Significance
Many membrane proteins are functioning in aggregations and associating with microdomains, which range from nanometers to micrometers in size. Therefore, it is indispensable to directly analyze these proteins and microdomains in native cell membranes at a single-molecule level. GLUT1 is a ubiquitously expressed protein, contributing to basal and growth factor-stimulated glucose uptake in many tissues. It is overexpressed in almost all tumors. Herein, by direct stochastic optical reconstruction microscopy, we previously mapped GLUT1 on native cell membranes and highlighted key contributions of the lipid raft, cytoskeleton, and glycosylation to the formation of clusters. Moreover, we elucidated that the clustered distribution of the transporter was associated with its activation, which is crucial to advance our understanding of the transporter’s spatial organization and activation mechanism.
Keywords: GLUT1, direct stochastic optical reconstruction microscopy, single molecule, cluster, activation
Abstract
The glucose transporter GLUT1, a plasma membrane protein that mediates glucose homeostasis in mammalian cells, is responsible for constitutive uptake of glucose into many tissues and organs. Many studies have focused on its vital physiological functions and close relationship with diseases. However, the molecular mechanisms of its activation and transport are not clear, and its detailed distribution pattern on cell membranes also remains unknown. To address these, we first investigated the distribution and assembly of GLUT1 at a nanometer resolution by super-resolution imaging. On HeLa cell membranes, the transporter formed clusters with an average diameter of ∼250 nm, the majority of which were regulated by lipid rafts, as well as being restricted in size by both the cytoskeleton and glycosylation. More importantly, we found that the activation of GLUT1 by azide or MβCD did not increase its membrane expression but induced the decrease of the large clusters. The results suggested that sporadic distribution of GLUT1 may facilitate the transport of glucose, implying a potential association between the distribution and activation. Collectively, our work characterized the clustering distribution of GLUT1 and linked its spatial structural organization to the functions, which would provide insights into the activation mechanism of the transporter.
Glucose is the primary source of energy and substrate for cells, and its transport process is important for both normal and diseased cellular metabolisms (1, 2). Previous studies have shown that the uptake of glucose and other carbohydrates through the cell plasma membrane is largely dependent on members of the glucose transport (GLUT) family (3). Humans have 14 such members, all of which are encoded by SLC2A genes (4). The first characterized glucose transporter, GLUT1, is widely expressed and responsible for the constant uptake of glucose (5, 6). Many researchers have been attracted to focus on its vital physiological and pathophysiological sense (7, 8), and its overexpression has become an important hypoxic marker in malignant tumors and a prognostic indicator for tumorigenesis (7, 9).
Recently, the structure and distribution pattern of GLUT1 has also drawn wide concern. Some studies have found that it is an inward-open uniporter with a single N-glycosylation site (10, 11), and some have showed a markedly punctate staining pattern of GLUT1 on cell membranes under deconvolution fluorescence microscopy (12). However, the diffraction-limited resolution made it very difficult to reveal the detailed structure of GLUT1. For example, issues on whether membrane GLUT1 forms clusters as a working unit in the same way as many other membrane proteins, such as GPI-anchored proteins, epidermal growth receptors (EGFRs), and Toll-like receptors (13–15), and which transmutation causes an acute increase of the maximal velocity (Vmax) for glucose uptake following exposure to osmotic or metabolic stimuli (12, 16), have not been clarified. Fortunately, super-resolution fluorescence microscopy, which breaks the diffraction barrier and achieves a lateral resolution in the tens of nanometers (17), has provided a particularly suitable tool to solve these problems. Meanwhile, it has been proven that multiprotein assemblies are dependent on cholesterol environment, and their separation and anchoring are related to the actin cytoskeleton (18, 19). Nonetheless, it is still unknown whether these factors have contributions to the spatial distribution of GLUT1.
Lipid rafts, also known as the detergent-resistant membranes (DRMs), are membrane domains containing high levels of cholesterol, sphingolipids, and specific proteins, which play a significant role in cell signaling and protein assembling (20, 21). Abundant evidence has proved that spatial recruitment and clustering of proteins and lipids into lipid rafts is a remarkable feature in a variety of signaling and transferring processes (22, 23), for instance insulin receptors, integrin, and T cell antigen receptors (22, 24). Even the members of GLUT family (GLUT4 and GLUT1) have been found to associate with DRMs (4, 25). However, due to the use of detergents for extracting lipid rafts in these experiments that broke the natural condition of cell membranes, the validity and accuracy of the colocalization between GLUT1 and lipid rafts is still debatable. Besides, actin as a major cytoskeleton protein is also found to be involved in almost all biological events, contributing to the mechanical properties and shapes of cells (26). Some ion channel proteins on cell membranes have been identified binding to actin directly or indirectly through the actin binding proteins (27). Nevertheless, whether actin filaments have an effect on the distribution of energy channel protein, GLUT1, remains unknown.
As an important glucose transporter, the activation and the transport of GLUT1 has been explored as well. Several studies have suggested that the activation of the transporter by metabolic stresses is mediated by translocating of GLUT between intracellular storage pools and the cell surface or involves activation (“unmasking”) of individual transporters preexisting in the plasma membranes (28, 29). Little is known about whether the activation changes the distribution pattern of GLUT1 and how the transporters assemble and organize with or without activation. The current uncertainty on these topics calls for new methods capable of directly monitoring the size and stability of GLUT1 clusters.
Herein, we applied direct stochastic optical reconstruction microscopy (dSTORM) (30, 31), one of the super-resolution imaging techniques, to observe the spatial distribution of GLUT1 on HeLa cell membranes, which are found to overexpress GLUT1 (SI Appendix, Fig. S1), then quantitatively analyze the distribution characteristics of GLUT1 clusters. By dual-color dSTORM imaging and inhibitor treatment, we elucidated the critical roles of lipid rafts, actin cytoskeleton, and glycosylation in cluster formation and stability. Moreover, combining dSTORM data with biochemistry results, we depicted the detailed changes of GLUT1 clustering and distribution under activation by different stimuli, which revealed a probable relationship between the activation and spatial distribution.
Results and Discussion
Mapping GLUT1 on Cell Membranes Using STORM.
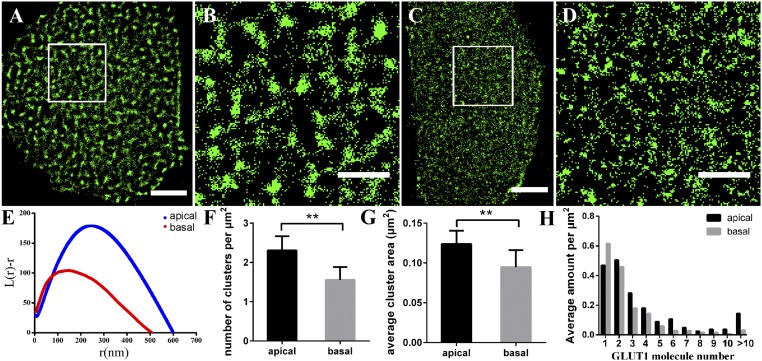
Considering the fluidity of the cell membrane, we used fixed HeLa cells to observe the distribution of GLUT1. When the fixation was not adequate, proteins on cell membranes would not be completely immobilized, causing the redistribution of proteins (32, 33). So, we first optimized the fixation time and found that the morphology of GLUT1 has no significant difference on cell membranes with different fixation time (SI Appendix, Fig. S2). Thus, 20 min for fixation was used in all subsequent experiments. Cultured HeLa cells present adherent growth and their adherent side and medium exposed side face different environments, which we think may influence the distribution of GLUT1. To test this idea, we used dSTORM to investigate the spatial distribution of GLUT1 on both the medium exposed side and adherent side (see Experimental Section and SI Appendix, Fig. S3 for detail). The reconstructed dSTORM images and the corresponding magnified pictures showed that GLUT1 tended to form elliptic and dense clusters on the medium exposed side (Fig. 1 A and B) but sparse clusters with irregular shapes on the adherent side (Fig. 1 C and D). The same phenomenon was also observed on OS-RC-2 cell (human renal carcinoma cell) membranes (SI Appendix, Fig. S4).
Fig. 1.
GLUT1 proteins form clusters of different sizes and amounts on HeLa medium exposed side and adherent side. (A–D) Reconstructed dSTORM images of GLUT1 on medium exposed side (A) and adherent side (C), and the corresponding magnified images (B and D). (Scale bars: A and C, 5 μm; B and D, 2 μm.) (E) Representative Ripley’s K function plots of GLUT1 on different membranes. Data are from 30 stochastically selected regions of 2 × 2 μm2 in 10 cells of three independent experiments. (F) The average number of GLUT1 clusters per μm2. (G) The average cluster area. (H) The number of sporadic GLUT1 and clusters containing different amounts of molecules per μm2. Data in F–H are obtained from 10 cells of three independent experiments (mean ± SD). **P < 0.01 (two-tailed unpaired t test).
To quantify the features of the clusters, we used Ripley’s K function (13) to analyze the spatial clustering in nanoscale domains (see SI Appendix, Fig. S5 for detail). The maxima of the L(r)-r (medium exposed side: 180 ± 20, adherent side: 101 ± 4) in Fig. 1E indicated that the degree of clustering on the medium exposed side was higher than that on the adherent side. The rmax value corresponding to the maximum of L(r)-r was defined as the average size of the analyzed clusters in the region of 2 × 2 μm2, which showed that GLUT1 formed clusters with an average diameter of 250 ± 20 nm on the medium exposed side and 137 ± 16 nm on the adherent side. We also extracted the information of the amount (Fig. 1F) and size (Fig. 1G) of the GLUT1 clusters on the medium exposed side and adherent side of HeLa cells. The results showed that the transporter formed much more and larger clusters on the medium exposed side than on the adherent side. To further clarify the sporadic GLUT1 and the molecular organization in clusters, we applied semiquantitative analysis (Experimental Section) to calculate the amount of distribution of sporadic GLUT1 (one molecule) and clustered GLUT1 containing more than two molecules (Fig. 1H). The statistical result showed that GLUT1 formed clusters with different numbers of molecules, ranging from 2 to more than 10, but a few were more than 25. Small clusters containing two to four molecules were in the majority. Moreover, more large clusters that consisted of more than four molecules were found to generate on the medium exposed side, while sporadic GLUT1 were more on the adherent side.
By comparison, we found that GLUT1 formed clusters with different numbers and sizes on HeLa medium exposed side and adherent side. High-density and large-diameter clusters were seen on the medium exposed side, while sparse and small clusters were observed on the adherent side. These discrepancies may be caused by the varying external environments experienced by different surfaces. The medium exposed side are entirely exposed to the external environment and are able to contact with more external factors, such as hormones and extracellular growth factors. As previous studies reported that growth factors like IGF and SCF can increase glucose metabolism (34), it is thus possible that the changes of glucose levels induced by extracellular factors could result in the difference of GLUT1 distribution on the medium exposed side and adherent side.
Interactions Between GLUT1 and Lipid Rafts.
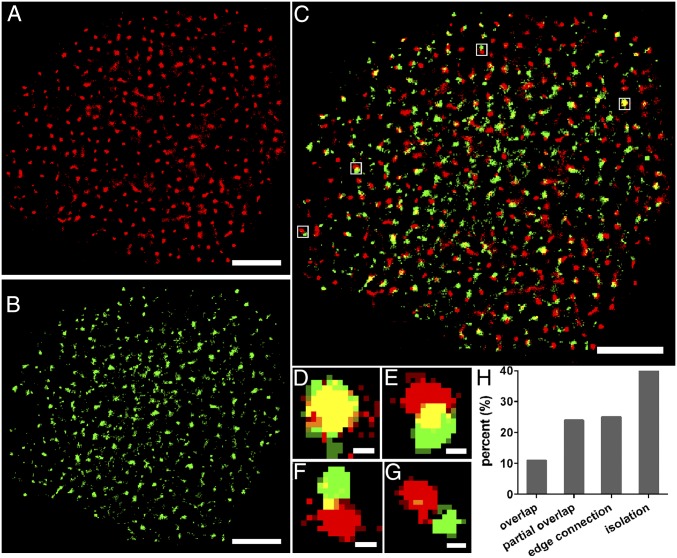
Many studies have been reported that GLUT1 is partly localized to DRM domains and that the transporter activity is sensitive to the phospholipid and cholesterol environment (12, 34). Besides, several studies have depicted that lipid rafts exist as nanodomains or microdomains on the cell membrane (14, 35), and our previous studies also observed lipid rafts with the size ranging from 10 to 200 nm (14, 36, 37), which was consistent with the average diameter of GLUT1 (∼200 nm). Accordingly, we hypothesized that GLUT1 clustering might be associated with lipid rafts. To verify this assumption, dual-color dSTORM imaging was performed to locate the lipid rafts and GLUT1 on the medium exposed side of HeLa cells (Fig. 2). The merged image of lipid rafts and GLUT1 (Fig. 2C) showed a degree of colocalization between the two. Mander’s coefficients (38), M1 and M2, were used to analyze said colocalization. These coefficients represented the amount of colocalized pixels relative to the total covered by each component. Based on their values, we sorted the positional relationships into four types: overlap (M1/M2 > 0.66, Fig. 2D), partial overlap (0.33 < M1/M2 <0.66, Fig. 2E), edge connection (0 < M1 and M2 < 0.33, Fig. 2F), and isolation (M1/M2 = 0, Fig. 2G), then we determined the percentage of the four localization states (Fig. 2H). Overlap and partial overlap were collectively referred to as colocalization. The data showed that 35% (overlap: 11%, partial overlap: 24%) of total GLUT1 was associated with lipid rafts, whereas the remaining might be localized in other lipid compositions of rafts or nonlipid rafts domains. This hinted us that the clusters’ formation was not only affected by lipid rafts and there might be other factors that contributed to the aggregation of GLUT1.
Fig. 2.
Dual-color dSTORM images revealing the relative spatial distribution of GLUT1 and lipid rafts on the HeLa membrane. (A and B) Reconstructed dSTORM images of lipid rafts with Alexa647-conjugated CT-B (A) and GLUT1 labeled with Alexa532-conjugated antibody (B) on the same cell membrane. (C) The merged image of A and B, showing significant colocalization of the two. (D–G) Enlarged dual-color dSTORM images of white Inset squares in C, displaying four types of location relationship: overlap (D), partial overlap (E), edge connection (F), and isolation (G). (H) The percentage of the four types of location states. Data are from 10 cells in three independent experiments. (Scale bars: A–C, 5 μm; D–G, 200 nm.)
Lipid Rafts Disruption Weakens GLUT1 Clustering.
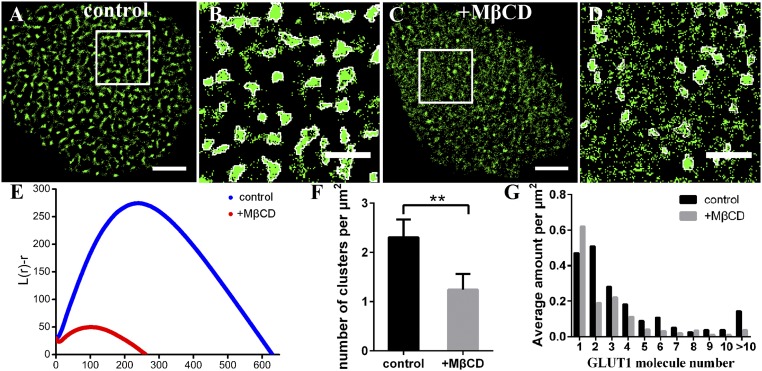
The reasons why GLUT1 clusters did not totally colocalize with GM1-enriched lipid rafts (CT-B labeled) may be two: (i) other compositions of rafts may colocalize with GLUT1 clusters; and (ii) other factors may participate in regulating clustering. Hence, we decided to further confirm the role of lipid rafts at first. We treated HeLa cells with 10 mM methyl-β-cyclodextrin (MβCD) for 20–30 min, which can remove the cholesterol from the lipid rafts (39), to investigate whether the alteration of the cholesterol environment could influence GLUT1 distribution. We found that a lot of GLUT1 clusters became smaller or even disappeared after adding MβCD (Fig. 3). The Ripley’s K-function plots indicated that the degree of clustering decreased dramatically and that the average diameter of the clusters dropped from ∼250 nm to ∼140 nm (Fig. 3E). Moreover, the number of clusters per unit area reduced from ∼2.3 ± 0.3 to ∼1.2 ± 0.3 (Fig. 3F), and clusters consisting of more than two molecules declined sharply (Fig. 3G). To rule out the destructive possibility of MβCD on membrane structure or other membrane constituents, we performed the experiment of cholesterol repletion (MβCD saturated with cholesterol at a MβCD:cholesterol molar ratio of 8:1). As shown in SI Appendix, Fig. S6A, lipid rafts returned to the clustered structure after repletion of cholesterol. The localizations and size of lipid rafts decreased sharply with MβCD treatment, while these values increased to the level of control cells after cholesterol repletion (SI Appendix, Fig. S6 B and C). Thus, these results indicated that MβCD had no effect on other constitutes except lipid rafts. In addition, we also validated that GLUT1 clusters formed again and the average cluster size recovered to that on control cell membranes (∼0.12 μm2) (SI Appendix, Fig. S6 D–F).
Fig. 3.
The changes of GLUT1 clusters after lipid rafts disruption. (A–D) Reconstructed dSTORM images of GLUT1 on control (A) and MβCD-treated (C) HeLa cell membranes and the corresponding magnified images with clusters circled in white (B and D). (Scale bars: A and C, 5 μm; B and D, 2 μm.) (E) Ripley’s K function plots of GLUT1 on control and MβCD-treated HeLa membranes. (F) The number of clusters per μm2. (G) The number of sporadic GLUT1 and clusters containing a different amount of molecules per μm2. Data are obtained from 10 cells of three independent experiments (mean ± SD). **P < 0.01 (two-tailed unpaired t test).
Dual-color imaging, together with the disruption and repletion of lipid rafts, demonstrated that the stable existence of most GLUT1 clusters is dependent on the integrality of lipid rafts. The destruction of lipid rafts may induce to partial or complete fragmentation of larger GLUT1 clusters. Of note, although the number of large clusters reduced after MβCD treatment, there were a portion of GLUT1 that still formed clusters. This finding was consistent with that only 35% of GLUT1 colocalized with lipid rafts, suggesting other mechanisms in regulation of its distribution.
The Limitation of the Actin Cytoskeleton in GLUT1 Clustering.
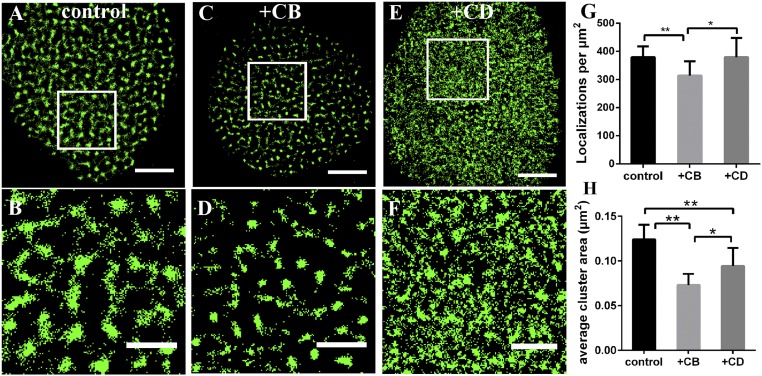
As above mentioned, there are other factors participating in GLUT1 clustering, and previous studies have reported that various membrane channel proteins directly interact with actin (27). Thus, to clarify whether the actin cytoskeleton plays a role in controlling the distribution and clustering of GLUT1, we first imaged GLUT1 on HeLa membranes treated by 20 μg/mL cytochalasin B (CB) for 30 min, which can depolymerize actin cytoskeleton. The average cluster area (Fig. 4) and the molecules within clusters (SI Appendix, Fig. S7A) decreased significantly compared with that on control cell membranes. However, CB not only inhibits actin filaments from generating networks, but also weakens glucose transport by interacting with the substrate efflux site (40). Our results also showed that CB-treated cells consumed less glucose than control (see Fig. 7). To exclude the effect of glucose transport on GLUT1 distribution, we used another inhibitor cytochalasin D (CD). Likewise, it inhibits actin polymerization but hardly affects the cellular permeability to sugars (41), which was supported by our data of glucose consumption as well (see Fig. 7). After CD treatment, the total localizations of GLUT1 did not change, whereas clusters became scattered and small (Fig. 4 and SI Appendix, Fig. S7B). These findings demonstrated that the disruption of actin filaments limited the ability of GLUT1 to form clusters, but did not change its total level, therefore verifying the role of actin cytoskeleton in transporter clustering.
Fig. 4.
Comparative analysis of the morphologies of GLUT1 after the disruption of actin cytoskeleton. (A–F) Reconstructed dSTORM images of GLUT1 on control (A), CB-treated (C) and CD-treated (E) cell membranes, and the corresponding magnified images (B, D, and F). (Scale bars: A, C, and E, 5 μm; B, D, and F, 2 μm.) (G) The number of localizations per μm2 on these three kinds of membranes. (H) The average cluster area. Data are obtained from 10 cells of three independent experiments (mean ± SD). *P < 0.5, **P < 0.01 (two-tailed unpaired t test).
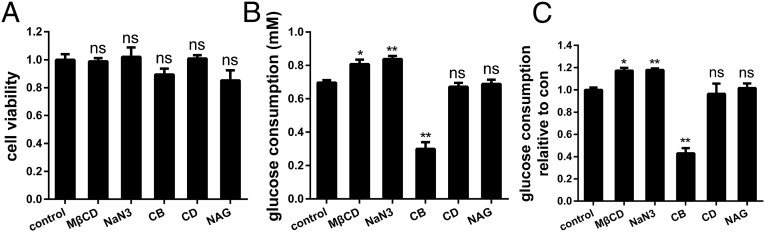
Fig. 7.
Glucose uptake assay with different treatments. (A) Normalized cell viability under the different conditions. (B) The glucose consumption of every group, which was normalized by living cell numbers. (C) The glucose consumption relative to control. Data are from three independent experiments (mean ± SD). *P < 0.05; **P < 0.01; ns, no significance (two-tailed unpaired t test).
The Impairment of N-Glycosylation Disruption in GLUT1 Clustering.
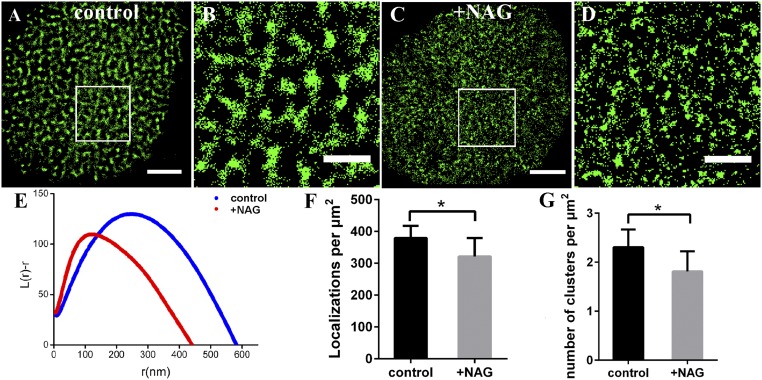
Glycosylation is a prevalent protein modification with a profound effect on protein stability, folding, and a multitude of biological processes (42). Since GLUT1 owns an N-glycosylation site, we presupposed that GLUT1 clustering would be related to glycosylation. To prove this, HeLa cells were pretreated with 50 μg/mL N-acetyl-β-d-gulcosaminides (NAG) for 30 min, which has an effective transglycosylation of N-acetyl-β-d-gulcosaminides, a common motif of N-linked sugar chains in proteins (36); then the nanoscale organizations of GLUT1 on cell membranes were investigated. The deglycosylation made GLUT1 clusters disperse, and almost all of the large clusters disappeared (Fig. 5 A–D). From the Ripley’s K function analysis (Fig. 5E), we found that the degree of clustering and the diameter of GLUT1 clusters fell down significantly (from ∼250 nm to ∼130 nm). Further statistical data indicated that there was a distinguishable decline in both the number of localizations (Fig. 5F) and clusters (Fig. 5G). Moreover, sporadic molecules and clusters containing two molecules increased, whereas other large clusters with more than two molecules fell down (SI Appendix, Fig. S7C). The reasons for the decrease of cluster size and number might be two: One is that deglycosylation directly inhibits the formation of clusters, and the other is that the lower level of total GLUT1 could not generate larger and more clusters as it could on control cell membranes. However, we could not determine which one or both were the right reasons. Even so, the results clearly supported the hypothesis that glycosylation was an indispensable element in maintaining of the distribution and clustering of GLUT1 on cell membranes.
Fig. 5.
The glycosylation regulates the GLUT1 clustering on HeLa cell membranes. (A–D) Reconstructed dSTORM images of GLUT1 on control (A) and NAG-treated (C) cell membranes, and the corresponding magnified images (B and D). (Scale bars: A and C, 5 μm; B and D, 2 μm.) (E) Ripley’s K function analysis of GLUT1 under the two conditions. (F) The average number of localizations per μm2. (G) The average number of GLUT1 clusters per μm2. Data are obtained from 10 cells of three independent experiments (mean ± SD). *P < 0.05 (two-tailed unpaired t test).
The Effect of GLUT1 Activation on Its Clustering.
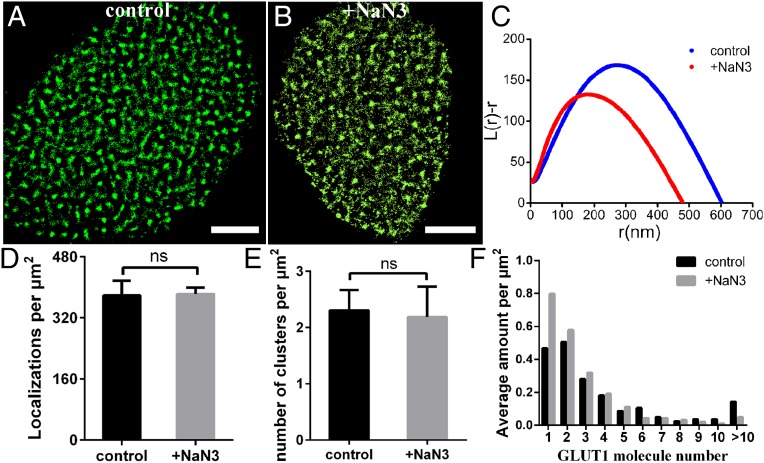
Exposure to osmotic or metabolic stimuli like MβCD or azide can increase the maximal transfer rate of GLUT1 (12, 34), we wondered what changes would be brought to GLUT1’s membrane distribution by activating. So, we first performed glucose uptake assay to confirm the activation of sodium azide and MβCD on GLUT1. As shown in Fig. 7, the glucose consumption increased, and the cell viability did not change after treatment with azide or MβCD, validating that these two reagents can effectively activate glucose transporter. Then we used 5 mM sodium azide to stimulate the adherent HeLa cells for 30 min before staining. From the reconstructed dSTORM images, most of the molecules on treated membranes seemed to form clusters in the same way as those on control membranes (Fig. 6 A and B). Further Ripley’s K function analysis and statistical results showed some differences in the size, number, and molecular organization of GLUT1 clusters. The total localizations of GLUT1 remained stable (Fig. 6D), and even the cluster number reduced slightly on azide-treated cell membranes (Fig. 6E). However, the degree of clustering and diameter of clusters reduced, which was only ∼170 ± 25 nm (Fig. 6C). Moreover, the number of monomer GLUT1 increased following activation, and small clusters with less than five molecules went up significantly (Fig. 6F). These results indicated that the activation of GLUT1 might change the molecular organization of clusters and inhibit the formation of large clusters on cell membranes.
Fig. 6.
NaN3 activation of GLUT1 changed the molecular organization of it clusters. (A and B) Reconstructed dSTORM images of GLUT1 on control (A) and NaN3-treated (B) cell membranes. (Scale bars: 5 μm.) (C) Ripley’s K function analysis of GLUT1 under the two conditions. (D) The average number of localizations per μm2. (E) The average number of GLUT1 clusters per μm2. (F) The number of sporadic GLUT1 and clusters containing different amount of molecules per μm2. Data are obtained from 10 cells in three independent experiments (mean ± SD). ns, no significance (two-tailed unpaired t test).
At this time, we focused on the results of MβCD treatment again. The cluster number decreased (Fig. 3F) and only monomer GLUT1 increased, but small clusters with less than four molecules were not as many as those on control cells (Fig. 3G). The results indicated that the inhibition of MβCD on GLUT1 clustering was stronger than that of sodium azide. It is easy to understand. MβCD has two functions, i.e., disrupting lipid rafts and activating glucose transporter, both of which can weaken the clustering. Additionally, we also tested the influence of CB, CD, and NAG treatment on the activity of glucose transport (Fig. 7). Only CB reduced the glucose uptake compared with control groups. CB has two antagonistic effects on clustering. One is inhibiting actin polymerization that could limit the aggregation of GLUT1, the opposite one is inhibiting GLUT1 activity that may induce the formation of clusters. Therefore, the changes of clustering features after adding CB were not as obvious as those with CD treatment (Fig. 4), because CD only affects the actin cytoskeleton. For NAG groups, we thought the glucose consumption would decrease due to the disruption of N-glycosylation, while it did not change as we expected. This might be because there is another family of glucose transporters, sodium-linked glucose transporters (SGLTs) (43), which are not affected by the deglycosylation of NAG. Together, the detailed molecular mechanism for the clustering of GLUT1 and glucose uptake is complex; even so, our findings revealed a potential relationship between GLUT1 clustering and activation and suggested that small clusters may be more beneficial to glucose transport.
Conclusions
In summary, by the combination of dSTORM imaging and proposed single molecule analysis methods, we observed that most of GLUT1 was aggregated in clusters on the HeLa membrane and found a precise spatial association between GLUT1 and lipid rafts, which resolved the debate surrounding on the localization of the transporter in membrane domains. Regarding the organizational mechanism of GLUT1 clusters, our study revealed that not only the lipid rafts’ environment can stabilize their existence, but also the actin cytoskeleton and N-glycosylation play important roles in the clusters’ formation. Furthermore, under the activation by MβCD and sodium azide, we found that many transporters were prone to distribute sporadically or form small clusters, which might facilitate glucose uptake. The ability to directly visualize the transporter’s distribution at a nanometric resolution provided crucial information of clustering features, clarified many regulatory factors, and captured a probable relationship between GLUT1 distribution and activation. With further studies such as mutation and simultaneously observation of multiple glucose transporters, these initial observations may form a significant step forward in our understanding of the molecular mechanism of GLUT clustering and glucose uptake.
Experimental Section
Cell Culture.
Cells were cultured in Dulbecco’s modified Eagle medium (DMEM; HyClone) supplemented with 10% FBS (Gibco) and antibiotics and maintained in a humidified environment with 5% CO2 at 37 °C. Before the imaging experiment, HeLa cells were divided into a dish where a clean coverslip was placed and cultured at least 24 h.
Glucose Uptake Analysis.
The glucose consumption of HeLa cells with different treatment was measured as described in SI Appendix, SI Materials and Methods.
Preparation of dSTORM Samples.
Cells were fixed, blocked, and labeled with antibodies and sealed on microscopes as described in SI Appendix, SI Materials and Methods.
Superresolution Imaging.
The raw data were captured by our home-built dSTORM and analyzed by Quickpalm as described in SI Appendix, SI Materials and Methods.
Data Analysis.
The spatial distribution of molecules was analyzed by Ripley’s K function and the molecular organization of clusters was estimated by semiquantitative analysis method as described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was financially supported by National Key R&D Program of China Grant 2017YFA0505300 (to H.W.), National Nature Science Foundation of China Grants 21727816, 21525314, 21721003 (to H.W.), 21703231 (to J.G.), 21503213 (to M.C.), and 31330082 (to J.J.), and Yunnan Provincial Science and Technology Department of China Grants 2017FA044 and 2013HA023 (to W.X.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.Y. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803859115/-/DCSupplemental.
References
- 1.Meireles P, et al. GLUT1-mediated glucose uptake plays a crucial role during Plasmodium hepatic infection. Cell Microbiol. 2017;19:e12646. doi: 10.1111/cmi.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto T, Jimi S, Migita K, Takamatsu Y, Hara S. Inhibition of glucose transporter 1 induces apoptosis and sensitizes multiple myeloma cells to conventional chemotherapeutic agents. Leuk Res. 2016;41:103–110. doi: 10.1016/j.leukres.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Schlößer HA, et al. Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer. 2017;20:83–91. doi: 10.1007/s10120-015-0577-x. [DOI] [PubMed] [Google Scholar]
- 4.Rungaldier S, Oberwagner W, Salzer U, Csaszar E, Prohaska R. Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin-1 in human erythrocyte membrane domains. Biochim Biophys Acta. 2013;1828:956–966. doi: 10.1016/j.bbamem.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, et al. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- 7.Deng D, et al. Crystal structure of the human glucose transporter GLUT1. Nature. 2014;510:121–125. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 8.Maiden MC, Davis EO, Baldwin SA, Moore DC, Henderson PJ. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987;325:641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- 9.Amann T, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–1552. doi: 10.2353/ajpath.2009.080596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakyo T, Kitagawa T. Differential localization of glucose transporter isoforms in non-polarized mammalian cells: Distribution of GLUT1 but not GLUT3 to detergent-resistant membrane domains. Biochim Biophys Acta. 2002;1567:165–175. doi: 10.1016/s0005-2736(02)00613-2. [DOI] [PubMed] [Google Scholar]
- 11.Sakyo T, Naraba H, Teraoka H, Kitagawa T. The intrinsic structure of glucose transporter isoforms Glut1 and Glut3 regulates their differential distribution to detergent-resistant membrane domains in nonpolarized mammalian cells. FEBS J. 2007;274:2843–2853. doi: 10.1111/j.1742-4658.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- 12.Barnes K, Ingram JC, Bennett MD, Stewart GW, Baldwin SA. Methyl-beta-cyclodextrin stimulates glucose uptake in Clone 9 cells: A possible role for lipid rafts. Biochem J. 2004;378:343–351. doi: 10.1042/BJ20031186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lillemeier BF, et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, et al. Mechanistic insights into EGFR membrane clustering revealed by super-resolution imaging. Nanoscale. 2015;7:2511–2519. doi: 10.1039/c4nr04962d. [DOI] [PubMed] [Google Scholar]
- 15.Magenau A, et al. Discreet and distinct clustering of five model membrane proteins revealed by single molecule localization microscopy. Mol Membr Biol. 2015;32:11–18. doi: 10.3109/09687688.2014.990997. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JZ, Behrooz A, Ismail-Beigi F. Regulation of glucose transport by hypoxia. Am J Kidney Dis. 1999;34:189–202. doi: 10.1016/s0272-6386(99)70131-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, et al. Regulation of EGFR nanocluster formation by ionic protein-lipid interaction. Cell Res. 2014;24:959–976. doi: 10.1038/cr.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chichili GR, Rodgers W. Clustering of membrane raft proteins by the actin cytoskeleton. J Biol Chem. 2007;282:36682–36691. doi: 10.1074/jbc.M702959200. [DOI] [PubMed] [Google Scholar]
- 19.Goswami D, et al. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen DM, Magenau A, Williamson D, Gaus K. The lipid raft hypothesis revisited–New insights on raft composition and function from super-resolution fluorescence microscopy. BioEssays. 2012;34:739–747. doi: 10.1002/bies.201200044. [DOI] [PubMed] [Google Scholar]
- 21.Brodbek L, Schmid F. Interplay of curvature-induced micro- and nanodomain structures in multicomponent lipid bilayers. Int J Adv Eng Sci Appl Math. 2016;8:111–120. [Google Scholar]
- 22.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: Lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 23.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 24.Aaron JS, Carson BD, Timlin JA. Characterization of differential Toll-like receptor responses below the optical diffraction limit. Small. 2012;8:3041–3049. doi: 10.1002/smll.201200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L, Chen J, Gao J, Wang H, Xiong W. Super-resolution microscopy reveals the insulin-resistance-regulated reorganization of GLUT4 on plasma membranes. J Cell Sci. 2017;130:396–405. doi: 10.1242/jcs.192450. [DOI] [PubMed] [Google Scholar]
- 26.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki S, Yui N, Noda Y. Actin directly interacts with different membrane channel proteins and influences channel activities: AQP2 as a model. Biochim Biophys Acta. 2014;1838:514–520. doi: 10.1016/j.bbamem.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Maraldi T, Fiorentini D, Prata C, Landi L, Hakim G. Stem cell factor and H2O2 induce GLUT1 translocation in M07e cells. Biofactors. 2004;20:97–108. doi: 10.1002/biof.5520200204. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JZ, Hayashi H, Ebina Y, Prohaska R, Ismail-Beigi F. Association of stomatin (band 7.2b) with Glut1 glucose transporter. Arch Biochem Biophys. 1999;372:173–178. doi: 10.1006/abbi.1999.1489. [DOI] [PubMed] [Google Scholar]
- 30.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henriques R, Mhlanga MM. PALM and STORM: What hides beyond the Rayleigh limit? Biotechnol J. 2009;4:846–857. doi: 10.1002/biot.200900024. [DOI] [PubMed] [Google Scholar]
- 32.Stanly TA, et al. Critical importance of appropriate fixation conditions for faithful imaging of receptor microclusters. Biol Open. 2016;5:1343–1350. doi: 10.1242/bio.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgert A, et al. Characterization of plasma membrane ceramides by super-resolution microscopy. Angew Chem Int Ed Engl. 2017;56:6131–6135. doi: 10.1002/anie.201700570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caliceti C, et al. Effect of plasma membrane cholesterol depletion on glucose transport regulation in leukemia cells. PLoS One. 2012;7:e41246. doi: 10.1371/journal.pone.0041246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pike LJ. Rafts defined: A report on the Keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, et al. Revealing the carbohydrate pattern on a cell surface by super-resolution imaging. Nanoscale. 2015;7:3373–3380. doi: 10.1039/c4nr05970k. [DOI] [PubMed] [Google Scholar]
- 37.Cai M, et al. Direct evidence of lipid rafts by in situ atomic force microscopy. Small. 2012;8:1243–1250. doi: 10.1002/smll.201102183. [DOI] [PubMed] [Google Scholar]
- 38.Zinchuk V, Grossenbacher-Zinchuk O. Recent advances in quantitative colocalization analysis: Focus on neuroscience. Prog Histochem Cytochem. 2009;44:125–172. doi: 10.1016/j.proghi.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Gaus K, et al. Domain-specific lipid distribution in macrophage plasma membranes. J Lipid Res. 2005;46:1526–1538. doi: 10.1194/jlr.M500103-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Shanahan MF. Characterization of cytochalasin B photoincorporation into human erythrocyte D-glucose transporter and F-actin. Biochemistry. 1983;22:2750–2756. doi: 10.1021/bi00280a024. [DOI] [PubMed] [Google Scholar]
- 41.Tannenbaum J, Tanenbaum SW, Godman GC. The binding sites of cytochalasin D. II. Their relationship to hexose transport and to cytochalasin B. J Cell Physiol. 1977;91:239–248. doi: 10.1002/jcp.1040910209. [DOI] [PubMed] [Google Scholar]
- 42.Clerc F, et al. Human plasma protein N-glycosylation. Glycoconj J. 2016;33:309–343. doi: 10.1007/s10719-015-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirayama BA, Wright EM. Glycosylation of the rabbit intestinal brush border Na+/glucose cotransporter. Biochim Biophys Acta. 1992;1103:37–44. doi: 10.1016/0005-2736(92)90054-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.