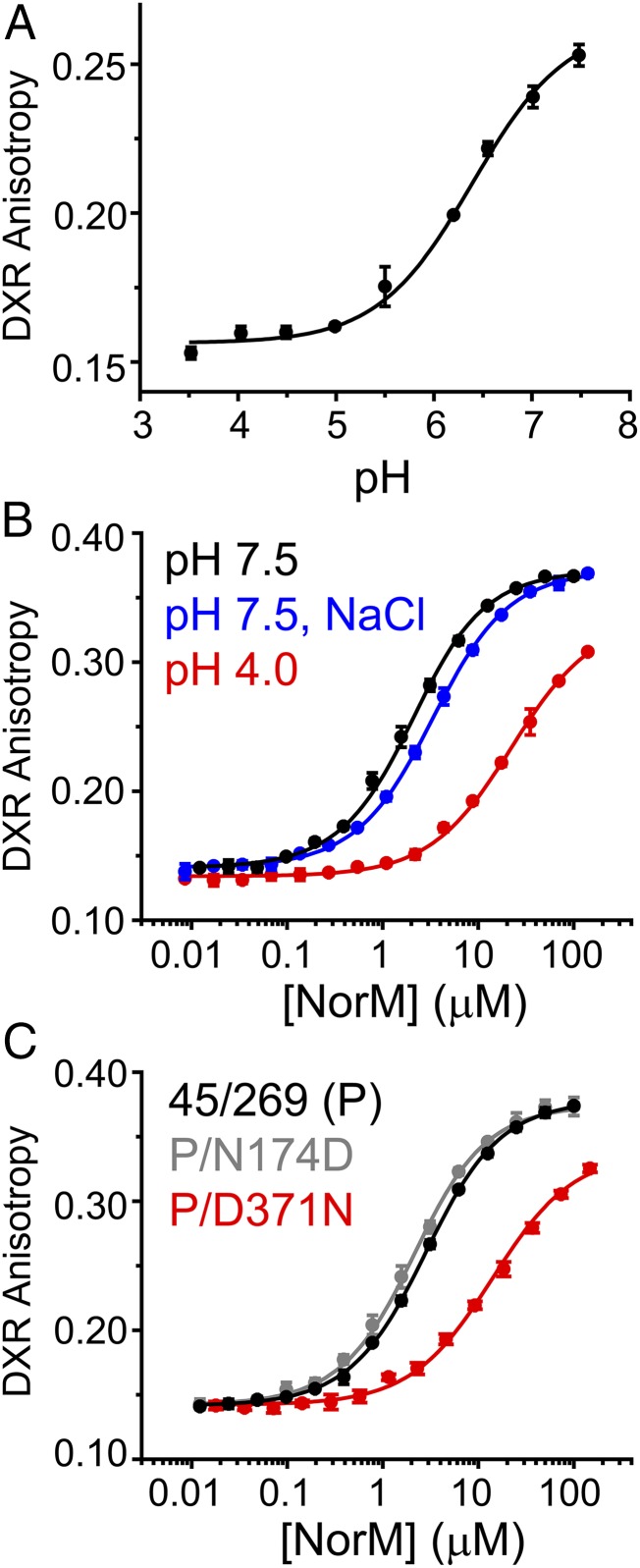
Fig. 4.
Role of CTD residues in H+-dependent DXR binding. (A) pH dependence of DXR binding to Cysless NorM-Vc observed through changes in DXR fluorescence anisotropy. Nonlinear least-squares fitting of the binding curve yielded a pKa of 6.4. (B) In contrast to DXR binding curves generated in the presence of 10 mM NaCl, drug binding was attenuated substantially in low-pH buffer. (C) Comparison of DXR binding curves (pH 7.5) for NTD and CTD background mutants relative to the parent construct. Introduction of D371N and E255Q (Table 1) strongly reduced DXR binding affinity.
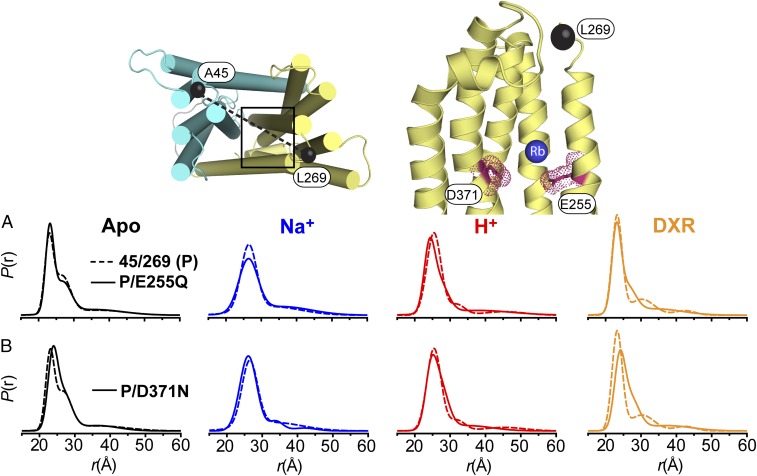
In contrast to background mutations in the NTD, which did not disrupt DXR binding relative to the parent construct, introduction of the CTD mutations E255Q and D371N, which abrogate potential protonation/deprotonation of their side chains, resulted in drastically reduced binding affinities similar to that observed for low-pH buffer (Fig. 4C and Table 1). E255 and D371 were proposed originally to participate in Na+ coordination based on a Rb+-bound crystal structure (15). However, E255Q and D371N did not preclude ion-driven conformational changes (Fig. 5 and SI Appendix, Fig. S6E), although a modest shift (approximately twofold) in was observed for E255Q (SI Appendix, Fig. S3). Interestingly, DXR-driven conformational changes were reduced in E255Q and D371N (Fig. 5 and SI Appendix, Fig. S6E), consonant with the reduction in DXR binding affinity for these mutants. Taken together, these results indicated that formation of the DXR-bound intermediate is dependent on the protonation/deprotonation of E255 and D371 side chains.
Fig. 5.
Conformational dynamics of CTD background mutants. The location of conserved E255 and D371 within the CTD is shown as sticks outlined by a space-filling representation, highlighting interactions with a bound Rb+ as observed in the crystal structure (PDB ID code 3MKU). The location of spin labels for the A45C/L269C pair is shown as black spheres connected by a dashed line. (A and B) P(r) of each background mutant (solid line) is compared with the P(r) of the parent construct (P, dashed line) for each biochemical condition.
Correlation of Ion/Drug-Dependent Structural Dynamics with DXR Resistance.
The sensitivity of P(r) to mutation of conserved residues (Figs. 3 and 5) provides a framework to correlate changes in transport activity to conformational dynamics. To characterize activity levels of NorM-Vc mutants, we determined the (5) for each mutant when expressed in an Escherichia coli strain devoid of seven endogenous multidrug transporters (38). The DXR resistance profile shown in SI Appendix, Fig. S9 identified active and impaired mutants relative to the parent A45C/L269C construct and vector control. D36N, E255Q, and D371N were highly susceptible to DXR toxicity, resulting in cell death at low drug concentrations, as expected (25, 26). Moreover, the data highlighted potentiated activity for the N174D, N178D, and T200N mutations relative to the parent construct. The altered activity profiles were verified to not result from changes in expression and membrane incorporation of the mutants (SI Appendix, Fig. S9).
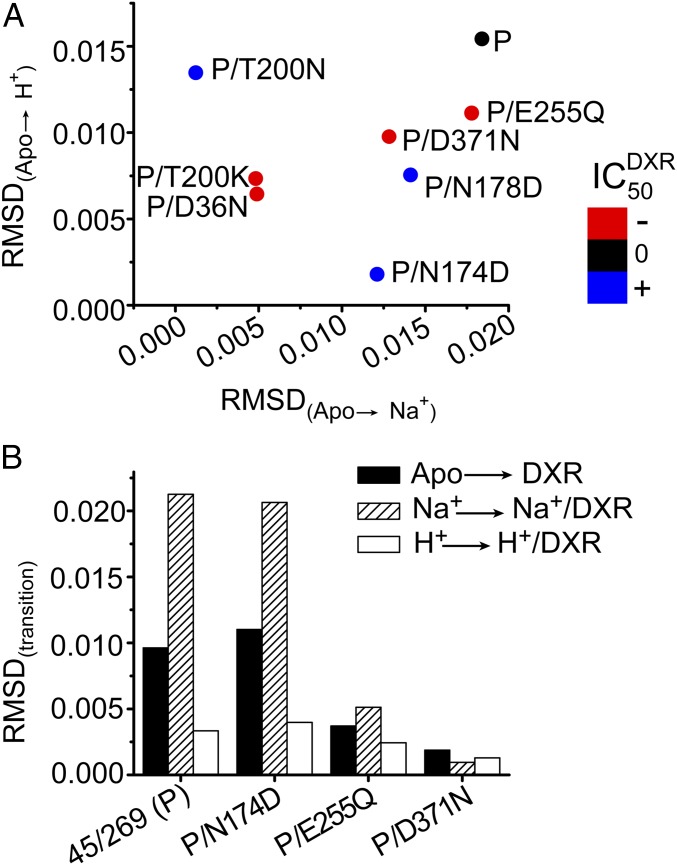
To quantitatively describe the ion- and substrate-dependent conformational changes of point mutants, we determined the root-mean-square deviation (rmsd) between P(r) values under distinct biochemical conditions (Methods). This parameter reflects the underlying changes in populations of intermediates induced by ligand binding. To relate the conformational dynamics to the activity profiles, we mapped the onto an rmsd plot describing the Na+- and H+-driven transitions (Fig. 6A). The distribution of the data reveals the origin of altered activity for the NTD mutants. For the impaired mutants D36N and T200K, the rmsd between the Apo state and the ion-bound intermediates was relatively small (Fig. 6A). In contrast, a larger rmsd for active constructs reflected a robust structural response to Na+, H+, or both (Fig. 6A).
Fig. 6.
Correlation of conformational dynamics with resistance to DXR toxicity. (A) Overlay of activity reported by differences in in background mutants with the ion-driven structural transitions from the Apo state characterized by the calculated rmsd between distance distributions. The change in of each mutant corresponds to the color of each point according to the color scale. The of the parent construct (P, A45C/L269C) was 0.91 ± 0.05 μg/mL. The ranges of for the impaired (red −) and activated (blue +) constructs were 0.23–0.26 and 1.79–2.12 μg/mL, respectively. The parent construct has a large rmsd for both ions relative to the Apo state. Severely impaired mutants D36N and T200K displayed highly reduced rmsd for both ions. (B) Relative to active constructs, reduced of E255Q (0.22 ± 0.01 μg/mL) and D371N (0.21 ± 0.01 μg/mL) is correlated with impaired structural transition to the DXR-bound state. In contrast to the presence of Na+, the drug-bound intermediate could not be formed at low pH as indicated by a small rmsd between H+ and H+/DXR states.
Although ions elicited a change in the P(r) of CTD mutants E255Q and D371N (Figs. 5 and 6A), the lack of significant activity for these mutants is presumably a consequence of their inability to extrude DXR. Not only does DXR fail to elicit a robust structural response (Fig. 6B) but the drug also has a substantially reduced binding affinity to these mutants (Table 1). This analysis reinforces that E255 and D371 facilitate DXR-dependent conformational changes. Together, the correlation of rmsd and establish the significance of the conformational dynamics on the periplasmic side of NorM-Vc in the mechanism of transport.
Discussion
The combined spectroscopic and functional analysis presented here illuminates important aspects of the structural basis of Na+ and H+ coupling in the transport cycle of NorM-Vc and defines the role of conserved residues in controlling conformational dynamics. Fundamental to the transport mechanism is the formation of unique Na+- and H+-dependent intermediates defined in part by distinct conformations of TM1. Na+ and H+ binding is supported by a network of polar residues in the NTD forming a site that was not identified in the crystal structures of NorM-Vc. In general, alignment of conserved residues sharing similar spatial organization within the NTD across MATE homologs (SI Appendix, Fig. S4) suggests that this binding site may have a common role in the energetics of ion-dependent conformational changes (39).
Direct correlation between NorM-Vc structural dynamics on the periplasmic side with drug resistance activity reveals how individual conserved residues modulate the transport energy landscape. Impairment of ion-driven changes in P(r) for D36N and T200K suggests that these mutants are trapped in specific conformations leading to compromised function. Inhibition of Na+- and H+-driven conformational changes by the D36N mutation, which modifies the conserved carboxylate moiety, implicates this Asp as a hub for both ions (Fig. 3). Similarly, the finding that T200N abrogates the Na+-dependent conformational transition suggests that this residue may be involved in Na+ coordination. These results are consistent with previous MD simulations identifying D36 as a site of entry for Na+ into NorM-Vc and coordination by T200 (34). Finally, the potentiated transport activities of N174D and N178D mutations imply lowered free-energy barrier between conformations, leading to more favorable structural transitions.
While a functional role for H+ binding has been suggested in the context of an H+-dependent MATE transporter (40), our results establish that protonation not only regulates conformational dynamics but is also involved in substrate release. Protonated NorM-Vc is refractory to DXR binding as evidenced by an order-of-magnitude loss in drug binding affinity and inhibition of DXR-driven conformational changes (Figs. 4 and 6B). This result is in contrast to the effect of Na+, which induced a relatively modest reduction in substrate KD, an observation consistent with previous experiments involving a spin-labeled derivative of daunorubicin (41). The correlation of pH-dependent binding with reduced binding affinity of E255Q and D371N mutants suggests that these CTD residues mediate mutually exclusive DXR and H+ binding. Multiple lines of evidence support this model. Recent MD simulations of NorM-Vc indicated that protonation of E255 or D371 is favorable in an aqueous environment (42). Substrate-binding studies of H+-dependent NorM from Pseudomonas stutzeri identified the corresponding E257 and D373 as critical residues in high-affinity binding of the fluorescent probe DAPI (43). Moreover, mutation of glutamate residues E273 and E389 in hMATE1 (SI Appendix, Fig. S4) altered affinity and pH-dependent substrate uptake in HEK-293 cells (44), suggesting conserved function of these carboxylates in the eukaryotic subfamily.
Although E255 and D371 play an essential role in DXR binding, the contribution of other residues to specific drug interactions is anticipated. A previous report indicated that ethidium binding to NorM-Vc was not affected by D371N, even though ethidium binding was associated with H+ release (26). Additionally, NorM-Ng displays reduced DXR binding affinity relative to NorM-Vc (approximately fourfold) despite retaining the conserved carboxylate moieties (SI Appendix, Fig. S10). A recent comparison of multiple MATE structures suggests that the substrate polyspecificity profile of the family may be linked to variable electrostatic charge distribution of an internal pocket formed between the NTD and CTD (45).
Collectively, our results are consistent with a model that involves ion entry into the NTD site inducing transitions between discrete conformations and culminating with DXR release from a binding site mediated in part by buried CTD carboxylates. In this model, ions modulate DXR affinity either indirectly via shifts in conformational preference (Na+) or through direct competition (H+) of the binding site. The pattern of DXR-dependent conformational changes implies that substrate binding drives movement of CTD elements, such as TM7. Facilitated in part by ion-driven changes in the conformation of TM1, transport of bound substrate can be accomplished through associated changes in hydration that increase the H+ concentration at the DXR binding site. Although the model does not exclude Na+ occupation of the crystallographically determined Rb+ binding site, it emphasizes the role of H+ in DXR dissociation. This interpretation rationalizes previous observations that substrate transport is coupled to both Na+ and H+ gradients (26), and delineates the contribution of conserved residues to the structural dynamics of MATE transporters.
Methods
Mutagenesis, Expression, Purification, and Labeling of NorM-Vc.
A previously generated Cysless NorM-Vc harbored within the pET19b vector encoding an N-terminal His10 tag under control of the inducible T7 promoter (41) was used as the template to introduce double-Cys pairs and background mutations via site-directed mutagenesis with complementary oligonucleotide primers. DNA sequencing using both T7 forward and reverse primers confirmed mutagenesis and the absence of undesired changes. Mutants are identified by the native residue and primary sequence position followed by the mutant residue. NorM-Vc was expressed in Escherichia coli BL21(DE3) cells and purified as described previously (41) with some modification. Briefly, cells were lysed in 20 mM Tris, pH 8, 20 mM NaCl, and 5% (vol/vol) glycerol buffer including 10 mM DTT and the membrane fraction isolated via ultracentrifugation. NorM-Vc was solubilized from membranes with 30 mM [1.5% (wt/vol)] DDM in the presence of 1 mM DTT. Following ultracentrifugation to remove insoluble material, the supernatant was batch incubated with Ni-NTA Superflow resin (Qiagen) in the presence of 30 mM imidazole for 2 h at 4 °C. NorM-Vc was eluted from the resin with buffer containing 300 mM imidazole. The sample was labeled with two rounds of 20-fold molar excess 1-oxyl-2,2,5,5-tetramethylpyrroline-3-methyl methanethiosulfonate (Enzo Life Sciences) per cysteine at 4 °C in the dark over a 4-h period. After a third addition of spin label, the sample was incubated on ice for 12 h. Unreacted spin label was removed by gel filtration chromatography over a Superdex 200 Increase 10/300 GL column (GE Healthcare) into 50 mM Tris/Mes, pH 7.5, 1 mM DDM, and 10% (vol/vol) glycerol buffer. Peak fractions were combined and concentrated by an Amicon Ultra (100-kDa molecular-weight cutoff) for binding studies and EPR and DEER analysis.
EPR and DEER Spectroscopy and Determination of Rmsd.
EPR spectra were collected at room temperature on a Bruker EMX spectrometer (X-band, 9.5 GHz) at an incident power of 10 mW and 1.6-gauss modulation amplitude. Scan width was 150 gauss. Distance measurements were carried out on a Bruker E580 pulsed EPR spectrometer at Q-band frequency (34 GHz) employing a standard four-pulse protocol at 83 K (28). Pulse lengths were 10–12 ns (π/2) for the probe pulse and 40 ns for the pump pulse. The frequency separation between probe and pump pulses was 63 MHz. The Na+ intermediate was generated by addition of 10 mM NaCl as informed from Na+ titration experiments. The drug-bound state was generated by addition of 0.95 mM DXR in the absence or presence of ions. To ascertain the role of H+, samples were titrated to pH 4 with an empirically determined amount of 0.28 M citric acid and confirmed by pH electrode measurement. Samples for DEER analysis were cryoprotected with 25% (wt/vol) glycerol and flash-frozen in liquid nitrogen. DEER signals were analyzed with home-written software operating in the Matlab (MathWorks) environment (46). The fitting routine assumes that the distance distribution P(r) is a sum of Gaussians. The number of Gaussians to sufficiently describe P(r) was statistically determined either by F test between fits of increasing Gaussian components or comparison of the resulting Bayesian information criterion for each fit. The confidence bands generated for the A45C/L269C pair as shown in SI Appendix, Fig. S7 were determined from calculated uncertainties of the fit parameters (46). Comparison of the experimental distance distributions with the NorM-Vc crystal structure [Protein Data Bank (PDB) ID code 3MKU] using molecular-dynamics simulations with dummy spin labels (MDDS) was facilitated by the DEER Spin-Pair Distributor at the CHARMM-GUI website (32, 47). The rmsd between specific states was determined by the sum of square difference for each point constructing P(r) according to the following equation: , where P(r)x represents the experimental distance distribution of a specific state and is the total number of points in P(r).
DXR Binding Assay.
The concentration of DXR HCl (AvaChem Scientific) solubilized in water was determined spectrophotometrically using an extinction coefficient () of 11,500 M−1⋅cm−1 at 480 nm. DXR (1 μM) was mixed with increasing concentrations of NorM-Vc in 25-μL total volume of gel filtration buffer and transferred to a 384-well black microplate (Greiner Bio-One). DXR anisotropy (λex = 485 nm, λem = 595 nm) was measured on a BioTek Synergy H4 microplate reader. Binding isotherms were measured in triplicate for most mutants. However, binding isotherms with Cysless and parent (A45C/L269C) constructs were measured at least five times to ascertain variability in protein preparations. The individual curves were fit to a single-site binding model in the program Origin (OriginLab) to determine KD. The average KD and SD for each construct or biochemical condition are reported in Table 1. To test pH-dependent binding, purified NorM-Vc was diluted 10-fold (3.4 μM final) into 50 mM buffer containing 1 μM DXR, 1 mM DDM, and 10% (vol/vol) glycerol. The buffer for each pH condition was made by mixing appropriate ratios of Tris base with either Mes hydrate or citric acid and confirmed by pH electrode measurement. Binding isotherms generated at pH 4 (Tris/citric acid) was performed with fresh stock NorM-Vc that had been exchanged into assay buffer by diluting the protein >30-fold on an Amicon concentrator.
DXR Resistance Assay.
Resistance to DXR toxicity was carried out as previously described (41) with modifications. Drug-sensitive Escherichia coli BL21(DE3) Δ7 cells, denoting the deletion of genes associated with multidrug resistance (macAB, yojHI, acrAB, acrEF, emrAB, emrKY, and mdtEF) (38), were transformed with empty pET19b vector, pET19b encoding Cysless, or mutant NorM-Vc. A dense overnight culture from a single transformant was used to inoculate 10 mL of LB broth (LabExpress) containing 0.1 mg/mL ampicillin (Gold Biotechnology) to a starting OD600 of 0.0375. Cultures were grown to OD600 of 0.3 at 37 °C and expression of the encoded construct was induced with 0.1 mM IPTG (Gold Biotechnology). Expression was allowed to continue at 37 °C for 2 h, after which the OD600 of the cultures was adjusted to 0.5. The cells were then used to inoculate (1:20 dilution, starting OD600 = 0.025) a sterile 96-well microplate (Greiner Bio-one) containing 50% LB broth, 0.1 mg/mL ampicillin, and 0.5 μg/mL DXR. Microplates were incubated at 37 °C with shaking at 200 rpm for 6 h, after which the cell density was measured on a BioTek Synergy H4 microplate reader and normalized to the Cysless construct. Each data point was performed in triplicate, and the experiment was repeated three times to obtain the mean and SEM. Statistical significance was assessed by an unpaired t test. The results are shown in SI Appendix, Fig. S1.
To determine the , cells expressing NorM-Vc mutants were grown as described above in 50 mL of LB broth and used to inoculate (1:20 dilution; starting OD600 of 0.025) a sterile 96-well microplate containing 50% LB broth, 0.1 mg/mL ampicillin, and 0−8 μg/mL DXR. Microplates were incubated at 37 °C with shaking at 200 rpm for 6 h, after which the cell density was measured on a BioTek Synergy H4 microplate reader. The cell density of the wells was normalized to the 0 μg/mL DXR well and plotted as a function of increasing DXR concentration to obtain a toxicity profile. The data were fit with a dose–response curve weighted by the SD of each data point to determine the . For most mutants, each data point was performed in triplicate and the experiment repeated three times. However, experiments with empty vector, Cysless, Cysless/D36N, Cysless/D371N, A45C/L269C (P), and P/D36N were repeated at least five times. The mean and SEM for each construct are shown in SI Appendix, Fig. S9. Statistical significance was determined by an unpaired t test. To confirm NorM-Vc expression, cells were normalized to the same density, harvested, and processed by sonication in 1 mL of lysis buffer. Cell debris was removed by centrifugation, and equal-volume samples for SDS/PAGE were made from the supernatant, which contains the membrane fraction. Following electrophoresis on a 12.5% acrylamide gel, the protein was visualized by InVision His-tag in gel stain (Novex).
Note Added in Proof.
Using a computational approach, the companion manuscript by Ficici et al. (39) identifies an Na+ binding site in the NTD of PfMATE, a NorM-Vc homolog, that is weakly specific against H+ and a putative H+ binding site in the CTD, consistent with the conclusions of this study.
Supplementary Material
Acknowledgments
We thank Dr. Reza Dastvan, Dr. Smriti Mishra, and Ms. Abigail Poff for critical reading and editing of the manuscript. This work was supported by NIH Grant R01 GM077659 (to H.S.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802417115/-/DCSupplemental.
References
- 1.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 2.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 3.Saier MH, Jr, Paulsen IT. Phylogeny of multidrug transporters. Semin Cell Dev Biol. 2001;12:205–213. doi: 10.1006/scdb.2000.0246. [DOI] [PubMed] [Google Scholar]
- 4.Du D, van Veen HW, Murakami S, Pos KM, Luisi BF. Structure, mechanism and cooperation of bacterial multidrug transporters. Curr Opin Struct Biol. 2015;33:76–91. doi: 10.1016/j.sbi.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Tal N, Schuldiner S. A coordinated network of transporters with overlapping specificities provides a robust survival strategy. Proc Natl Acad Sci USA. 2009;106:9051–9056. doi: 10.1073/pnas.0902400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaatz GW, McAleese F, Seo SM. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob Agents Chemother. 2005;49:1857–1864. doi: 10.1128/AAC.49.5.1857-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuroda T, Tsuchiya T. Multidrug efflux transporters in the MATE family. Biochim Biophys Acta. 2009;1794:763–768. doi: 10.1016/j.bbapap.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 8.McAleese F, et al. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob Agents Chemother. 2005;49:1865–1871. doi: 10.1128/AAC.49.5.1865-1871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda S, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006;17:2127–2135. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka M, et al. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci USA. 2005;102:17923–17928. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonezawa A, Inui K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br J Pharmacol. 2011;164:1817–1825. doi: 10.1111/j.1476-5381.2011.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci. 2006;27:587–593. doi: 10.1016/j.tips.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Brown MH, Paulsen IT, Skurray RA. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol. 1999;31:394–395. doi: 10.1046/j.1365-2958.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu M. Structures of multidrug and toxic compound extrusion transporters and their mechanistic implications. Channels (Austin) 2016;10:88–100. doi: 10.1080/19336950.2015.1106654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X, et al. Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature. 2010;467:991–994. doi: 10.1038/nature09408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu M, Radchenko M, Symersky J, Nie R, Guo Y. Structural insights into H+-coupled multidrug extrusion by a MATE transporter. Nat Struct Mol Biol. 2013;20:1310–1317. doi: 10.1038/nsmb.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M, et al. Structures of a Na+-coupled, substrate-bound MATE multidrug transporter. Proc Natl Acad Sci USA. 2013;110:2099–2104. doi: 10.1073/pnas.1219901110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mousa JJ, et al. MATE transport of the E. coli-derived genotoxin colibactin. Nat Microbiol. 2016;1:15009. doi: 10.1038/nmicrobiol.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, et al. Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature. 2013;496:247–251. doi: 10.1038/nature12014. [DOI] [PubMed] [Google Scholar]
- 20.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- 22.Hipolito CJ, Tanaka Y, Katoh T, Nureki O, Suga H. A macrocyclic peptide that serves as a cocrystallization ligand and inhibits the function of a MATE family transporter. Molecules. 2013;18:10514–10530. doi: 10.3390/molecules180910514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radchenko M, Symersky J, Nie R, Lu M. Structural basis for the blockade of MATE multidrug efflux pumps. Nat Commun. 2015;6:7995. doi: 10.1038/ncomms8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mousa JJ, Newsome RC, Yang Y, Jobin C, Bruner SD. ClbM is a versatile, cation-promiscuous MATE transporter found in the colibactin biosynthetic gene cluster. Biochem Biophys Res Commun. 2017;482:1233–1239. doi: 10.1016/j.bbrc.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka M, et al. Identification of essential amino acid residues of the NorM Na+/multidrug antiporter in Vibrio parahaemolyticus. J Bacteriol. 2005;187:1552–1558. doi: 10.1128/JB.187.5.1552-1558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Y, Nair A, van Veen HW. Multidrug transport protein norM from Vibrio cholerae simultaneously couples to sodium- and proton-motive force. J Biol Chem. 2014;289:14624–14632. doi: 10.1074/jbc.M113.546770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claxton DP, Kazmier K, Mishra S, Mchaourab HS. Navigating membrane protein structure, dynamics, and energy landscapes using spin labeling and EPR spectroscopy. Methods Enzymol. 2015;564:349–387. doi: 10.1016/bs.mie.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeschke G. DEER distance measurements on proteins. Annu Rev Phys Chem. 2012;63:419–446. doi: 10.1146/annurev-physchem-032511-143716. [DOI] [PubMed] [Google Scholar]
- 29.McHaourab HS, Steed PR, Kazmier K. Toward the fourth dimension of membrane protein structure: Insight into dynamics from spin-labeling EPR spectroscopy. Structure. 2011;19:1549–1561. doi: 10.1016/j.str.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiemann O, Prisner TF. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q Rev Biophys. 2007;40:1–53. doi: 10.1017/S003358350700460X. [DOI] [PubMed] [Google Scholar]
- 31.Radchenko M, Nie R, Lu M. Disulfide cross-linking of a multidrug and toxic compound extrusion transporter impacts multidrug efflux. J Biol Chem. 2016;291:9818–9826. doi: 10.1074/jbc.M116.715227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam SM, Roux B. Simulating the distance distribution between spin-labels attached to proteins. J Phys Chem B. 2015;119:3901–3911. doi: 10.1021/jp510745d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, Ji C, Zhang JZ. Insights on Na+ binding and conformational dynamics in multidrug and toxic compound extrusion transporter NorM. Proteins. 2014;82:240–249. doi: 10.1002/prot.24368. [DOI] [PubMed] [Google Scholar]
- 34.Vanni S, Campomanes P, Marcia M, Rothlisberger U. Ion binding and internal hydration in the multidrug resistance secondary active transporter NorM investigated by molecular dynamics simulations. Biochemistry. 2012;51:1281–1287. doi: 10.1021/bi2015184. [DOI] [PubMed] [Google Scholar]
- 35.Leung YM, Holdbrook DA, Piggot TJ, Khalid S. The NorM MATE transporter from N. gonorrhoeae: Insights into drug and ion binding from atomistic molecular dynamics simulations. Biophys J. 2014;107:460–468. doi: 10.1016/j.bpj.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claxton DP, et al. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17:822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mchaourab HS, Lietzow MA, Hideg K, Hubbell WL. Motion of spin-labeled side chains in T4 lysozyme. Correlation with protein structure and dynamics. Biochemistry. 1996;35:7692–7704. doi: 10.1021/bi960482k. [DOI] [PubMed] [Google Scholar]
- 38.Yamanaka H, Kobayashi H, Takahashi E, Okamoto K. MacAB is involved in the secretion of Escherichia coli heat-stable enterotoxin II. J Bacteriol. 2008;190:7693–7698. doi: 10.1128/JB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ficici E, Zhou W, Castellano S, Faraldo-Gómez JD. Broadly conserved Na+-binding site in the N-lobe of prokaryotic multidrug MATE transporters. Proc Natl Acad Sci USA. 2018;115:E6172–E6181. doi: 10.1073/pnas.1802080115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishima W, et al. Mechanisms for two-step proton transfer reactions in the outward-facing form of MATE transporter. Biophys J. 2016;110:1346–1354. doi: 10.1016/j.bpj.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steed PR, Stein RA, Mishra S, Goodman MC, McHaourab HS. Na+-substrate coupling in the multidrug antiporter norm probed with a spin-labeled substrate. Biochemistry. 2013;52:5790–5799. doi: 10.1021/bi4008935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krah A, Zachariae U. Insights into the ion-coupling mechanism in the MATE transporter NorM-VC. Phys Biol. 2017;14:045009. doi: 10.1088/1478-3975/aa5ee7. [DOI] [PubMed] [Google Scholar]
- 43.Nie L, et al. Identification of the high-affinity substrate-binding site of the multidrug and toxic compound extrusion (MATE) family transporter from Pseudomonas stutzeri. J Biol Chem. 2016;291:15503–15514. doi: 10.1074/jbc.M116.728618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto T, Kanamoto T, Otsuka M, Omote H, Moriyama Y. Role of glutamate residues in substrate recognition by human MATE1 polyspecific H+/organic cation exporter. Am J Physiol Cell Physiol. 2008;294:C1074–C1078. doi: 10.1152/ajpcell.00504.2007. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka Y, Iwaki S, Tsukazaki T. Crystal structure of a plant multidrug and toxic compound extrusion family protein. Structure. 2017;25:1455–1460.e2. doi: 10.1016/j.str.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Stein RA, Beth AH, Hustedt EJ. A straightforward approach to the analysis of double electron-electron resonance data. Methods Enzymol. 2015;563:531–567. doi: 10.1016/bs.mie.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jo S, Kim T, Iyer VG, Im W. CHARMM-gui: A web-based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.