Summary
Immune cell‐mediated destruction of salivary glands is a hallmark feature of Sjögren syndrome. Similar to the female predominance in humans, female non‐obese diabetic (NOD) mice develop spontaneous salivary gland autoimmunity. However, in both humans and mice it is unclear what factors contribute to the initial immune infiltration of the salivary glands. Here, we used an adoptive transfer model of Sjögren syndrome to determine if female mice harbor a sex‐specific defect in salivary‐gland‐protective regulatory T (Treg) cells. Transfer of cervical lymph node (LN) cells from female NOD mice into sex‐matched NOD‐severe combined immunodeficient (SCID) recipients resulted in sialadenitis, regardless of the presence or absence of Treg cells. In contrast, transfer of cervical LN cells from male NOD mice into sex‐matched NOD‐SCID recipients only resulted in sialadenitis when Treg cells were depleted before transfer, suggesting that male NOD mice have functional salivary‐gland‐protective Treg cells. Notably, the host environment affected the ability of Treg cells to prevent sialadenitis with testosterone promoting salivary gland protection. Treg cells from male mice did not protect against sialadenitis in female recipients. Testosterone treatment of female recipients of bulk cervical LN cells decreased sialadenitis, and Treg cells from female mice were capable of protecting against development of sialadenitis in male recipients. Hence, our data demonstrate that female NOD mice develop sialadenitis through a defect in salivary‐gland‐protective Treg cells that can be reversed in the presence of testosterone.
Keywords: autoimmunity, regulatory T cells, salivary gland, sex‐specific, sialadenitis, Sjögren syndrome
Abbreviations
- FACS
fluorescence‐activated cell sorting
- GFP
green fluorescent protein
- IL‐17
interleukin‐17
- LN
lymph node
- NOD
non‐obese diabetic
- SCID
severe combined immunodeficiency
- TCR
T‐cell receptor
- Treg cell
regulatory T cell
Introduction
Sjögren syndrome is an autoimmune disease that predominantly affects women and is characterized by dysfunction of the salivary and lacrimal glands as a result of immune cell‐mediated destruction.1 Factors contributing to the initiation of the autoimmune attack are poorly defined owing to the prolonged time (years) between initiation of autoimmunity and eventual diagnosis.2 Hence, alternative models are required to fully understand the mechanisms that lead to the initial autoimmune attack. The non‐obese diabetic (NOD) mouse is a well‐established model of Sjögren syndrome with spontaneous development of salivary and lacrimal gland autoimmunity.3, 4 Similar to Sjögren syndrome in humans, salivary gland autoimmunity in NOD mice occurs spontaneously with a female predominance.5, 6, 7 In contrast, male NOD mice are protected from the development of salivary gland inflammation,6, 7 but the factors responsible for these sex‐specific differences in sialadenitis are not known.
CD4+ Foxp3+ regulatory T (Treg) cells suppress immune cell activation and are required for maintaining tolerance to self‐antigens.8 The importance of Treg cells is shown by the widespread autoimmunity found in mice and humans that lack this population because of mutations in the gene encoding the transcription factor Foxp3.8 In non‐autoimmune‐prone mouse strains, genetic manipulations resulting in decreased Treg cells led to the development of sialadenitis,9, 10 suggesting that Treg cells play a dominant role in preventing salivary gland autoimmunity in healthy individuals. However, whether Treg cells play a role in preventing salivary gland autoimmunity in male NOD mice has not been evaluated. Here we use an adoptive transfer model of Sjögren syndrome to dissect the role of Treg cell dysfunction and sex hormones in the female‐specific development of autoimmune sialadenitis.
Materials and methods
Mice
Male and female NOD/ShiLtJ (NOD), castrated and sham‐castrated male NOD, and NOD.CB17‐Prkdc scid/J (non‐obese diabetic– severe combined immunodeficient; NOD‐SCID) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). NOD mice expressing the bicistronic Foxp3‐green fluorescent protein (Foxp3GFP) reporter construct knocked into the endogenous Foxp3 locus11 (NOD), were a kind gift from Vijay Kuchroo (Harvard University, Cambridge, MA) and were previously described.12 Mice used for phenotypic analyses of salivary gland infiltrating cells were 14‐ to 15‐week‐old females and 19‐ to 21‐week‐old males. Donor and recipient mice for transfer studies were 6–12 weeks old. Mice used for in vivo CD25 depletion studies were 5–6 weeks old at the start of antibody treatment. Mice were maintained and used in accordance with the Institutional Animal Care and Use Committee Guidelines of the University of Iowa and the Children's Hospital of Philadelphia.
Histological characterization of salivary and lacrimal glands
Inflammation of submandibular salivary glands and exorbital lacrimal glands was quantified as previously described.12, 13 Briefly, glands were formalin‐fixed, processed and embedded in paraffin. Five‐micrometre sections were stained with haematoxylin & eosin and analysed by standard light microscopy. Inflammation was quantified by a blinded observer using standard focus scoring as previously described12, 13 with focus score reported as number of foci (aggregates of 50 or more mononuclear cells) per 4‐mm2 tissue area. Tissue areas were measured using either Nikon nis‐elements br 3.1 software or imagej software14 as previously described.12, 13 Representative light microscopic images were obtained using Nikon nis‐elements imaging software (Nikon Instruments Inc., Melville, NY).
Lymphocyte isolation
Lymphocytes were isolated from cervical lymph nodes (LNs) or submandibular salivary glands by dissociating the tissues with the end of a 3‐ml syringe plunger through 70‐μm (for LNs) or 40‐μm (for salivary glands) nylon mesh in RPMI‐1640 (Life Technologies, Waltham, MA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, (Life Technologies) and 50 μm β‐mercaptoethanol (Sigma‐Aldrich, St Louis, MO) (complete RPMI). Cervical LNs in this study include mandibular, accessory mandibular and superficial parotid LNs.15 For dissection of submandibular salivary glands, the overlying cervical LNs were first removed followed by careful dissection of submandibular salivary glands from surrounding parotid gland tissue and then removal of the intimately associated sublingual salivary glands to ensure that the glands of interest were specifically isolated from surrounding lymphoid tissues and other salivary tissue. Red blood cells were lysed using ACK lysing buffer (Lonza, Mapleton, IL). Peripheral blood samples were collected and mononuclear cells were isolated with Histopaque‐1083 (Sigma‐Aldrich) as previously described.12
Flow cytometry and fluorescence‐activated cell sorting
Lymphocytes were analysed by flow cytometry using a BD LSR II or BD FACSCanto (BD Biosciences, San Jose, CA) for acquisition and flowjo software (Treestar Inc., Ashland, OR) for analysis. The lymphocyte gate was established by gating on forward‐scatter area by side‐scatter area followed by forward‐scatter area by forward‐scatter width to exclude singlets. Additional gating is specified in the figure legends. For sorting of Treg cells, cells from Foxp3GFP reporter NOD mice were labelled with fluorophore‐conjugated anti‐CD4 antibody and purified by fluorescence‐activated cell sorting (FACS) based on CD4 and Foxp3GFP expression using a FACSAria II sorter (BD Biosciences). The non‐Treg cells were sorted in a similar way to include all other cells within the lymphocyte singlet gate that were not CD4+ Foxp3+ (i.e. all CD4+ Foxp3− and CD4− lymphocytes). For all sorts, the purified CD4+ Foxp3+ Treg cell population was > 95% pure and the purified non‐Treg cell populations contained < 2% CD4+ Foxp3+ cells. Antibodies were purchased from BD Biosciences, eBioscience (San Diego, CA), or BioLegend (San Diego, CA). Antibody clones included the following: CD3ε (145‐2c11), CD4 (GK1.5 or RM4‐5), CD8α (53‐6.7), Foxp3 (FJK‐16s), CD19 (1D3), T‐cell receptor‐β (TCR‐β; H57‐597).
In vivo Treg cell depletion
To deplete Treg cells in vivo, mice were treated for 4 weeks with weekly intraperitoneal injections of 0·5 mg anti‐CD25 monoclonal antibody (clone PC‐61.5.3) or rat IgG1 isotype control antibody (clone HRPN) (Bio X Cell, West Lebanon, NH).
Adoptive transfer model of Sjögren syndrome
The adoptive transfer model was performed as previously described.12, 13 Briefly, bulk cervical LN cells were isolated, pooled and 5 × 106 cells were transferred intravenously to NOD‐SCID recipient mice. Five to seven weeks later salivary and lacrimal glands were analysed histologically and lymphocytes were analysed by flow cytometry. For Treg cell depletion studies, 5 × 106 non‐Treg cells were transferred alone or with CD4+ Foxp3+ Treg cells at a physiological ratio based on the pre‐sort donor non‐Treg : Treg ratio. Donor and recipient mice were 6–12 weeks old. All donors and recipients tested negative for glucosuria at time of tissue harvest. Testosterone treatment of female NOD‐SCID recipient mice was performed as previously described.13 Briefly, placebo pellets or those containing testosterone (45 mg/pellet, 90‐day release) (Innovative Research of America, Sarasota, FL) were implanted subcutaneously in the subscapular region of female NOD‐SCID mice 1 week before adoptive transfer of donor cells.
Statistical analysis
Statistical analyses were performed with prism software version 7.00 (GraphPad, San Diego, CA). Mann–Whitney U‐test was used for two‐group comparisons of non‐normally distributed data (focus scores) and unpaired Student's t‐test was used for two‐group comparisons of data that approximated normal distribution (flow cytometry data). Paired Student's t‐test was used to compare flow cytometry data between two different sites within the same mouse. All two‐group comparisons were two‐tailed. Unpaired Student's t‐tests were corrected for multiple comparisons where appropriate using the Holm–Sidak method. Paired Student's t‐test was used for two‐group comparisons of cells isolated from different locations within the same individual mice. P‐values < 0·05 were considered significant.
Results
Sex‐specific development of sialadenitis in the NOD mouse model of Sjögren syndrome
The NOD mouse is a well‐established model of Sjögren syndrome. Inflammation of the salivary glands is sex‐specific with sialadenitis spontaneously developing in female but not in male NOD mice (Fig. 1a,b and refs 6, 7). Similar to human Sjögren syndrome,16, 17, 18 salivary gland infiltrates in affected NOD mice, even at early stages, are dominated by CD4 T cells but also include CD8 T cells and B cells (Fig. 1c,d and refs 19, 20, 21). Among the abundant CD4+ T cells, CD4+ Foxp3+ Treg cells were detected in the salivary glands of female NOD mice in proportions comparable to those in the salivary‐gland‐draining cervical LNs (Fig. 1d). Surprisingly, similar proportions of B cells and T‐cell subsets were found in male salivary glands (Fig. 1c,d; see Supplementary material, Fig. S1). Although the proportions of these cells were comparable between gland and LN, the number of cells recovered from the salivary glands was routinely 50‐ to 150‐fold lower than that recovered from the cervical LNs. Hence, in female NOD mice, salivary gland inflammation was not associated with a selective exclusion of Treg cells from the glands; however, the possibility remained that a salivary‐gland‐protective subset of Treg cells was either absent or was present but dysfunctional. Similarly, the lack of spontaneous sialadenitis in male NOD mice suggested that male NOD mice may have functional salivary‐gland‐protective Treg cells.
Figure 1.
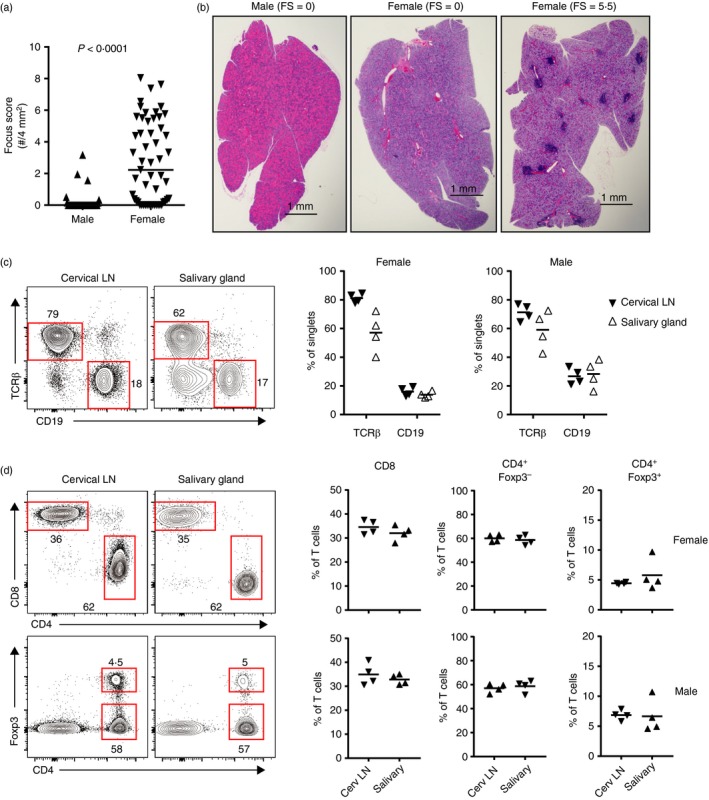
Sex‐specific development of sialadenitis in the non‐obese diabetic (NOD) mouse model of Sjögren syndrome. (a) Quantification of spontaneous sialadenitis in male (n = 40) and female (n = 55) NOD mice (aged 6–12 weeks). Each data‐point represents an individual mouse and lines represent medians. P‐value determined by Mann–Whitney U‐test. (b) Representative haematoxylin & eosin‐stained salivary gland sections from 15‐ to 17‐week‐old male (left) and female (middle and right) NOD mice with focus scores (no. inflammatory mononuclear cell foci per 4 mm2 tissue) as indicated. Bars = 1 mm. (c, d) Representative flow cytometry plots of gland‐infiltrating T cells isolated from cervical lymoph nodes (LNs) or salivary glands of 14‐ to 15‐week‐old female NOD mice. Plots were gated on singlet lymphocytes (c) or further gated on T cells (TCR‐β + CD19−) (d). For representative gating see Supplementary material (Fig. S1). Numbers represent the frequency of cells in each gate. Graphs depict cumulative quantification of data from 14‐ to 15‐week‐old females (n = 4) and 19‐ to 21‐week‐old males (n = 4) as indicated above (c) or to the right (d) of graphs. Symbols represent individual mice. Lines are means. Comparisons between LNs and salivary glands were not significant as determined by paired Student's t‐test.
In vivo Treg cell depletion is sufficient to drive dacryoadenitis but not sialadenitis
To determine if salivary‐gland‐protective Treg cells prevent sialadenitis in male NOD mice, we used an in vivo Treg cell depletion model, taking advantage of the expression of CD25 on the majority of Treg cells.22 Mice were injected with an anti‐CD25 antibody or isotype control antibody for four consecutive weeks and salivary and lacrimal glands were analysed for inflammation 9 weeks after the initial injection. Flow cytometric analyses of cervical LN cells demonstrated a significant reduction in CD4+ Foxp3+ Treg cells in male NOD mice treated with the anti‐CD25 antibody relative to those treated with the isotype control antibody, indicating that anti‐CD25 antibody treatment was effective at reducing this population (Fig. 2a). However, this Treg cell depletion in male NOD mice did not promote development of sialadenitis (Fig. 2b). In contrast, lacrimal gland inflammation (which spontaneously develops in male NOD mice) was comparable whether Treg cells were depleted or not (Fig. 2c). Longitudinal analyses of peripheral blood cells from these treated male mice demonstrated that the anti‐CD25 antibody treatment was effective in decreasing Treg cells throughout the course of the experiment (Fig. 2d).
Figure 2.
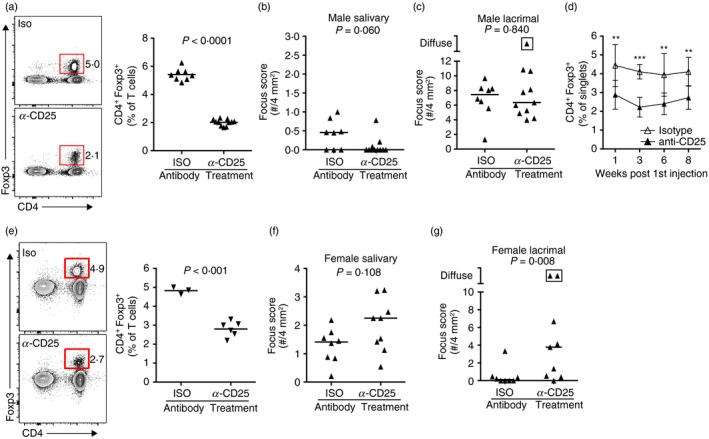
In vivo regulatory T (Treg) cell depletion results in dacryoadenitis in female non‐obese diabetic (NOD) mice but not sialadenitis in male NOD mice. (a) Representative flow cytometry plots of cells isolated from the cervical lymph nodes (LNs) of male NOD mice that received treatment with anti‐CD25 antibody (n = 11) or isotype control antibody (n = 8) for four consecutive weeks starting at week 0. Plots were gated on singlet T cells (CD3ε +). Graphs depict cumulative quantification of data from two independent experiments. Symbols represent individual mice, bars represent means. P‐value determined by unpaired Student's t‐test. (b, c) Quantification of sialadenitis (b) or dacryoadenitis (c) in male mice from (a). Each data‐point represents an individual mouse and lines represent medians. P‐values determined by Mann–Whitney U‐test. Boxed symbol represents diffuse inflammation in which foci coalesced and could not be accurately enumerated. (d) Cumulative quantification of the percentage of CD4+ Foxp3+ Treg cells present in the peripheral blood of mice from (a) to (c) at the indicated time following the initial antibody treatment. Data are pooled from two independent experiments. Symbols represent means and bars represent the standard deviations. P‐value determined by multiple t‐tests with correction for multiple comparisons using the Holm–Sidak method. **P < 0·01, ***P < 0·001. (e) Representative flow cytometry plots of cells isolated from the cervical LNs of female NOD mice that received treatment with anti‐CD25 antibody or isotype control antibody for four consecutive weeks starting at week 0. Plots were gated on singlet T cells (CD3ε +). Graphs show data from one experiment (anti‐CD25: n = 6; isotype: n = 3). Symbols represent individual mice, bars represent means. P‐value determined by unpaired Student's t‐test. (f and g) Quantification of sialadenitis (f) or dacryoadenitis (g) in female NOD mice treated with anti‐CD25 antibody (n = 9) or isotype control antibody (n = 8). Each data‐point represents an individual mouse and lines represent medians pooled from two independent experiments. Boxed symbols as in (c). P‐values determined by Mann–Whitney U‐test.
To confirm that in vivo anti‐CD25 antibody treatment does not antagonize sialadenitis development and was sufficient to promote autoimmunity, we performed this treatment in female NOD mice and assessed the development of sialadenitis and dacryoadenitis. Sialadenitis occurs spontaneously in female NOD mice, but dacryoadenitis does not occur spontaneously in female NOD mice because of the presence of lacrimal‐gland‐protective Treg cells.13 Similar to males, flow cytometric analyses of female cervical LN cells showed a significant reduction in the percentage of CD4+ Foxp3+ Treg cells in anti‐CD25 antibody‐treated females (Fig. 2e). Comparable sialadenitis was observed in female NOD mice treated with the isotype control antibody or the anti‐CD25 antibody (Fig. 2f). Notably, although little to no dacryoadenitis was observed in female NOD mice treated with the isotype control antibody, treatment with the Treg cell‐depleting antibody induced dacryoadenitis (Fig. 2g). These data confirm our earlier findings that lacrimal‐gland‐protective Treg cells are present in female NOD mice13 and suggest that the partial Treg cell depletion capable of driving dacryoadenitis in females does not drive sialadenitis in male NOD mice.
Male NOD mice have salivary‐gland‐protective Treg cells
Male NOD mice do not spontaneously develop sialadenitis (Fig. 1). This may be a result of (i) the lack of functional pathogenic effector cells (including specific T‐cell subsets, B cells, antigen‐presenting cells and other pathogenic populations), (ii) the resistance of male salivary glands to being targeted in an autoimmune attack, or (iii) the presence of functional salivary‐gland‐protective Treg cells. The lack of sialadenitis in male NOD mice upon in vivo Treg cell depletion (Fig. 2b) may suggest that the latter is less likely; however, in vivo Treg cell depletion with anti‐CD25 antibody induced only partial Treg cell depletion (Fig. 2a,d). Although such partial depletion was sufficient to drive dacryoadenitis in female NOD mice, the possibility remained that male NOD mice have a Treg cell population capable of protecting salivary glands even in the face of partial depletion. To address these possibilities, we used an adoptive transfer model in which cervical LN cells were isolated from NOD donor mice and transferred intravenously to lymphocyte‐deficient NOD‐SCID recipient mice, as previously described.12, 13 Similar to female‐specific sialadenitis that develops spontaneously in NOD mice, transfer of bulk cervical LN cells from NOD donors to NOD‐SCID recipients resulted in significantly greater sialadenitis in female‐to‐female transfers compared with male‐to‐male transfers (Fig. 3a). Hence, similar to the lack of spontaneous sialadenitis in male NOD mice, male NOD‐SCID recipient mice were protected from sialadenitis in our transfer model.
Figure 3.
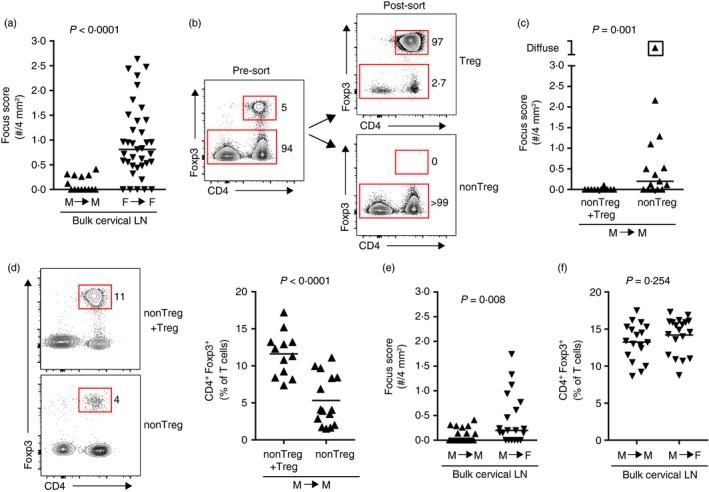
Salivary‐gland‐protective regulatory T (Treg) cells are present in male non‐obese diabetic (NOD) mice. (a) Quantification of sialadenitis in male (n = 14) and female (n = 37) non‐obese diabetic–severe combined immunodeficient (NOD‐SCID) recipients of bulk cervical lymph node (LN) cells from sex‐matched NOD donors, pooled from four or seven transfers, respectively. Each data‐point represents an individual mouse and lines represent medians. P‐value determined by Mann–Whitney U‐test. (b) Representative flow cytometry plots depicting Treg and non‐Treg cells from cervical LNs of male Foxp3GFP reporter NOD mice before (pre‐sort) or after (post‐sort) FACS purification. Plots were gated on singlet lymphocytes. Numbers indicate the percentage of cells within each gate. (c) Quantification of inflammation of salivary glands in male NOD‐SCID recipients of male non‐Treg cells with Treg cells added back (non‐Treg + Treg; n = 12) or non‐Treg cells alone (non‐Treg; n = 15), sorted as in (b). Data are pooled from three independent experiments. Each data‐point represents an individual mouse and lines represent medians. P‐value determined by Mann–Whitney U‐test. (d) Representative flow cytometry plots of cells isolated from the cervical LNs of recipients in (c). Plots were gated on singlet T cells (CD19− CD3ε +). Numbers indicate the percentage of cells within each gate. Graph depicts cumulative data pooled from three independent experiments. Symbols represent individual mice, lines are means. P‐value determined by unpaired Student's t‐test. (e) Quantification of sialadenitis in male (n = 23) and female (n = 20) NOD‐SCID recipients of male bulk cervical LN cells, pooled from four independent transfers. Each data‐point represents an individual mouse, lines represent medians. P‐value determined by Mann–Whitney U‐test. (f) Quantification of Treg cells in cervical LNs of recipients as in (e). Data were pooled from three independent experiments. Symbols represent individual mice, lines are means. P‐value determined by unpaired Student's t‐test.
To determine if a more complete depletion of Treg cells would promote sialadenitis in male mice we used male NOD mice expressing a Foxp3GFP reporter to purify Treg cells and non‐Treg cells from the cervical LNs of male NOD mice by FACS (Fig. 3b). We then transferred either non‐Treg cells alone or non‐Treg cells with Treg cells added back at physiological ratios (non‐Treg + Treg) to male NOD‐SCID recipients. As with our bulk transfers (Fig. 3a) little to no sialadenitis was observed in recipients of non‐Treg + Treg cells, whereas recipients of the Treg cell‐depleted non‐Treg donor cells developed significantly more sialadenitis, including one recipient that developed so much inflammation that individual foci coalesced and could not be accurately enumerated (designated diffuse) (Fig. 3c). Flow cytometric analyses of T‐cell populations in the cervical LNs of recipient mice showed repopulation of the Treg cells at takedown but at a significantly decreased proportion compared with recipients of non‐Treg + Treg cells (Fig. 3d). Although the overall degree of inflammation (i.e. focus scores) was slightly lower in some mice compared with that in female recipient mice (Fig. 3c compared with Fig. 3a), these results demonstrate that male NOD mice have (i) effector T cells that can target salivary glands, (ii) salivary glands that are not intrinsically resistant to being targeted by pathogenic T cells, and (iii) a population of Treg cells that prevent sialadenitis.
To determine if these salivary‐gland‐protective Treg cells were capable of preventing sialadenitis in female mice, we transferred bulk cervical LN cells (which include Treg cells) from male NOD donors to either male or female NOD‐SCID recipients. Despite little to no sialadenitis in male recipients, the same pool of donor cells caused significantly more sialadenitis in female recipients (Fig. 3e). This was not the result of a decrease in proportion of Treg cells in female recipients (Fig. 3f). Hence, male NOD mice have salivary‐gland‐protective Treg cells capable of preventing sialadenitis in male, but not female, recipient mice.
Female NOD mice develop sialadenitis in the presence or absence of Treg cells
The presence of salivary‐gland‐protective Treg cells in male NOD mice that were incapable of preventing transfer of sialadenitis to female NOD‐SCID mice (Fig. 3e) suggested that sialadenitis in females may be the result of dysfunction of salivary‐gland‐protective Treg cells. Accordingly, transfer of female non‐Treg cells or non‐Treg + Treg cells to female NOD‐SCID recipients resulted in a similar degree of sialadenitis regardless of the presence or absence of Treg cells in the donor population (Fig. 4a). Similar to male‐to‐male transfers (Fig. 3d), Treg cells were partially repopulated in recipients of non‐Treg cells at takedown but were still significantly lower than in recipients of non‐Treg + Treg cells (Fig. 4b). Hence, in female NOD mice, Treg cells were incapable of significantly modulating the degree of sialadenitis.
Figure 4.
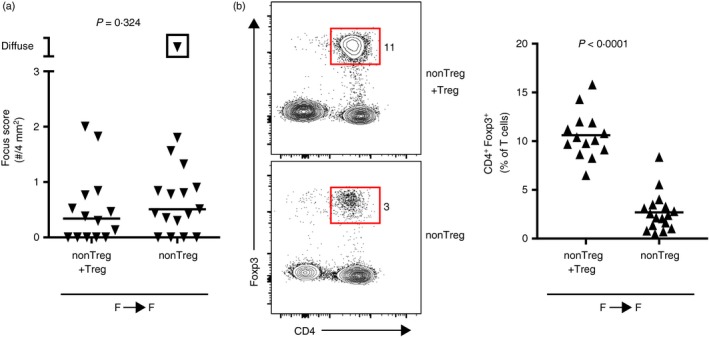
Females develop sialadenitis regardless of the presence or absence of regulatory T (Treg) cells. (a) Quantification of inflammation of salivary glands in female non‐obese diabetic–severe combined immunodeficient (NOD‐SCID) recipients of female non‐Treg cells with Treg cells added back (non‐Treg + Treg; n = 14) or non‐Treg cells alone (non‐Treg cells; n = 17). Data are pooled from three independent experiments. Each data‐point represents an individual mouse and lines represent medians. P‐value determined by Mann–Whitney U‐test. (b) Representative flow cytometry plots of cells isolated from the cervical lymph nodes (LNs) of recipients in (a). Plots were gated on singlet T cells (CD19− CD3ε +). Numbers indicate the percentage of cells within each gate. Graph depicts cumulative data pooled from two independent experiments. Symbols represent individual mice, lines are means. P‐value determined by unpaired Student's t‐test.
Female Treg cells prevent sialadenitis in male mice
The ability of male salivary‐gland‐protective Treg cells to function in males but not in females (Fig. 3e) prompted us to determine if this was also true for female salivary‐gland‐protective Treg cells: can female Treg cells prevent sialadenitis in males but not in females? To address this question, we isolated bulk cervical LN cells from female NOD donors and transferred them into male or female NOD‐SCID recipients. In contrast to the development of sialadenitis in female recipients, male recipients of the same population of female donor cells developed significantly less sialadenitis (Fig. 5a). A similar proportion of Treg cells was observed in male and female recipients, suggesting that this was not the result of an increase in the proportion of Treg cells in the male recipients (Fig. 5b). We considered the possibility that the sex‐based difference in salivary gland disease development was due to sex hormones. Previous studies in NOD mice had shown that altering female sex hormones through castration did not modulate the development of sialadenitis in females,6 whereas castration of males increased development of sialadenitis (Fig. 5c).6, 23 Similar proportions of Treg cells were observed in castrated and sham‐castrated mice, indicating that this effect was not the result of differences in the frequency of Treg cells in the absence of testosterone (Fig. 5d). Rather, this was associated with morphological differences in salivary glands, with castrated male salivary glands adopting a more female‐like histological appearance (see Supplementary material, Fig. S2). We, therefore, chose to determine if testosterone was a key protective factor in the development of sialadenitis in our transfer model. We transferred bulk cervical LN cells from female donor mice to testosterone‐treated or placebo‐treated female NOD‐SCID recipient mice and found that testosterone‐treated recipients developed significantly less sialadenitis (Fig. 5e). No difference in the percentage of Treg cells was observed between testosterone‐treated or placebo‐treated recipients, again demonstrating that the protective effect was not the result of differences in the proportion of Treg cells repopulating the recipient mice (Fig. 5f). To determine if Treg cells were required for the prevention of sialadenitis when female cervical LN cells were transferred to a testosterone‐rich male environment, we transferred FACS‐purified non‐Treg cells or non‐Treg + Treg cells from female NOD donors into male NOD‐SCID recipients. As expected, recipients of non‐Treg cells had a significantly decreased proportion of Treg cells within the cervical LNs at takedown (Fig. 5g). Strikingly, sialadenitis developed in recipients of non‐Treg cells but not in those receiving non‐Treg + Treg cells (Fig. 5h). The male environment protects from sialadentitis by promoting salivary‐gland‐protective Treg cells. Collectively, these data indicate that salivary‐gland‐protective Treg cells are present in the cervical LNs of both male and female NOD mice, yet they fail to prevent the development of autoimmune sialadenitis in a female host.
Figure 5.
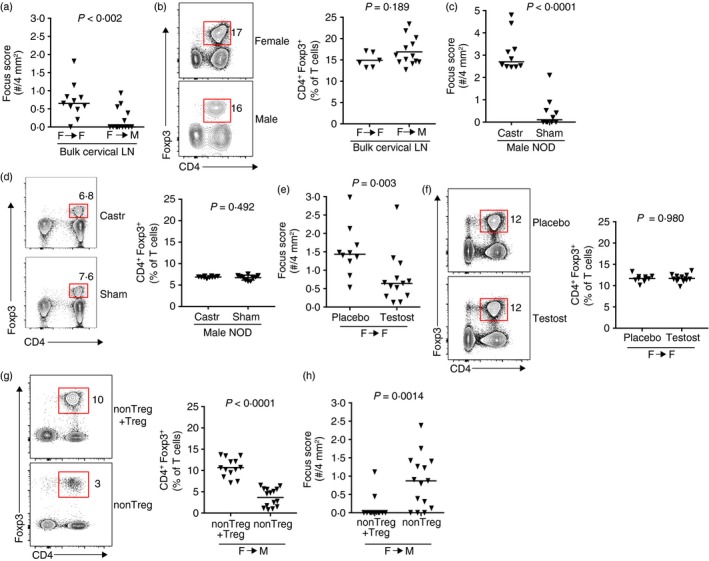
Female salivary gland‐protective regulatory T (Treg) cells prevent sialadenitis in male mice. (a) Quantification of inflammation of salivary glands in female (n = 11) or male (n = 14) non‐obese diabetic–severe combined immunodeficient (NOD‐SCID) recipients of female bulk cervical lymph node (LN) cells. Data are pooled from two or three independent experiments, respectively. Each data‐point represents an individual mouse and lines represent medians. P‐value determined by Mann–Whitney U‐test. (b) Representative flow cytometry plots of cells isolated from the cervical LNs of recipients in (a). Plots were gated on singlet T cells (CD19− TCR β + or B220− CD3ε +). Numbers indicate the percentage of cells within each gate. Graph depicts cumulative data from one (Female) or two (Male) independent experiments. Symbols represent individual mice, lines are means. P‐value determined by unpaired Student's t‐test. (c) Quantification of inflammation in salivary glands from 15‐week‐old castrated (n = 10) or sham‐castrated (n = 10) male NOD mice. Symbols represent individual mice pooled from two independent experiments. Lines are medians. P‐value by Mann–Whitney U‐test. (d) Representative flow cytometry plots of cells isolated from castrated or sham‐castrated male NOD mice in (c). Plots were gated on singlet T cells (CD19− CD3ε +). Numbers indicate the percentage of cells within the indicated Treg cell gate. Graph depicts cumulative data pooled from two independent experiments. P‐value by unpaired Student's t‐test. (e) Quantification of inflammation in salivary glands from female NOD‐SCID recipients of female NOD cervical LN cells. Recipients were treated with testosterone (n = 13) or placebo (n = 10) before cell transfer. Data are pooled from two independent experiments. Each data‐point represents an individual mouse and lines represent medians. P‐value determined by Mann–Whitney U‐test. (f) Representative flow cytometry plots of cells isolated from recipient mice in (e). Plots were gated on singlet T cells (B220− CD3ε +). Numbers indicate the percentage of cells within each gate. Graph depicts cumulative data from two independent experiments. Symbols represent individual mice, lines are means. P‐value determined by unpaired Student's t‐test. (g) Representative flow cytometry plots of cells isolated from the cervical LNs of male NOD‐SCID recipients of female non‐Treg + Treg cells (n = 13) or non‐Treg cells (n = 15). Plots were gated on singlet T cells (CD19− CD3ε + or CD19− TCR β +). Numbers indicate the percentage of cells within each gate. Graph depicts cumulative data from two independent experiments. Symbols represent individual mice, lines are means. P‐value determined by unpaired Student's t‐test. (h) Quantification of sialadenitis in male NOD‐SCID recipients in (g). Data are pooled from two independent experiments. Each data‐point represents an individual mouse and lines represent medians. P‐values determined by Mann–Whitney U‐test.
Discussion
Treg cells suppress immune cell activation and are required to prevent autoimmunity.8 Although the exact nature of Treg cell‐mediated prevention of autoimmunity is complex and not completely understood, multiple studies demonstrate that Treg cells function in an organ‐specific manner.24, 25, 26 This was observed in early studies in rats in which T cells isolated from rats with intact thyroid glands were capable of preventing experimental autoimmune thyroiditis and experimental autoimmune diabetes, whereas T cells isolated from rats in which the thyroid was ablated in utero failed to protect against the development of experimental autoimmune thyroiditis but could still confer protection against experimental autoimmune diabetes.24 In a mouse model of autoimmune prostatitis, CD4 T cells could only suppress disease if they were isolated from male mice, but not if they were from female mice or prostate‐deficient male mice.25 Induced Treg cells from NOD mice could only provide protection against type 1 diabetes when their cognate antigen was present in vivo.26 These studies suggest that the organ‐specific protective function of Treg cells is based on antigen specificity. In further support of antigen‐specific function of Treg cells, Treg cells with distinct TCRs were enriched within different anatomically located LN groups27 and preferentially homed to their LN of origin following adoptive transfer.28 Functionally, several studies provide evidence that organ‐protective Treg cells are enriched within organ‐draining LNs.29, 30, 31 These studies formed the basis for our recent studies using transfers of cells from cervical LNs (which drain lacrimal and salivary glands) to demonstrate that protection from dacryoadenitis in female NOD mice was associated with functional lacrimal‐gland‐protective Treg cells. Here we show that male NOD mice were protected from sialadenitis because of the presence of salivary‐gland‐protective Treg cells. In contrast, Treg cells were incapable of preventing sialadenitis in female NOD mice or female NOD‐SCID recipients, accounting for the female‐specific susceptibility to salivary gland autoimmunity. Collectively, our studies suggest that the organ‐specific autoimmunity observed in NOD mice is the result of factors that disrupt organ‐specific Treg cell function.
The fact that the organ‐specific Treg cell dysfunction was not transferable between males and females (e.g. female salivary‐gland‐protective Treg cells were dysfunctional in females but functional in males) suggested that the factors driving disease development were either reversible or were Treg cell extrinsic. This could be due to direct effects on non‐Treg immune cells (e.g. effector T cells or antigen‐presenting cells) that allow them to resist Treg cell‐mediated suppression or due to effects on other cell types, such as cells within the target organs themselves (e.g. acinar or ductal epithelial cells). In the NOD mouse, salivary gland autoimmunity is thought to develop in three distinct, yet continuous phases: (i) defects in salivary gland organogenesis and apoptosis of salivary gland acinar cells; (ii) lymphocytic autoimmune attack on glands; (iii) gland destruction and dysfunction.19, 32, 33, 34 Therefore it seems plausible that the factors driving disease could affect both immune and non‐immune cell types at different stages of disease development. Notably, salivary gland epithelial cells in female NOD‐SCID mice exhibited histological changes in the absence of inflammation with a decrease in the acinar to ductal cell ratio over time.35 This may be the result of increased epithelial cell apoptosis independent of inflammation as salivary gland epithelial cells in NOD‐SCID mice express a similar increase in pro‐apoptotic molecules to that found in NOD mice.36 Whether these changes are specific to females even in NOD‐SCID mice has not been formally explored, but the histological differences in haematoxylin & eosin‐stained sections of submandibular salivary glands from female and male NOD mice (Fig. 1b) were also apparent in NOD‐SCID mice (see Supplementary material, Fig. S2 and data not shown) suggesting that the sex differences in salivary gland morphology are not a sequela of the autoimmune process but may instead be a relevant driving factor. The strikingly similar morphological differences in sections of salivary glands from castrated and sham‐castrated male NOD mice (see Supplementary material, Fig. S2) to those in female and male NOD and NOD‐SCID mice suggest that androgens are a key factor in these differences. But exactly how such morphological changes reflect a predisposition to sialadenitis is not yet known.
Sex hormones are obvious candidates for driving the sex‐specific differences in autoimmunity that are observed in the NOD mouse model of Sjögren syndrome. In our study, treatment of female mice with testosterone reduced the degree of salivary gland inflammation, suggesting that testosterone functions in a protective manner in the context of salivary gland inflammation. Similar effects have been observed in other mouse models of Sjögren syndrome, including in the NZB/NZW F1 mouse model in which treatment with testosterone reduced the number of lymphocytic foci in the salivary gland.37 Similarly, castrated male NOD mice showed significantly elevated levels of sialadenitis relative to sham‐castrated controls (Fig. 5c).6, 23 In contrast to protective effects on salivary gland autoimmunity, testosterone promotes dacryoadenitis in NOD mice.6, 13, 38 Castration of male NOD mice protected from spontaneous dacryoadenitis,6, 38 and testosterone treatment of female NOD‐SCID recipients of female cervical LN cells promoted dacryoadenitis.13 Collectively, these studies demonstrate that the effects of testosterone on salivary and lacrimal gland autoimmunity in NOD mice are opposite and that testosterone is a key factor that drives lacrimal gland autoimmunity while protecting from salivary gland autoimmunity. Interestingly, in NOD mice modified to express the C57BL/10 mouse‐derived MHC locus (H2b haplotype) in place of the NOD H2g7 haplotype, female mice develop both salivary and lacrimal gland inflammation.39 Hence, comparing disease mechanisms in these NOD.B10 mice and wild‐type NOD mice may provide insight into both hormonal and non‐hormonal factors driving salivary and lacrimal gland disease.
In contrast to testosterone, the lack of an effect on sialadenitis or dacryoadenitis in ovariectomized female NOD mice suggests that female sex hormones do not influence disease in salivary or lacrimal glands.6 However, reports in other models suggest that oestrogen may play a protective role. For example, aromatase knockout mice, which fail to synthesize oestrogens, develop sialadenitis.40 In a day 3 thymectomy model, ovariectomized females developed more sialadenitis than sham controls.37 Further, the increase in ductal cell death observed in the salivary glands of ovariectomized mice suggests that oestrogen promotes the survival of these cells37 and so may play a protective role in disease development. In contrast, oestrogen promotes interleukin‐17 (IL‐17) production41 and increased proportions of IL‐17‐producing cells have been associated with more severe sialadenitis in the C57BL/6.NOD‐AEC1AEC2 mouse model of Sjögren syndrome.42 Therefore, oestrogens appear to have different effects on salivary gland inflammation and the exact role is probably modified by other factors, including genetic and strain‐specific effects, and possibly also the microbiome.23
Our transfer studies here demonstrated the presence of functional salivary‐gland‐protective Treg cells in male NOD mice, and our previous studies demonstrated functional lacrimal‐gland‐protective Treg cells in female NOD mice. It was somewhat surprising to note that the in vivo Treg cell depletion was not sufficient to drive salivary gland autoimmunity in male NOD mice. This may be because of the incomplete depletion of Treg cells by this method; however, similar partial Treg cell depletion in female NOD mice resulted in lacrimal gland autoimmunity. This may reflect a fundamental difference in mechanisms of lacrimal versus salivary gland autoimmunity. Alternatively, this may reflect an underlying increased propensity for female NOD mice to develop autoimmune manifestations such that a partial Treg cell depletion was able to drive the females to develop autoimmunity of a normally unaffected target organ (e.g. lacrimal glands). In general, the mammalian immune response in females is stronger than that in males, as demonstrated by greater pro‐inflammatory responses, T‐cell proliferation and antibody responses in females upon infection or vaccination.43 This was also observed in several mouse models of Sjögren syndrome. In the NOD.H2h4 mouse model, female mice have more severe sialadenitis and an increased number of CD45 cells, including CD4 T cells, in salivary gland infiltrates.44 A comparison of sex differences in sialadenitis and dacryoadenitis also showed more severe disease in females of several non‐NOD strains.7 Hence the female host environment appears to favour effector cells in the balance between effector and regulatory cells. Therefore, transient, incomplete depletion of Treg cells in females may more easily allow development of autoimmunity, whereas in a male host, a higher threshold must be overcome for an autoimmune attack to occur, and partial depletion of Treg cells is simply not sufficient to overcome this barrier. Along these lines, recent studies in humans found increased frequency of an extra X chromosome in males and females with Sjögren syndrome45, 46 supporting the role of X chromosome genes in increasing risk of autoimmunity. Similarly, epigenetic DNA methylation changes may contribute to an increased dose effect by allowing access to genes on the inactivated X chromosome in females with normal karyotypes.47
The ability of Treg cells within male or female donor cervical LN cells to prevent the co‐transferred effector T cells from mediating salivary gland autoimmunity in a male host but not in a female raises the question as to which effector cell population may be modulated by Treg cells to prevent salivary gland autoimmunity. In the C57BL/6.NOD‐AEC1AEC2 model of Sjögren syndrome, evidence for a sexually dimorphic role for IL‐17 in salivary gland autoimmunity has been observed.42 Genetic ablation of IL‐17 restored salivary gland function in both sexes, but greater effects were observed in females relative to males, including a greater reduction in the immune cell infiltrate into the gland, and so a greater reduction in overall focus score.42 Notably, splenocytes from oestrogen‐treated mice have increased IL‐17 expression and secrete higher levels of IL‐17 ex vivo.41 Hence, it is possible that pathogenic IL‐17‐producing effector T cells may be modulated by Treg cells in a male environment, whereas this mechanism is not sufficient to protect against autoimmunity in females. Another consideration is the possibility that host cells within the recipient mice, such as epithelial cells or antigen‐presenting cells, may be selectively resistant to Treg cell‐mediated suppression within the female host. These possibilities remain to be explored.
NOD mice display sex‐specific differences in the development of salivary and lacrimal gland autoimmunity that can be, in part, attributed to sex hormones. A key outstanding question is which cells are targeted by sex hormones in the promotion or prevention of organ‐specific autoimmunity. The androgen receptor and oestrogen receptors are expressed within salivary and lacrimal glands,48, 49, 50 suggesting that hormones can impact the cells that make up the glands. Studies on salivary glands from non‐autoimmune‐prone mice demonstrate that significant gene expression differences exist between males and females, affecting genes involved in processes such as development, growth, transcription, metabolism, signal transduction and receptor activity, among others.51, 52 Some of these differences can be directly attributed to the action of hormones as testosterone treatment affected thousands of genes in the salivary glands of orchidectomized male BALB/c mice.53 Further support for the effect of sex hormones on salivary glands is observed in female or castrated males, where rapid morphological changes in the salivary glands are observed upon androgen treatment.54, 55 Similar morphological differences are clearly apparent when comparing male and female salivary glands from NOD mice or NOD‐SCID mice (Fig. 1b; see Supplementary material, Fig. S2) or castrated and sham‐castrated male NOD mice (see Supplementary material, Fig. S2). Despite these reports on the effects of sex hormones on the salivary glands themselves, the specific molecular mechanisms by which these changes promote or protect from autoimmunity is unclear. Hence, using the NOD mouse model to address these questions to delineate the mechanisms by which hormones exert their effects to drive salivary and lacrimal gland autoimmunity will further our understanding of the role of sex hormones in Sjögren syndrome and other autoimmune diseases.
Author contributions
JYB and SML performed experiments, analysed data, and wrote the paper. XW and PAK performed experiments and analysed data.
Disclosures
The authors have no competing financial or commercial conflicts of interest to disclose.
Supporting information
Figure S1. Representative flow cytometry plots to depict gating strategy for salivary (top) and cervical lymph node (bottom) cell analyses as in Fig. 1.
Figure S2. Testosterone induces changes in salivary gland morphology in non‐obese diabetic (NOD) and non‐obese diabetic– severe combined immunodeficient (NOD‐SCID) mice.
Acknowledgements
The authors gratefully acknowledge assistance from members of the flow cytometry core facility and the animal husbandry and veterinary staff in the office of animal research at the University of Iowa, members of the Karandikar laboratory for helpful discussion, and Dr Vijay Kuchroo for the NOD‐Foxp3GFP reporter mice. This work was supported by the National Institutes of Health (EY022344 to SML) and by start‐up funds from the Stead Family Department of Pediatrics (SML). The data presented herein were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center and Iowa City Veterans Affairs Medical Center.
References
- 1. Mavragani CP, Moutsopoulos HM. Sjogren's syndrome. Annu Rev Pathol 2014; 9:273–85. [DOI] [PubMed] [Google Scholar]
- 2. Theander E, Jonsson R, Sjostrom B, Brokstad K, Olsson P, Henriksson G. Prediction of Sjogren's syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol 2015; 67:2427–36. [DOI] [PubMed] [Google Scholar]
- 3. Delaleu N, Nguyen CQ, Peck AB, Jonsson R. Sjogren's syndrome: studying the disease in mice. Arthritis Res Ther 2011; 13:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee BH, Gauna AE, Pauley KM, Park YJ, Cha S. Animal models in autoimmune diseases: lessons learned from mouse models for Sjogren's syndrome. Clin Rev Allergy Immunol 2012; 42:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanagi K, Haneji N, Ishimaru N, Saito I, Hayashi Y. Analysis of T cell receptor Vβ usage in the autoimmune sialadenitis of non‐obese diabetic (NOD) mice. Clin Exp Immunol 1997; 110:440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunger RE, Carnaud C, Vogt I, Mueller C. Male gonadal environment paradoxically promotes dacryoadenitis in nonobese diabetic mice. J Clin Invest 1998; 101:1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toda I, Sullivan BD, Rocha EM, Da Silveira LA, Wickham LA, Sullivan DA. Impact of gender on exocrine gland inflammation in mouse models of Sjogren's syndrome. Exp Eye Res 1999; 69:355–66. [DOI] [PubMed] [Google Scholar]
- 8. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30:531–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma R, Zheng L, Guo X, Fu SM, Ju ST, Jarjour WN. Novel animal models for Sjogren's syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell‐deficient mice. J Autoimmun 2006; 27:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka Y, Sotome T, Inoue A, Mukozu T, Kuwabara T, Mikami T et al SATB1 Conditional knockout results in Sjogren's syndrome in mice. J Immunol 2017; 199:4016–22. [DOI] [PubMed] [Google Scholar]
- 11. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235–8. [DOI] [PubMed] [Google Scholar]
- 12. Barr JY, Wang X, Meyerholz DK, Lieberman SM. CD8 T cells contribute to lacrimal gland pathology in the nonobese diabetic mouse model of Sjogren syndrome. Immunol Cell Biol 2017; 95:684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lieberman SM, Kreiger PA, Koretzky GA. Reversible lacrimal gland‐protective regulatory T‐cell dysfunction underlies male‐specific autoimmune dacryoadenitis in the non‐obese diabetic mouse model of Sjogren syndrome. Immunology 2015; 145:232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods 2006; 312:12–9. [DOI] [PubMed] [Google Scholar]
- 16. Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren's syndrome. J Autoimmun 2010; 34:400–7. [DOI] [PubMed] [Google Scholar]
- 17. Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL‐18 and Th17 cells in salivary glands of patients with Sjogren's syndrome, and amplification of IL‐17‐mediated secretion of inflammatory cytokines from salivary gland cells by IL‐18. J Immunol 2008; 181:2898–906. [DOI] [PubMed] [Google Scholar]
- 18. Mingueneau M, Boudaoud S, Haskett S, Reynolds TL, Nocturne G, Norton E et al Cytometry by time‐of‐flight immunophenotyping identifies a blood Sjogren's signature correlating with disease activity and glandular inflammation. J Allergy Clin Immunol 2016; 137:1809–21.e12. [DOI] [PubMed] [Google Scholar]
- 19. Robinson CP, Cornelius J, Bounous DE, Yamamoto H, Humphreys‐Beher MG, Peck AB. Characterization of the changing lymphocyte populations and cytokine expression in the exocrine tissues of autoimmune NOD mice. Autoimmunity 1998; 27:29–44. [DOI] [PubMed] [Google Scholar]
- 20. Roescher N, Lodde BM, Vosters JL, Tak PP, Catalan MA, Illei GG et al Temporal changes in salivary glands of non‐obese diabetic mice as a model for Sjögren's syndrome. Oral Dis 2012; 18:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou J, Jin JO, Kawai T, Yu Q. Endogenous programmed death ligand‐1 restrains the development and onset of Sjögren's syndrome in non‐obese diabetic mice. Sci Rep 2016; 6:39105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor α‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. [PubMed] [Google Scholar]
- 23. Hansen CH, Yurkovetskiy LA, Chervonsky AV. Cutting edge: commensal microbiota has disparate effects on manifestations of polyglandular autoimmune inflammation. J Immunol 2016; 197:701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med 1999; 189:877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taguchi O, Kontani K, Ikeda H, Kezuka T, Takeuchi M, Takahashi T et al Tissue‐specific suppressor T cells involved in self‐tolerance are activated extrathymically by self‐antigens. Immunology 1994; 82:365–9. [PMC free article] [PubMed] [Google Scholar]
- 26. Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo . J Immunol 2008; 181:4516–22. [DOI] [PubMed] [Google Scholar]
- 27. Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen‐specific peripheral shaping of the natural regulatory T cell population. J Exp Med 2008; 205:3105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lieberman SM, Kim JS, Corbo‐Rodgers E, Kambayashi T, Maltzman JS, Behrens EM et al Site‐specific accumulation of recently activated CD4+ Foxp3+ regulatory T cells following adoptive transfer. Eur J Immunol 2012; 42:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wheeler KM, Samy ET, Tung KS. Cutting edge: normal regional lymph node enrichment of antigen‐specific regulatory T cells with autoimmune disease‐suppressive capacity. J Immunol 2009; 183:7635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KS. Cutting edge: autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease‐specific regulatory T cells. J Immunol 2008; 180:4366–70. [DOI] [PubMed] [Google Scholar]
- 31. Green EA, Choi Y, Flavell RA. Pancreatic lymph node‐derived CD4+CD25+ Treg cells: highly potent regulators of diabetes that require TRANCE‐RANK signals. Immunity 2002; 16:183–91. [DOI] [PubMed] [Google Scholar]
- 32. Nguyen CQ, Peck AB. Unraveling the pathophysiology of Sjogren syndrome‐associated dry eye disease. Ocul Surf 2009; 7:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cha S, van Blockland SC, Versnel MA, Homo‐Delarche F, Nagashima H, Brayer J et al Abnormal organogenesis in salivary gland development may initiate adult onset of autoimmune exocrinopathy. Exp Clin Immunogenet 2001; 18:143–60. [DOI] [PubMed] [Google Scholar]
- 34. Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA et al Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren's syndrome. Proc Natl Acad Sci U S A 1998; 95:7538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robinson CP, Yamamoto H, Peck AB, Humphreys‐Beher MG. Genetically programmed development of salivary gland abnormalities in the NOD (nonobese diabetic)‐scid mouse in the absence of detectable lymphocytic infiltration: a potential trigger for sialoadenitis of NOD mice. Clin Immunol Immunopathol 1996; 79:50–9. [DOI] [PubMed] [Google Scholar]
- 36. Masago R, Aiba‐Masago S, Talal N, Zuluaga FJ, Al‐Hashimi I, Moody M et al Elevated proapoptotic Bax and caspase 3 activation in the NOD.scid model of Sjogren's syndrome. Arthritis Rheum 2001; 44:693–702. [DOI] [PubMed] [Google Scholar]
- 37. Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjogren's syndrome through fas‐mediated apoptosis. Am J Pathol 1999; 155:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takahashi M, Ishimaru N, Yanagi K, Haneji N, Saito I, Hayashi Y. High incidence of autoimmune dacryoadenitis in male non‐obese diabetic (NOD) mice depending on sex steroid. Clin Exp Immunol 1997; 109:555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kiripolsky J, Shen L, Liang Y, Li A, Suresh L, Lian Y et al Systemic manifestations of primary Sjogren's syndrome in the NOD.B10Sn‐H2(b)/J mouse model. Clin Immunol 2017; 183:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shim GJ, Warner M, Kim HJ, Andersson S, Liu L, Ekman J et al Aromatase‐deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren's syndrome. Proc Natl Acad Sci U S A 2004; 101:12628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan D, Dai R, Karpuzoglu E, Ahmed SA. Estrogen increases, whereas IL‐27 and IFN‐γ decrease, splenocyte IL‐17 production in WT mice. Eur J Immunol 2010; 40:2549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voigt A, Esfandiary L, Nguyen CQ. Sexual dimorphism in an animal model of Sjögren's syndrome: a potential role for Th17 cells. Biol Open 2015; 4:1410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 44. Cihakova D, Talor MV, Barin JG, Baldeviano GC, Fairweather D, Rose NR et al Sex differences in a murine model of Sjogren's syndrome. Ann N Y Acad Sci 2009; 1173:378–83. [DOI] [PubMed] [Google Scholar]
- 45. Harris VM, Sharma R, Cavett J, Kurien BT, Liu K, Koelsch KA et al Klinefelter's syndrome (47,XXY) is in excess among men with Sjogren's syndrome. Clin Immunol 2016; 168:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu K, Kurien BT, Zimmerman SL, Kaufman KM, Taft DH, Kottyan LC et al X chromosome dose and sex bias in autoimmune diseases: increased prevalence of 47,XXX in systemic lupus erythematosus and Sjogren's syndrome. Arthritis Rheumatol 2016; 68:1290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mougeot JC, Noll B, Bahrani Mougeot FK. Sjogren's syndrome X‐chromosome dose effect: an epigenetic perspective. Oral Dis 2018; doi: 10.1111/odi.12825. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48. Toda I, Wickham LA, Sullivan DA. Gender and androgen treatment influence the expression of proto‐oncogenes and apoptotic factors in lacrimal and salivary tissues of MRL/lpr mice. Clin Immunol Immunopathol 1998; 86:59–71. [DOI] [PubMed] [Google Scholar]
- 49. Leimola‐Virtanen R, Salo T, Toikkanen S, Pulkkinen J, Syrjanen S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000; 36:131–7. [DOI] [PubMed] [Google Scholar]
- 50. Richards SM, Sullivan DA. Do genetic alterations in sex steroid receptors contribute to lacrimal gland disease in Sjögren's syndrome? Open Endocrinol J 2009; 3:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Richards SM, Jensen RV, Liu M, Sullivan BD, Lombardi MJ, Rowley P et al Influence of sex on gene expression in the mouse lacrimal gland. Exp Eye Res 2006; 82:13–23. [DOI] [PubMed] [Google Scholar]
- 52. Treister NS, Richards SM, Lombardi MJ, Rowley P, Jensen RV, Sullivan DA. Sex‐related differences in gene expression in salivary glands of BALB/c mice. J Dent Res 2005; 84:160–5. [DOI] [PubMed] [Google Scholar]
- 53. Treister NS, Richards SM, Suzuki T, Jensen RV, Sullivan DA. Influence of androgens on gene expression in the BALB/c mouse submandibular gland. J Dent Res 2005; 84:1187–92. [DOI] [PubMed] [Google Scholar]
- 54. Harvey H. Sexual dimorphism of submaxillary glands in mice in relation to reproductive maturity and sex hormones. Physiol Zool 1952; 25:205–22. [Google Scholar]
- 55. Botts S, Jokinen M, Gaillard ET, Elwell MR, Mann PC. Salivary, harderian, and lacrimal glands In: Maronpot RR, Boorman GA, Gaul BW, eds. Pathology of the Mouse: Reference and Atlas, 1st edn St. Louis, MO: Cache River Press, 1999: 49–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative flow cytometry plots to depict gating strategy for salivary (top) and cervical lymph node (bottom) cell analyses as in Fig. 1.
Figure S2. Testosterone induces changes in salivary gland morphology in non‐obese diabetic (NOD) and non‐obese diabetic– severe combined immunodeficient (NOD‐SCID) mice.


