Significance
We constructed a library of miniecosystems that can translate the information from antibody phage display directly into signals of biological function, thereby allowing for rapid selection of antibodies with the function of interest. Compared with the conventional phage display platform that can only isolate antibodies based on their binding affinity toward antigens, our new method bypasses the step of affinity-based selection, and the selection is based purely on the activity of antibodies in a biological system without concern for their relative affinity for antigens. This new method bridges the gap, which has existed for almost three decades, between affinity- and activity-based antibody selection for phage display of combinatorial antibody libraries, thus advancing antibody drug discovery.
Keywords: antibody selection, miniecosystem, phage display
Abstract
We describe a method for the rapid selection of functional antibodies. The method depends on the cocultivation of Escherichia coli that produce phage with target eukaryotic cells in very small volumes. The antibodies on phage induce selectable phenotypes in the target cells, and the nature of the antibody is determined by gene sequencing of the phage genome. To select functional antibodies from the diverse antibody repertoire, we devised a selection platform that contains millions of picoliter-sized droplet ecosystems. In each miniecosystem, the bacteria produce phage displaying unique members of the antibody repertoire. These phage interact only with eukaryotic cells in the same miniecosystem, making phage available directly for activity-based antibody selection in biological systems.
There is a growing interest in the use of therapeutic antibodies for many diseases, including infectious diseases, inflammation, and cancer (1–3). While hybridomas and display technologies for the selection of monoclonal antibodies have been available for decades, the development of methods for rapid discovery of functional therapeutic antibodies still encounters bottlenecks in both academic laboratories and the pharmaceutical industry.
Therapeutic antibodies not only engage in the binding of antigens, but also may exhibit biological functions upon binding. For example, the binding of antibodies to membrane proteins may induce conformational changes in them, thereby selectively modulating downstream signaling pathways. Given that our immune system or combinatorial antibody libraries contain a highly diverse antibody repertoire (as many as 1011 members), one can potentially find antibodies that bind to any protein of interest, only some of which are functional. The problem is how to find the desired antibodies in a time- and cost-effective manner from such a diverse antibody repertoire (4).
Currently, antibody phage display is widely used as a selection platform for the discovery of antibodies that bind to antigens. Typically, the antibody library is screened against an immobilized antigen of interest to isolate antibodies that can bind to antigens (5). In most cases, however, a variety of antibodies targeting different epitopes of the antigen are selected from the antibody repertoire, and thus an additional step is needed to evaluate the biological functions of the isolated antibodies individually (6). This process is usually tedious, costly, and time-consuming and limits the rapid discovery of therapeutic antibodies.
In the study reported here, we devised a system that can translate the information from phage display libraries directly into signals of biological function for each member of the antibody repertoire, thereby allowing for rapid selection of antibodies with the function of interest. Our method bypasses the bottleneck of the conventional phage display platform that can only isolate antibodies based on their binding affinity toward antigens. The design of this selection system was inspired by ecosystems on earth. Living organisms in an ecosystem may produce something that affects biological processes of others in the same space. For example, the gut of each person is an ecosystem in which bacteria secrete enzymes and other molecules to modulate the function of epithelial cells and immune cells in the gut (7). Each person has his or her own gut ecosystem distinct from others, and these ecosystems among people are independent. Inspired by this, we envisioned a library of miniecosystems containing both bacteria and mammalian cells that can be used for selecting functional antibodies. The central idea is that the bacteria in each miniecosystem produce phages displaying a unique member of the antibody repertoire. These phages interact with mammalian cells in the same ecosystem. Because each miniecosystem is in a singular package in which the phenotype of the mammalian cells is linked by packaging to the genotype of the phage-producing bacteria, the nature of the selected antibody can be extracted from the miniecosystems in which mammalian cells display a phenotype of interest.
Results
Development of Picoliter-Sized Ecosystems for Rapid Discovery of Functional Antibodies.
We constructed a library of miniecosystems in which two different organisms live together for paracrine-based selection of therapeutic antibodies/polypeptides. One organism acts as a “producer,” making something functional that acts on the other organism, the “recipient,” in the same ecosystem, resulting in an observable phenotype in the recipient. When bacteria are cocultured with mammalian cells, for example, the bacteria may produce phages displaying an agonist antibody that activates receptors on mammalian cells in the same community.
Before turning the idea of miniecosystems into a general route for the rapid discovery of functional antibodies, we need to be concerned with the quantity of miniecosystems that we can handle at the laboratory level. Indeed, if each miniecosystem had a volume of 100 µL (the size of each well in a 96-well plate), then the number of miniecosystems that could be dealt with would be very limited. This would present a problem, considering that each miniecosystem represents only one member of the highly diverse antibody repertoire. However, reducing the size of the miniecosystem to a volume of 10 pL would allow us to handle up to 108 miniecosystems in a test tube of 1.5 mL. Thus, we devised a selection platform in which millions of picoliter-sized ecosystems are generated for the rapid discovery of therapeutic antibodies that exhibit biological functions of interest on binding to the antigen.
We used a microfluidic device to generate millions of droplets, each of which contains both mammalian cells and bacteria. Thus, each droplet becomes a miniecosystem in which the bacteria make phages, each displaying a unique antibody of the diverse antibody repertoire that can only interact with mammalian cells in the same droplet. Importantly, the system is clonal, because each droplet contains one bacterium and one reporter cell. If the antibody is functional, it can induce a change of phenotype in the mammalian cell. Using instruments such as flow cytometers, we can analyze millions of droplet ecosystems that represent a diverse antibody repertoire and select droplets in which the mammalian cells display a phenotype of interest. In this way, we can directly select functional antibodies (Fig. 1).
Fig. 1.
Cartoon of a library of miniecosystems for the selection of active/functional antibodies. Millions of droplets, each containing both mammalian cells and bacteria, are generated with a microfluidic device. Each droplet becomes a miniecosystem in which the bacteria make phages displaying a unique antibody of the diverse antibody repertoire, which can only interact with mammalian cells in the same droplet. The droplets can be selected based on the phenotype of mammalian cells, and the gene sequence of the antibody can be extracted from the selected droplets.
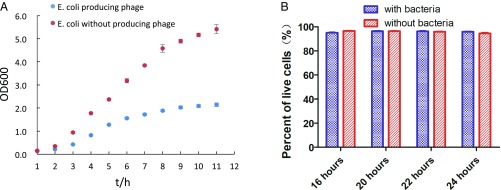
When designing the miniecosystem, the first challenge that we encountered was how to make the bacteria and mammalian cells live productively in the same space. Bacteria have a much shorter doubling time than mammalian cells. When cocultivating bacteria with mammalian cells, the bacteria population may grow too fast and may be harmful to the mammalian cells. If this were the case, using the miniecosystem as a selection platform could pose a problem, because the mammalian cells must be alive to respond to the biomolecules produced by bacteria. As shown in Fig. 2A, however, bacteria growth slows when phage production starts. This may be due in part to the switch of the bacteria’s synthetic machinery from proliferation to phage production. Moreover, we found that mammalian cells were still alive at 24 h after cocultivation with phage-producing bacteria (Fig. 2B).
Fig. 2.
The growth rate of E. coli and viability of mammalian cells when cocultured with E. coli. (A) Growth curve of E. coli with or without phage production. Bacterial growth transits from exponential to stationary phase within a few hours. (B) Viability of mammalian cells when cocultivated with bacteria.
Function of Antibodies Displayed on the Phage Surface Is Not Jeopardized by the Phage Itself.
When designing the miniecosystem, we were concerned as to whether the antibodies on the phage surface exhibit similar activity compared with free antibodies in solution. To address this question, we chose a TrkB antibody known to activate the TrkB receptor in mammalian cells and tested the activity of the phage displaying the TrkB antibody. The anti-TrkB scFv was fused to the N terminus of phage gene 3-encoded protein (p3) using a GGGGS flexible linker. A reporter cell, which should show fluorescence in response to the activation of TrkB receptor, was treated with either the purified TrkB agonist antibody or phage displaying the same antibody. To our surprise, the antibody on the phage surface was much more potent in activating the membrane receptors compared with the free antibody in solution (Fig. 3). This may be due to a “chelate effect” that involves the relatively weak hydrophobic interaction of roughly 2,700 copies of the phage surface protein p8 (gene 8-encoded) with the animal cell surface membrane, in addition to the specific stronger binding of phage p3-displayed scFv to cell surface antigens.
Fig. 3.
Direct activation of mammalian cells with phage displaying an agonist antibody. The reporter cells (mammalian cells) were treated with either an agonist antibody or a phage displaying the agonist antibody. The activation status of reporter cells was quantified by flow cytometry. Phage displaying the agonist antibody is approximately 100 times stronger than the antibody itself in solution.
Kinetic Issues When Cocultivating Mammalian Cells with Phage-Producing Bacteria.
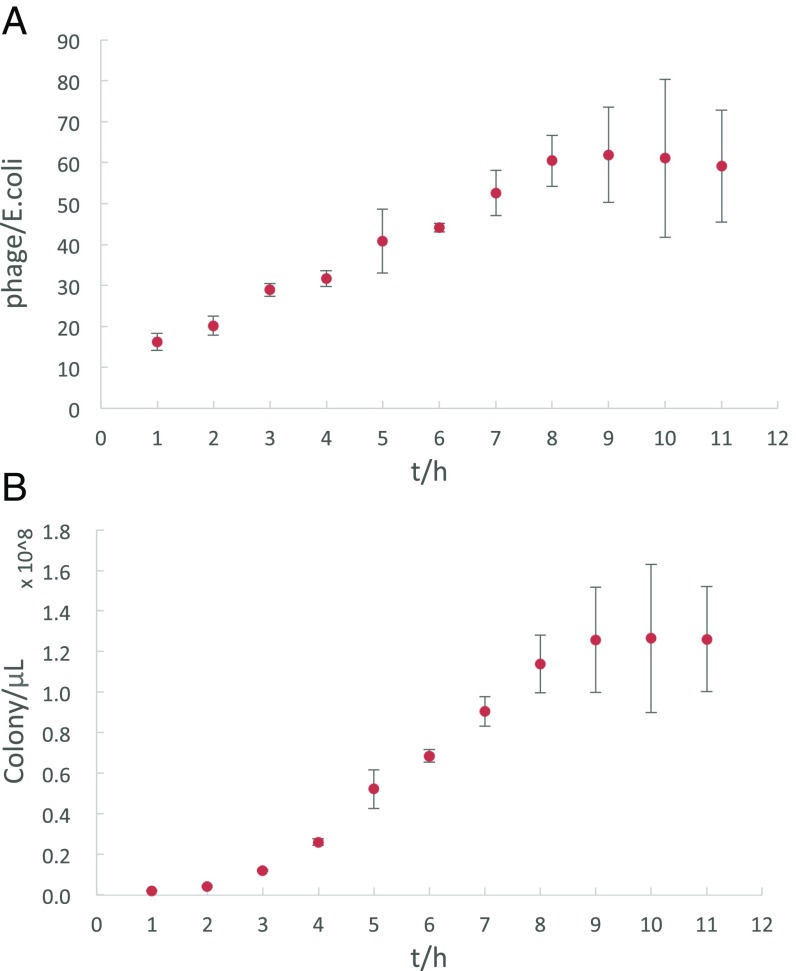
When cocultivating phage-producing bacteria with mammalian cells, a consideration is whether the bacteria can make enough phage-displayed antibodies for the activation of receptors on mammalian cells. As shown in Fig. 4A, each bacterium can make approximately 60 phages on average in approximately 8 h, and the phage concentration can be as high as 0.2 nM in solution. If we consider the chelating effect of the phage, the effective concentration of phage-displaying antibodies could be much higher than 0.2 nM. Thus, it is reasonable to hypothesize that mammalian cells may be activated when cocultivated with phage-producing bacteria in a confined space.
Fig. 4.
The kinetics of phage production. (A) Average number of phages produced per E. coli. Each E. coli can produce more than 60 phages on average within 8 h. (B) Total number of phages produced in a 1-μL culture of E. coli.
To test this hypothesis, we analyzed the receptor activation when cocultivating TrkB reporter cells (mammalian cells) with bacteria that produce phage displaying a TrkB agonist antibody. An scFv-expressing phagmid plus a helper phage were used to produce phage-displayed scFv in Escherichia coli (8). The scFv was fused to the N terminus of the gene 3-encoded protein using a GGGGS flexible linker. In this way, the scFv was displayed on the phage surface when the phage was made and released from E. coli, enabling the interaction of the phage scFv with membrane proteins on the mammalian cell surface. The mammalian cells used in this study were engineered to have a fluorescence-based reporter system that reports on the TrkB activation status (9). As shown in Fig. 5, the mammalian cells were activated when bacteria produced agonist antibody-bearing phage, as opposed to control phage.
Fig. 5.
Coculture of mammalian cells and bacteria: a pilot study for paracrine-based antibody selection. In this ecosystem, E. coli produce multiple copies of phages that act on mammalian cells in the same community. The mammalian cells were engineered to have a reporter system by infecting CellSensor NFAT-bla CHO-K1 cells with lentivirus expressing TrkB as described previously (9). If the phage displays an agonist antibody, the mammalian cell would be turned on and show fluorescence (Pacific blue) on flow cytometry. (A) Mammalian cells cultured together with E. coli that produce a whole phage library are not activated. (B) Mammalian cells cultured together with E. coli that produce a random phage are not activated. (C) Mammalian cells cultured together with E. coli that produce TrkB agonist scFv-bearing phage are activated and show fluorescence (Pacific blue).
Selecting Agonist Antibodies from a Diverse Repertoire of Antibodies Using Miniecosystems.
Nature has evolved a smart, economic strategy for biomolecular interactions by confining the molecules in a small space (e.g., a cell, an organelle) to help bring the molecules to an optimal concentration and increase the likelihood of molecules encountering one another for either noncovalent binding or biochemical reactions. For example, for an E. coli whose volume is as small as 10−15 L, only 100 copies of cytosolic molecules are needed to reach an effective concentration of 1 µM; however, for an in vitro reaction/selection container such as a 96-well plate, as many as 1013 copies of molecules are required to reach the same concentration in a volume of 100 µL. Inspired by nature, we envisioned a platform that uses picoliter-sized droplets in which as few as 1,000 copies of molecules are required for a concentration of 1 nM, which may be useful for studying cell–cell interactions, cell–environment communication, and the cell secretome. Another advantage of using picoliter-sized droplets is that the small volume of droplets allows us to manage a large number of samples (up to 108) simultaneously, making it possible to characterize the activity of each member of a diverse biomolecule repertoire in one test tube.
With this in mind, we used a microfluidic device to create millions of picoliter-sized droplets containing both bacteria and mammalian cells for activity-based antibody selection. The bacteria in each droplet make phages with an antibody representing one member of the antibody repertoire. We hypothesize that if the antibody is active/functional, the mammalian cells may show a phenotype of interest in response to the binding of phage-bearing antibodies in the droplet. Thus, the active/functional antibodies may be selected from an antibody repertoire via the analysis of individual droplets using such modalities as FACS.
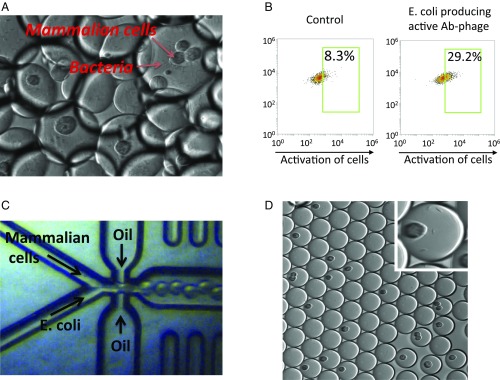
To test this idea, we first encapsulated both phage-producing bacteria and mammalian cells in picoliter-sized droplets and then analyzed the phenotype of mammalian cells in response to biomolecules produced by the bacteria. The generation of this type of droplet can be achieved simply by vortexing a mixture of bacteria/mammalian cells with fluorinated oils (Fig. 6A). The mammalian cells are engineered to have a reporter system that will express fluorescence proteins when receptors on the cell surface are activated by an agonist. Following the incubation of droplets at 37 °C overnight, we analyzed the fluorescence of mammalian cells in each droplet. As shown in Fig. 6B, when mammalian cells are cocultured with E. coli that produce an agonist antibody-bearing phage, rather than a control phage, the mammalian cells show fluorescence, suggesting that the active/functional antibody is selectable based on the phenotype of indicator cells in the droplet.
Fig. 6.
Selection of active/functional antibodies using droplet ecosystems. (A) Image of droplets containing both mammalian cells and phage-producing bacteria. The droplets were generated by vortexing a mammalian cells/bacteria mixture with fluorinated oil. (B) The reporter cells (mammalian cells that will show fluorescence when activated) were encapsulated with E. coli that produce either an agonist antibody-bearing phage or a control phage in droplets. The droplets were incubated at 37 °C overnight, and the fluorescence of mammalian cells in the droplets was analyzed by flow cytometry. (C) Millions of droplets containing both mammalian cells and bacteria were generated using a microfluidic device. (D) Image of droplets generated using the microfluidic device. (Inset) Image of one droplet containing both mammalian cells and bacteria.
The foregoing studies demonstrated that cocultivation of bacteria producing an agonist antibody-bearing phage and indicator cells in the same droplet can lead to activation of the cells. However, the variation in the size of the droplets produced by vortexing makes comparison of the activity of various antibody clones difficult. Therefore, we turned to microfluidic procedures to generate remarkably uniform-sized droplets (Fig. 6C). Millions of droplets containing both eukaryotic cells and E. coli were generated (Fig. 6D). We were able to modulate the number of cells per droplet and optimize the percentage of droplets containing both eukaryotic cells and E. coli. In this way, the interaction between the eukaryotic cells and antibody-displaying phage produced by the E. coli potentially could be analyzed directly with techniques such as FACS, because when double emulsions are used, the droplets are uniform and stable. Given that the traditional FACS instruments run on the water phase, we are currently adapting our droplet system to the double-emulsion format that makes them suitable for FACS.
Discussion
In this study, we have devised a simple, easy-to-use method that bridges the gap, which has existed for almost three decades, between affinity- and activity-based antibody selection for phage display of combinatorial antibody libraries, thereby advancing antibody drug discovery. Phage display is a popular tool for the development of therapeutic antibodies in the pharmaceutical industry. In most cases, therapeutic antibodies have biological functions; for example, antibodies bind to target proteins and act as antagonists, allosteric regulators, or agonists (10, 11). The main challenge with the use of phage panning for drug discovery is that the panning is based purely on the affinity between the antibody and the antigen (SI Appendix, Fig. S1), rather than on the activity/function of antibodies in a biological system. For a typical phage panning process with a library diversity as large as 1011, each antibody member of the library is present at only 100 copies on average in a volume of 1 mL. Thus, the concentration of each member is 10−18 M, which is approximately 109-fold lower than the EC50 of an active antibody drug. For this reason, while phage panning is very effective as an affinity-based selection tool to isolate high-affinity binders from a large antibody repertoire, it has been very difficult to apply this tool to directly select antibodies based on their activity/function in a biological system. This affinity-activity gap accounts for a large portion of the total spending in antibody drug discovery, because so much effort must be devoted to mining the “gold” post-phage panning, including the analysis of the activity/function of antibody candidates individually in mammalian cells. To address this challenge, we have developed an ecosystem by culturing mammalian cells together with phage-producing bacteria in small droplets, making phage available directly for activity-based antibody selection in biological systems.
Most molecular biologists have encountered problems when their tissue cultures become contaminated with organisms such as fungi and bacteria. In our system, we turn this problem into an advantage by deliberately cocultivating prokaryotic cells, making something useful together with animal cell targets. At first glance, one may have several concerns about this approach. First, the process must be clonal, so that what the prokaryotic cells are generating can be related to the phenotype observed in the animal cells. Then there is the issue of different growth rates of the two types of cells; for example, overgrowth of the prokaryotic cells may overtake the culture, ultimately killing the target cells. In phage systems, however, this unequal growth may not be a problem, because when E. coli begin to produce phage, their own growth slows as their metabolic machinery is diverted to the production of phage. This phenomenon is similar to what happens in infected animal cells; for instance, when HeLa cells are infected with polio virus, all the cells’ synthetic machinery is coopted for the growth of virus (12). Finally, something generated by agents such as E. coli might induce a phenotype; however, this is not a real problem, because what could be better than finding new substances that induce phenotypes in animal cells? Of course, one would need to be cognizant of molecules such as endotoxins, but this can be controlled for.
The droplet ecosystem is a significant advancement over traditional methods for antibody drug discovery for two reasons. First, traditional methods may put the selection for active/functional antibodies at risk. For example, the affinity-based phage panning usually enriches high-affinity binders, which in many cases do not have biological functions such as blocking ligand binding or inducing conformational changes of receptors. More importantly, potentially functional antibodies with relatively low affinity may be lost in the regular phage panning process. In contrast, the droplet ecosystem bypasses the step of affinity-based selection, and the selection is based purely on the activity/function of antibodies in a biological system with no concern for their relative affinity for antigens. Therefore, it is more likely to be successful for antibody drug development, where active/functional antibodies may have relatively lower affinities than nonfunctional binders.
Second, our droplet ecosystem is much more efficient than traditional methods for antibody drug development. Conventional phage panning can generate a vast collection of phage-bearing antibody molecules that bind to proteins of interest, but only a few of these may have biological functions when binding to the target. The development of an active/functional antibody is a costly and time-consuming process that usually requires the expression of a large amount of postpanning antibody candidates individually in mammalian cells for functional analysis. Although our laboratory has designed a powerful autocrine-based selection tool, the selection process still requires multiple rounds of lentiviral antibody library construction and takes months to identify an active/functional antibody (9, 13, 14). In contrast, because our new droplet ecosystem is an activity-based selection system, active/functional antibodies may be selected directly from a phage library within a couple of days without the arduous and lengthy postpanning procedure.
The droplet ecosystem is a replicating clonal packaging system that provides an ideal selection platform for combinatorial antibody libraries. Like the acquired immune system, the combinatorial antibody library contains a highly diverse repertoire of antibodies that can be presented on the phage surface. Using water-in-oil droplets, we generated millions of miniecosystems containing both mammalian cells and phage-producing bacteria. A key feature of these miniecosystems is that the antibody repertoire is selectable because the genotype and phenotype are linked via the packaging. The bacteria in each droplet produce phages displaying unique antibodies accessible by the mammalian cells in the same droplet. If the antibody is active/functional, the droplets are selected based on the phenotype of the mammalian cells. In this way, the bacteria bearing the active/functional antibody gene are selected from the repertoire to be replicated and/or amplified. Given that the bacteria are inside the droplets, the droplet ecosystem maintains its clonality in the selection process. Since each member of the antibody repertoire is incorporated in the droplets individually and mammalian cells are exposed only to the bacteria in the same droplets, the droplet ecosystem becomes a selectable package by linking the phenotype of mammalian cells to the genotype of the bacteria/phage.
Another key feature of this droplet ecosystem is that it is a paracrine-based selection system. Two different organisms live together in a water-in-oil droplet. One organism acts as a producer, making something useful for the other organism in the same community, resulting in a phenotype on the recipient. Because this ecosystem is in a selectable package, there may be many applications other than antibody phage display. For example, we may culture mammalian cells together with yeast or E. coli displaying protein candidates in a droplet for selection, or culture two different mammalian cells in a single droplet for studying secretomes.
Given that our droplet ecosystem is a selectable system that links phenotype with genotype, the system may also be used for the selection of useful organic compounds from DNA-encoded chemical libraries (DELs). Here one simply replaces E. coli with beads bearing multiple copies of DNA-encoded organic compounds. DNA-encoded chemical libraries are made by an iterative process in which DNA is added after each step to encode its nature (15, 16). When mammalian cells are cultured with DNA-encoded organic compounds in droplets, the droplets may be selected based on the phenotype of mammalian cells, and the nature of the organic compound can be decoded by sequencing the DNA barcode. Both the platforms of phage display and the DNA-encoded chemical library use DNA as an identifier barcode for determining the nature of each member of the library. The difference is that phages can be replicated and cloned such that the nature of the antibody present in each selected phage can be determined by replication followed by sequencing. Thus, replication allows the maintenance of clonality. Although unlike antibodies on phages, selected chemical compounds cannot replicate, they are nevertheless clonal because there is only one type of compound in each droplet. Since the system is clonal from the outset, and the nature of the organic compound and how it was made are knowable from the information contained in the DNA code, replication is not necessary. When there is complete information about how an organic molecule was constructed, it can be easily synthesized. Thus, since the molecule exists and how it was made is known, organic synthesis in this instance is the equivalent of biological replication.
Finally, the system described here is not limited to the discovery of antibodies or organic compounds from DELs. It can be deployed in any situation where there is an interaction between two or more components and even in classical organic chemistry itself. For instance, imagine the advantage of taking a reaction where A interacts with B and dividing it into millions of femtoliter compartments that vary in, for example, the nature of the solvent.
Materials and Methods
Growth Curve of E. coli When Infected with Phage.
E. coli XL1-blue was transformed with the pCGMT phagmid containing an scFv-gene III fusion chosen at random from a combinatorial antibody library. The vector pCGMT-based scFv library was reported previously (17). A single colony was chosen and cultured in SB medium containing 50 µg/mL carbenicillin and 10 µg/mL tetracycline at 37 °C until OD600 = 0.5. Helper phage or no reagents (control) was added to the culture. After shaking at 250 rpm at 37 °C for 0.5 h, the culture was centrifuged at 3,000 × g for 10 min, and the pellets were resuspended and diluted to OD600 = 0.1 with FreeStyle CHO Expression Medium containing 50 µg/mL carbenicillin, 10 µg/mL tetracycline, and 70 µg/mL kanamycin. The diluted E. coli was cultured at 37 °C with shaking at 250 rpm, and OD600 was measured every 1 h.
Direct Activation of Mammalian Cells with Phage Displaying an Agonist Antibody.
The phage displaying a TrkB receptor agonist antibody was produced, and the titer was measured using a previously reported method. The mammalian cells were engineered to have a reporter system by infecting CellSensor NFAT-bla CHO-K1 cells with lentivirus expressing TrkB. The cells were seeded at a density of 0.1 million cells/well in a 24-well plate at 24 h before the treatment. The cells were incubated in medium containing either the purified TrkB receptor agonist antibody or the purified phage displaying the same antibody for 5 h at 37 °C, and then treated with CCF4-AM according to the manufacturer’s protocol. The activation status of the cells was quantified by flow cytometry.
Kinetics of Phage Production in E. coli Culture.
E. coli XL1-blue was transformed with the pCGMT phagmid. A single colony was chosen and cultured in SB medium containing 50 µg/mL carbenicillin and 10 µg/mL tetracycline at 37 °C until OD600 = 0.5, after which helper phage was added to culture medium. After shaking at 250 rpm at 37 °C for 0.5 h, the culture was centrifuged at 3,000 × g for 10 min. The pellets were then resuspended and diluted to OD600 = 0.1 with FreeStyle CHO Expression Medium containing 50 µg/mL carbenicillin, 10 µg/mL tetracycline, and 70 µg/mL kanamycin. The diluted E. coli was cultured continuously at 37 °C with shaking at 250 rpm. An aliquot of culture was collected every 1 h and centrifuged at 3,000 × g for 10 min, and the supernatants were diluted serially for the measurement of phage titers.
Coculture of Mammalian Cells and Phage-Producing Bacteria in a 15-mL Tube.
E. coli XL1-blue was transformed with the pCGMT phagmid containing the gene of TrkB agonist scFv, a library of scFv genes, or an scFv gene chosen at random from the library. The transformed E. coli was cultured in SB medium containing 50 µg/mL carbenicillin and 10 µg/mL tetracycline at 37 °C until OD600 = 0.3, after which the helper phage was added to the culture medium. After shaking at 250 rpm at 37 °C for 0.5 h, the culture was centrifuged at 3,000 ×g for 10 min, and then the pellets were resuspended and diluted to OD600 = 0.6 with FreeStyle CHO Expression Medium containing 50 µg/mL carbenicillin, 10 µg/mL tetracycline, and 70 µg/mL kanamycin. To 1 mL of diluted E. coli was added 1 million TrkB receptor reporter cells. The mixture was incubated at 37 °C with shaking at 250 rpm for 5 h, after which the cells were then treated with CCF4-AM according to the manufacturer’s protocol. The activation status of the cells was quantified by flow cytometry.
Coculture of Mammalian Cells and Phage-Producing Bacteria in Droplets.
E. coli XL1-blue was transformed with the pCGMT phagmid containing the gene of the TrkB receptor agonist scFv, or a random scFv gene picked from the library. The transformed E. coli was cultured in SB medium containing 50 µg/mL carbenicillin and 10 µg/mL tetracycline at 37 °C until OD600 = 0.3, after which helper phage was added to culture medium. After shaking at 250 rpm at 37 °C for 0.5 h, the culture was centrifuged at 3,000 × g for 10 min, and then the pellets were resuspended and diluted to OD600 = 0.2 with FreeStyle CHO Expression Medium containing 50 µg/mL carbenicillin, 10 µg/mL tetracycline, and 70 µg/mL kanamycin. To 0.2 mL of diluted E. coli was added 0.2 million of TrkB receptor reporter cells that will express fluorescence proteins if the TrkB receptor is activated by an agonist. The mixture was vortexed with 0.6 mL of 2% fluorosurfactant/HFE-7500 3M Novec engineered oil at highest strength for 1 min, and then cultured at 37 °C with shaking at 250 rpm overnight. The activation status of the cells was quantified by flow cytometry.
Supplementary Material
Acknowledgments
This work was supported by the JPB Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806718115/-/DCSupplemental.
References
- 1.Lerner RA. Combinatorial antibody libraries: New advances, new immunological insights. Nat Rev Immunol. 2016;16:498–508. doi: 10.1038/nri.2016.67. [DOI] [PubMed] [Google Scholar]
- 2.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 3.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu JK. The history of monoclonal antibody development: Progress, remaining challenges and future innovations. Ann Med Surg (Lond) 2014;3:113–116. doi: 10.1016/j.amsu.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbas CF, 3rd, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: The gene III site. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yea K, Xie J, Zhang H, Zhang W, Lerner RA. Selection of multiple agonist antibodies from intracellular combinatorial libraries reveals that cellular receptors are functionally pleiotropic. Curr Opin Chem Biol. 2015;26:1–7. doi: 10.1016/j.cbpa.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18:2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbas CF, Burton DR, Scott JK, Silverman GJ, editors. Phage Display: A Laboratory Manual. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2001. pp. 1.1–1.37. [Google Scholar]
- 9.Zhang H, et al. Selecting agonists from single cells infected with combinatorial antibody libraries. Chem Biol. 2013;20:734–741. doi: 10.1016/j.chembiol.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Miersch S, Kuruganti S, Walter MR, Sidhu SS. A panel of synthetic antibodies that selectively recognize and antagonize members of the interferon alpha family. Protein Eng Des Sel. 2017;30:697–704. doi: 10.1093/protein/gzx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miersch S, Maruthachalam BV, Geyer CR, Sidhu SS. Structure-directed and tailored diversity synthetic antibody libraries yield novel anti-EGFR antagonists. ACS Chem Biol. 2017;12:1381–1389. doi: 10.1021/acschembio.6b00990. [DOI] [PubMed] [Google Scholar]
- 12.Crawford N, Fire A, Samuels M, Sharp PA, Baltimore D. Inhibition of transcription factor activity by poliovirus. Cell. 1981;27:555–561. doi: 10.1016/0092-8674(81)90397-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, et al. Phenotype-information-phenotype cycle for deconvolution of combinatorial antibody libraries selected against complex systems. Proc Natl Acad Sci USA. 2011;108:13456–13461. doi: 10.1073/pnas.1111218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J, Zhang H, Yea K, Lerner RA. Autocrine signaling-based selection of combinatorial antibodies that transdifferentiate human stem cells. Proc Natl Acad Sci USA. 2013;110:8099–8104. doi: 10.1073/pnas.1306263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerner RA, Brenner S. DNA-encoded compound libraries as open source: A powerful pathway to new drugs. Angew Chem Int Ed Engl. 2017;56:1164–1165. doi: 10.1002/anie.201612143. [DOI] [PubMed] [Google Scholar]
- 16.Neri D, Lerner RA. DNA-encoded chemical libraries: A selection system based on endowing organic compounds with amplifiable information. Annu Rev Biochem. 2018 doi: 10.1146/annurev-biochem-062917-012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao C, et al. Making chemistry selectable by linking it to infectivity. Proc Natl Acad Sci USA. 1997;94:11777–11782. doi: 10.1073/pnas.94.22.11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.