Summary
The expansion of myeloid‐derived suppressor cells (MDSCs) correlates with tumorigenesis in colorectal cancer (CRC). Here, we found a significant association between CD33+ MDSC number and Yes‐associated protein 1 (YAP1) and phosphatase and tensin homologue (PTEN) levels in CRC patients (P < 0·05). Moreover, the CD33+ MDSCs, YAP1 and PTEN were identified as predictors for the prognosis of CRC patients (P < 0·05). Notably, CD33+ MDSCs were an independent survival predictor for CRC patients through a Cox model analysis. In vitro data determined that the expression levels of YAP1 and PTEN in CRC‐derived cell lines were associated with CRC‐derived MDSC induction, and the blockade of YAP1 and PTEN decreased CRC‐derived MDSC induction. A mechanistic analysis revealed that YAP1 promoted CRC‐derived MDSC induction by suppressing PTEN expression to up‐regulate COX‐2, P‐AKT and P‐p65 in CRC‐derived cells, leading to secretion of the cytokine granulocyte–macrophage colony‐stimulating factor. Our findings establish a novel mechanism of pro‐tumorigenic MDSC induction mediated by ectopic YAP1 and PTEN expression in CRC.
Keywords: colorectal cancer, myeloid‐derived suppressor cell, prognosis, phosphatase and tensin homologue, yes‐associated protein 1
Abbreviations
- COX‐2
cyclo‐oxygenase subunit 2
- CRC
colorectal cancer
- DFS
disease‐free survival
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- HR
hazard ratio
- IL‐1β
interleukin‐1β
- MDSCs
myeloid‐derived suppressor cells
- miR29
microRNA 29
- NLRP3
NACHT, LRR and PYD domains‐containing protein 3
- OS
overall survival
- P‐AKT
phosphorylation of protein kinase B
- PBMC
peripheral blood mononuclear cells
- P‐p65
phosphorylation of REL‐associated protein
- PTEN
phosphatase and tensin homologue
- siRNA
small interfering RNA
- YAP1
yes‐associated protein 1
Introduction
Colorectal cancer (CRC) is one of the most prevalent malignancies worldwide, ranking as the third cause of all cancer‐related deaths.1, 2 The pathogenesis of CRC is complex. The clonal evolution of tumours by sequential mutation causes the genotypic heterogeneity of tumour cells, and many oncogenes and metastasis‐related genes have been identified in CRC.1, 3, 4 Yes‐associated protein 1 (YAP1) is a downstream effector in the Hippo‐YAP signalling pathway. Recent work has verified that YAP1 is critical for the proliferative response observed during intestinal inflammation as well as in sporadic CRC, and YAP1 signalling is generally activated in CRC and CRC‐derived cell lines.5, 6, 7 Meanwhile, the deletion of tumour suppressors, such as phosphatase and tensin homologue (PTEN) or tumour protein p53, is common and has been linked to the aetiology of CRC.8, 9 However, the complex molecular mechanisms of CRC development and progression are not yet fully understood.
The tumour microenvironment outside tumour cells is a complex mixture of tumour‐associated fibroblasts, infiltrating immune cells and signalling molecules, such as cytokines.10, 11 Among the infiltrating immune cells, myeloid‐derived suppressor cells (MDSCs) represent a phenotypically heterogeneous population of immature myeloid cells that support tumours by promoting immunological anergy and tolerance.12, 13 In a previous study, we identified that the MDSC population is expanded in the peripheral blood and tumour tissues of CRC patients, where it promotes tumour cell growth and suppresses T‐cell proliferation.14 Recently, some studies have identified that the ectopic expression of YAP1 and PTEN may be associated with tumour‐promoting inflammation in some cancers, including CRC.15, 16 However, the molecular mechanism remains unclear.
In this study, we explored the correlations between the tumour expression of YAP1 and PTEN and the expansion of CD33+ MDSCs as well as their clinical relevance in CRC patients. We further investigated the molecular mechanisms of pro‐tumorigenic MDSC induction, which is mediated by ectopic YAP1 and PTEN expression in CRC.
Materials and methods
Patients and cell lines
Paraffin‐embedded CRC tissues were collected from 145 newly diagnosed CRC patients who did not accept any preoperative chemoradiotherapy at the time of diagnosis at the Sun Yat‐Sen University Cancer Centre, Guangzhou, China, 2005–2006 (see Supplementary material, Table S1). Among the 145 CRC patients, 84 (57·9%) were male, the median age was 56·4 years (range 20–85 years), 60 (41·4%) were Duke stage A–B, 100 (69·0%) were at a well‐to‐moderate histological stage, 69 (47·6%) exhibited metastasis, 83 (57·2%) showed progressive disease, and 80 (55·2%) had died by the end of the follow‐up period. Written informed consent to participate was obtained from each patient. This study was approved by the Research Ethics Committee of the Sun Yat‐Sen University Cancer Centre. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www.researchdata.org.cn) with the approval RDD number RDDB2017000053.
The human LS174T, LOVO, SW620 and SW480 CRC cell lines were maintained in our laboratory and cultured in RPMI‐1640 medium supplemented with 10% fetal bovine serum (Guangdong Succhi Shiqi Pharmaceutical, Guangdong, China).
Immunohistochemical staining
Serial sections were cut continuously at a thickness of 4 μm. After deparaffinization, sections were analysed by immunohistochemical analysis using a mouse anti‐human CD33 antibody (1 : 50; Abcam, Cambridge, UK), rabbit anti‐human YAP1 antibody (1 : 300; Proteintech, Rosemont, IL) and monoclonal rabbit anti‐human PTEN antibody (1 : 100; Cell Signaling Technology, Danvers, MA). The slides were observed independently by two pathologists. The samples were scored as 0, 1, 2 or 3 when the following percentages of cells were positive for YAP1 or PTEN: < 5% (0 score); ≥ 5% but < 10% (1 score); ≥ 10% but < 50% (2 score); or ≥ 50% (3 score). CD33 staining was evaluated by counting positively stained cells in 10 separate 400× fields. Rabbit IgG1 (DAKO, Copenhagen, Denmark), mouse IgG1 (DAKO) and normal goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) were used as negative controls.
Western blot analysis
Western blot analysis was performed using a rabbit anti‐human YAP1 antibody, rabbit anti‐human YAP1 antibody, rabbit anti‐human P‐AKT (eBioscience, San Diego, CA), rabbit anti‐human P‐p65 (eBioscience), rabbit anti‐human cyclo‐oxygenase subunit 2 (COX‐2) (eBioscience), rabbit anti‐human NACHT, LRR and PYD domains‐containing protein 3 (NLRP3) (Proteintech) or rabbit anti‐human interleukin‐1β (IL‐1β) (Proteintech) at different concentrations. The protein bands were visualized using an ECL detection kit (PerkinElmer Life Science, Waltham, MA). glyceraldehyde‐3‐phosphate dehydrogenase (Cell Signaling Technology) was included as a control.
Functional MDSC induction in vitro, flow cytometry and proliferation assay
CD33+ cells were enriched using human CD33 MicroBeads (Miltenyi Biotec, Bergisch‐Gladbach, Germany) according to the manufacturer's instructions; the cells were separated from the peripheral blood mononuclear cells (PBMCs) of healthy donors. The CD33+ cells were co‐cultured with CRC‐derived SW480, SW620 or LS174T cells in 24‐well plates in a Transwell system (0·4‐μm pore; Corning, New York, NY) at a ratio of 1 : 5 for 48 hr. A total of 1 × 106 MDSCs in serum‐free medium were loaded into the lower chamber; 5 × 106 cancer cells were added to the upper chamber. CD33+ cells maintained in medium alone were used as a control. After 48 hr of incubation, the MDSC population was determined by flow cytometry, as described in our previous study.17 In brief, the MDSC phenotypes were gated within the HLA‐DR− cell population that expressed both the CD33 and CD11b antigens. The stained cells were detected using a Cytomics FC 500 MPL flow cytometry system (Beckman Coulter, Inc., Fullerton, CA) and analysed with CXP software (Beckman Coulter). Human monoclonal antibodies against HLA‐DR−, CD33, CD11b, CD8 and CD4 conjugated with different fluorescent dyes were purchased from BD Pharmingen (San Jose, CA) or eBioscience. To investigate the suppressive function of MDSCs on T‐cell proliferation, the harvested MDSCs were co‐cultured with T cells from the PBMCs (after depleting adhering cells) of healthy donors labelled with carboxyfluorescein succinimidyl ester (Molecular Probes) at a 1 : 1 ratio in an OKT3‐coated plate for 3 days. The fluorescence intensity was then detected by flow cytometry.
Silencing PTEN and YAP1 expression in CRC‐derived cells
YAP1 and PTEN expression in LS174T and SW620 CRC cells was silenced by RNA interference [small interfering RNA (siRNA) ‐PTEN, siRNA‐YAP1]; 21‐nucleotide siRNA duplexes were purchased from GenePharma Bio (Shanghai, China) and transiently transfected into CRC‐derived cells using Lipofectamine 2000 (Invitrogen, Grand Island, NY) following the manufacturer's instructions. An siRNA targeting control gene (siNC) was included in this study. The sequences of siRNA‐PTEN are as follows: siPTEN 001 sense strand 5′‐TGCAGCAATTCACTGTAAA‐3′; siPTEN 002 sense strand 5′‐GAGCGTGCAGATAATGACA‐3′; and siPTEN 003 sense strand 5′‐GGCGCTATGTGTATTATTA‐3′. The sequences of siRNA‐YAP1 are as follows: siYAP1 001 sense strand 5′‐GCGTAGCCAGTTACCAACA‐3′; siYAP1 002 sense strand 5′‐CAGTGGCACCTATCACTCT‐3′; and siYAP1 003 sense strand 5′‐GGTGATACTATCAACCAAA‐3′. In addition, we used a small molecule inhibitor verteporfin (10 nm; Melonepharma, Dalian, LN, China) to inhibit YAP1 function in the LS174T and SW620 CRC cells.
Enzyme‐linked immunosorbent assay
Enzyme‐linked immunosorbent assays for IL‐6, granulocyte–macrophage conlony‐stimulating factor (GM‐CSF), IL‐1β and tumour necrosis factor‐α were performed using human IL‐6 (R&D Systems, Nasdaq, MN), GM‐CSF Instant (R&D Systems) and human IL‐1β kits (R&D Systems) according to the manufacturers’ protocols. The absorption at 450 nm was detected using a 96‐well plate reader (Bio‐Rad, Hercules, CA).
Statistical analyses
All data are presented as the mean ± SEM. The significance of the differences between mean values was determined using SPSS 13·0 software (SPSS, Chicago, IL). Two‐group comparisons were tested using Student's t‐test, and the relationships among PTEN, YAP1, CD33 and clinical pathological features were tested using Pearson's chi‐square test. Optimal cut‐offs for YAP1, PTEN and CD33+ cells were assessed by performing a minimum P‐value estimation using X‐title software (Version 3.6.1, Yale University, New Haven, CT), and relapse after a response, death or the date of the last follow up was used as an endpoint. Using the validation cohort, Kaplan–Meier survival curves were constructed using the log‐rank test to evaluate overall survival (OS) and disease‐free survival (DFS) at the optimal cut‐off point. A Cox multivariate analysis (Cox regression) was performed and included biological markers known to be predictive factors, including Duke stage and histological grade. A value of P < 0·05 was considered significant.
Results
The tumour expression of YAP1 and PTEN in the tumour microenvironment and the correlation with the abundance of CD33+ MDSCs in CRC patients
Our previous study identified that MDSC populations were expanded in the peripheral blood and tumour tissues of CRC patients and promoted tumour progression.14 In the current study, to investigate the related molecular mechanisms of CRC‐induced MDSC expansion in CRC, we first identified increased CD33+ MDSC numbers and YAP1 expression levels along with decreased PTEN expression levels in tumour tissues compared with those of tumour‐adjacent tissues from the same CRC patients (Fig. 1a–d, P < 0·05, n = 40). The density of CD33+ MDSCs was significantly positively correlated with YAP1 expression (P = 0·005, R = 0·205, n = 145) but negatively associated with PTEN expression (P = 0·040, R = −0·152, n = 145). In addition, the level of YAP1 was prominently negatively associated with that of PTEN (P = 0·042, R = −0·149, n = 145) in the CRC microenvironment (Fig. 1e–g).
Figure 1.
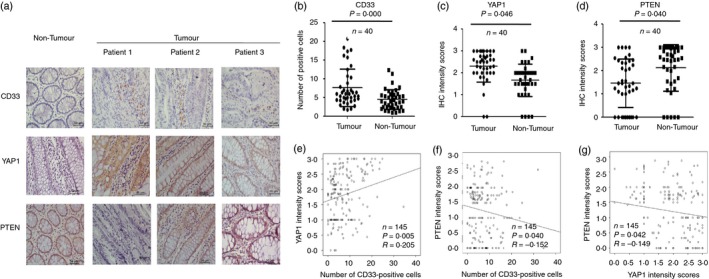
The levels of CD33+ myeloid‐derived suppressor cells (MDSCs) and Yes‐associated protein 1 (YAP1) and phosphatase and tensin homologue (PTEN) expression in patients with colorectal cancer (CRC). (a) Immunohistochemical analysis of CD33, YAP1 and PTEN in tumour and tumour‐adjacent tissues from the same CRC patients. (b–d) Statistical analysis revealed that the levels of CD33+ MDSCs and YAP1 were significantly higher in tumour tissues than in tumour‐adjacent tissues from the same patient, whereas the level of PTEN was significantly lower in tumour tissues than in tumour‐adjacent tissues from the same patient (n = 40, P < 0·05). (e–g) Correlation analysis between YAP1 or PTEN and tumour‐infiltrating CD33+ cells. Our observations indicated that YAP1 expression was significantly positively associated with the number of tumour‐infiltrating CD33+ cells (e, R = 0·205, P = 0·005, n = 145), whereas PTEN expression was significantly negatively associated with the number of tumour‐infiltrating CD33+ cells (g, R = −0·152, P = 0·040, n = 145) and YAP1 expression (f, R = −0·149, P = 0·042, n = 145). The statistical analysis was performed using Pearson's correlation coefficient and linear regression. R, Spearman's correlation; P, significance of correlation.
Relationships among YAP1, PTEN and CD33+ MDSC levels and clinical features
The median density of each immunohistochemical variant was used to separate the patients into high‐level and low‐level groups according to the levels of YAP1, PTEN and CD33+ MDSCs. The correlations among the levels of YAP1, PTEN and CD33+ MDSCs and clinical features are listed in Table 1. The density of CD33+ MDSCs and YAP1 expression were both significantly positively associated with adverse clinical features, including an advanced Duke stage, a poor histological grade and metastasis (P < 0·05), whereas the level of PTEN was significantly associated with favourable clinical features, including an early Duke stage and a good histological grade. In addition, the level of PTEN was related to the sex of the patients; females had a higher level of PTEN than males (P < 0·05).
Table 1.
Associations among tumour tissue phosphatase and tensin homologue (PTEN), Yes‐associated protein 1 (YAP1) and CD33 levels and the clinical parameters of patients with colorectal cancer in 2005
| Clinicopathological parameter | High level of CD33 (%) | P value | High level of YAP1 (%) | P value | High level of PTEN (%) | P value |
|---|---|---|---|---|---|---|
| Age | 0·788 | 0·263 | 0·860 | |||
| < 60 years | 43 (64·1) | 23 (33·3) | 34 (51·5) | |||
| ≥ 60 years | 44 (62·0) | 31 (42·5) | 35 (50·0) | |||
| Gender | 0·576 | 0·264 | 0·014 a | |||
| Female | 35 (60·3) | 20 (32·8) | 36 (63·2) | |||
| Male | 52 (65·0) | 34 (42·0) | 33 (41·8) | |||
| Duke stage | 0·027 a | 0·001 a | 0·023 a | |||
| A–B | 31 (52·5) | 13 (22·0) | 36 (62·1) | |||
| C–D | 56 (70·9) | 41 (49·4) | 33 (42·3) | |||
| Histological grade | 0·010 a | 0·001 a | 0·005 a | |||
| Well‐moderate | 51 (56·0) | 27 (29·0) | 52 (59·8) | |||
| Poor | 34 (79·1) | 25 (58·1) | 15 (34·1) | |||
| Metastasis | 0·000 a | 0·000 a | 0·124 | |||
| Yes | 57 (87·7) | 38 (55·9) | 29 (43·9) | |||
| No | 30 (41·1) | 16 (21·6) | 40 (57·1) |
P < 0·05, as determined by the Pearson χ2 test. High/low PTEN, YAP1, and CD33 values are based on the median density value.
The levels of YAP1, PTEN and CD33+ MDSCs and patient survival
The 10‐year DFS and OS rates for all 145 patients were 38·4% and 44·8%, respectively, in this study (Fig. 2a). When the survival outcomes of patients were compared according to the levels of CD33+ MDSCs and YAP1 expression, the high‐level CD33+ MDSC (63·0% of total) and YAP1 (38·0% of total) expression groups showed poorer DFS and OS than did the low‐level CD33+ MDSC (DFS: P = 0·000; OS: P = 0·000; Fig. 2b,c) and YAP1 (DFS: P = 0·000; OS: P = 0·000; Fig. 2d,e) expression groups. In contrast, when the survival outcomes of patients were compared according to the levels of PTEN expression, the high‐level PTEN (50·7% of total) expression group demonstrated favourable DFS and OS compared with the low‐level PTEN expression group (DFS: P = 0·036; OS: P = 0·037; Fig. 2f,g).
Figure 2.
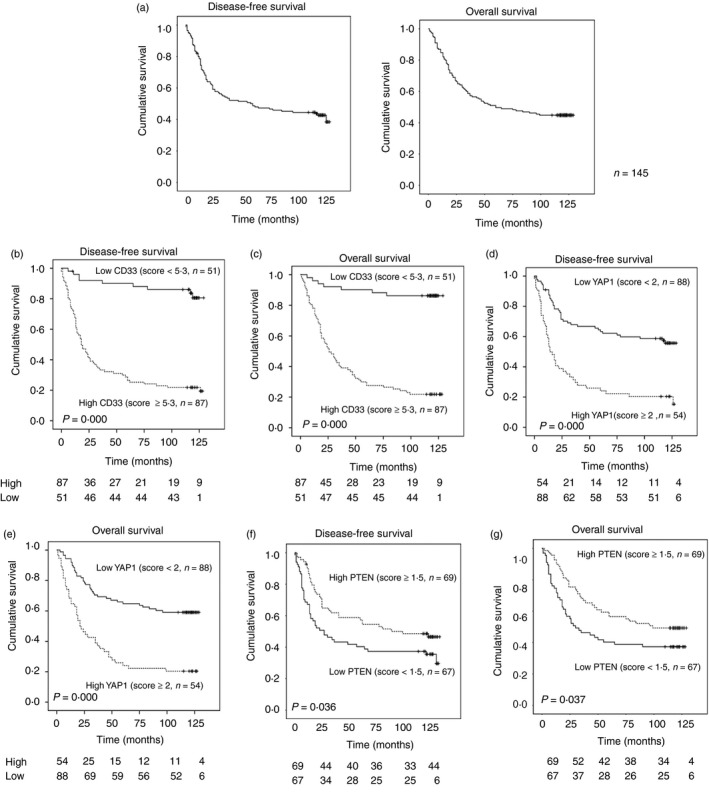
The prognostic values of Yes‐associated protein 1 (YAP1), phosphatase and tensin homologue (PTEN) and CD33+ myeloid‐derived suppressor cells (MDSCs) in patients with colorectal cancer (CRC). (a) The disease‐free survival (DFS) and overall survival (OS) curves of CRC patients in this study. (b–g) Samples from CRC patients were divided into two groups based on low or high expression of the indicated markers, including CD33, YAP1 and PTEN. DFS and OS were significantly negatively associated with the levels of tumour‐infiltrating CD33+ MDSCs (b, DFS: P = 0·000; c, OS: P = 0·000) and YAP1 (d, DFS: P = 0·000; e, OS: P = 0·000) but were positively associated with the level of PTEN (f, DFS: P = 0·036; g, OS: P = 0·037), Cut‐off value = median.
In the univariate analysis of potential prognostic indicators of DFS and OS, in addition to the density of CD33 [P = 0·000; hazard ratio (HR) = 7·95; 95% CI = 3·343–16·03 and P = 0·000; HR = 10·01; 95% CI = 4·573–21·913], YAP1 (P = 0·000; HR = 3·034; 95% CI = 1·956–4·705 and P = 0·000; HR = 3·067; 95% CI = 1·961–4·798) and PTEN (P = 0·039; HR = 0·629; 95% CI = 0·405–0·978 and P = 0·040; HR = 0·625; 95% CI = 0·399–0·979), the Duke stage (P = 0·000; HR = 4·445; 95% CI = 2·625–7·529 and P = 0·000; HR = 3·986; 95% CI = 2·349–6·764), the histological grade (P = 0·000; HR = 0·297; 95% CI = 0·189–0·469 and P = 0·000; HR = 0·275; 95% CI = 0·174–0·435) and the presence of metastasis (P = 0·000; HR = 16·730; 95% CI = 9·082–30·816 and P = 0·000; HR = 13·797; 95% CI = 7·527–25·289) were significant prognostic indicators for the DFS and OS of CRC patients. After adjusting for the listed key clinical prognostic factors and using a multivariate Cox regression analysis (Table 2), the Duke stage (P = 0·018, HR = 2·196, 95% CI = 1·143–4·220 and P = 0·019, HR = 2·197, 95% CI = 1·139–4·237), histological grade (P = 0·004, HR = 0·465, 95% CI = 0·275–0·783 and P = 0·000, HR = 0·341, 95% CI = 0·199–0·585), metastasis (P = 0·000, HR = 7·283, 95% CI = 3·500–15·158 and P = 0·000, HR = 5·455, 95% CI = 2·622–11·350) and CD33 scores (P = 0·001, HR = 4·245, 95% CI = 1·830–9·850 and P = 0·000, HR = 6·582, 95% CI = 2·481–17·460) were independent predictors for the DFS and OS of CRC patients. Taken together, these data indicated that tumour‐infiltrating CD33+ MDSCs had independent prognostic value in CRC patients, but YAP1 and PTEN did not.
Table 2.
Univariate and multivariate Cox regression analyses for the disease‐free survival and overall survival of colorectal cancer patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Overall survival | ||||
| Age (< 60/≥ 60) | 1·121 (0·723–1·737) | 0·611 | ||
| Gender (Female/Male) | 0·693 (0·439–1·095) | 0·116 | ||
| Dukes stage (A–B/C–D) | 3·986 (2·349–6·764) | 0·000 a | 2·370 (1·189–4·721) | 0·014 a |
| Histological grade (Well‐to‐moderate/Poor) | 0·275 (0·174–0·435) | 0·000 a | 0·303 (0·179–0·514) | 0·000 a |
| Metastasis (Yes/No) | 13·797 (7·527–25·289) | 0·000 a | 4·125 (1·943–8·757) | 0·000 a |
| YAP1 score (Low/High) | 3·067 (1·961–4·798) | 0·000 a | 1·166 (0·698–1·949) | 0·558 |
| PTEN score (Low/High) | 0·625 (0·399–0·979) | 0·040 a | 0·672 (0·403–1·122) | 0·129 |
| CD33 score (Low/High) | 10·010 (4·573–21·913) | 0·000 a | 6·582 (2·481–17·460) | 0·000 a |
| Disease‐free survival | ||||
| Age (< 60/≥ 60) | 1·114 (0·724–1·713) | 0·624 | ||
| Gender (Female/Male) | 0·720 (0·461–1·125) | 0·149 | ||
| Dukes stage (A,B/C,D) | 4·445 (2·625–7·529) | 0·000 a | 2·295 (1·165–4·521) | 0·016 a |
| Histological grade (Well‐to‐moderate/Poor) | 0·297 (0·189–0·469) | 0·000 a | 0·441 (0·266–0·732) | 0·002 a |
| Metastasis (Yes/No) | 16·730 (9·082–30·816) | 0·000 a | 6·028 (2·834–12·825) | 0·000 a |
| YAP1 score (Low/High) | 3·034 (1·956–4·705) | 0·000 a | 1·166 (0·692–1·963) | 0·564 |
| PTEN score (Low/High) | 0·629 (0·405–0·978) | 0·039 a | 0·785 (0·469–1·314) | 0·358 |
| CD33 score (Low/High) | 7·950 (3·943–16·030) | 0·000 a | 4·245 (1·830–9·850) | 0·001 a |
CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; OS, overall survival; PTEN, phosphatase and tensin homologue; YAP1, Yes‐associated protein 1.
Significant difference.
CRC‐mediated functional MDSC induction is associated with YAP1 and PTEN expression
We continued to detect the expression of YAP1 and PTEN in CRC cell lines. We found that YAP1 and PTEN were highly expressed in SW620 cells compared with LS174T, LOVO and SW480 cells (Fig. 3a). Therefore, as in our previous study, we analysed the induction of MDSCs mediated by co‐culture with colorectal cells and observed that an increased percentage of CD33+ CD11b+ HLA‐DR− MDSCs was induced by SW620, LS174T or SW480 cells (Fig. 3b). Moreover, the suppression of SW620‐induced and LS174T‐induced MDSCs on the proliferation of T cells, including CD4+ and CD8+ T cells, was stronger than that of control CD33+ cells in medium alone (Fig. 3c).
Figure 3.
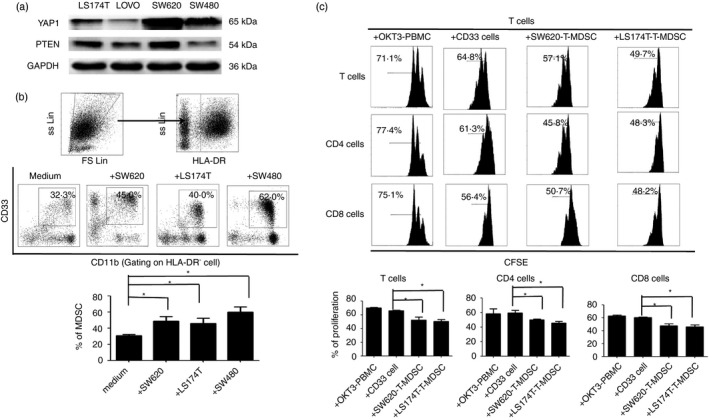
The levels of Yes‐associated protein 1 (YAP1) and phosphatase and tensin homologue (PTEN) in colorectal cancer (CRC) ‐derived cells and their associations with CRC‐mediated myeloid‐derived suppressor cell (MDSC) induction. (a) Western blot analysis showing the expression of YAP1 and PTEN in the CRC cell lines LS174T, LOVO, SW620 and SW480. (b) CD33+ cells were isolated from healthy peripheral blood mononuclear cells using human CD33 MicroBeads and were co‐cultured with the CRC cell lines LS174T, LOVO, SW620 or SW480. Gating strategy for the assessment of the MDSC population used flow cytometry. HLA‐DR− cells were gated from live cells, and CD33+ CD11b+ cells were further gated as MDSCs. The representative dot plots show the percentages of HLA‐DR− CD33+ CD11b+ MDSCs induced from CD33+ cells by co‐culture with LS174T, LOVO, SW620 or SW480 cells in a Transwell system for 48 hr. The CD33+ cells in medium alone were included as a control. (c) FACS density plots and statistical analysis showed that the CRC‐induced MDSCs displayed stronger suppression against T cells, including CD4+ and CD8+ cells. Representative FACS density plots from one of five experiments are shown. *P < 0·05 compared with the control treatment.
YAP1 and PTEN modulate CRC‐derived MDSC induction through regulating GM‐CSF secretion
YAP1 and PTEN expression was blocked by siRNA targeting of YAP1 or PTEN, or the YAP1 inhibitor verteporfin in CRC‐derived cells in this study. We found that the blockade of YAP1 decreased the expression of both YAP1 and PTEN, whereas the blockade of PTEN decreased the expression of PTEN but not YAP1 (Fig. 4a). These observations indicate that YAP1 is an upstream gene of PTEN and can suppress PTEN expression. Moreover, the percentage of CD33+ CD11b+ HLA‐DR− MDSCs induced by SW620 or LS174T cells was decreased with YAP1 knockdown; in contrast, the percentage of CD33+ CD11b+ HLA‐DR− MDSCs induced by SW620 or LS174T cells increased once PTEN was knocked down. Further blockade of PTEN recovered the decrease of CRC‐derived MDSC induction after siYAP1 interference (Fig. 4b, and see Supplementary material, Fig. S1A). These observations indicate that YAP1 promotes CRC‐mediated MDSC induction by suppressing PTEN expression in CRC‐derived cells.
Figure 4.
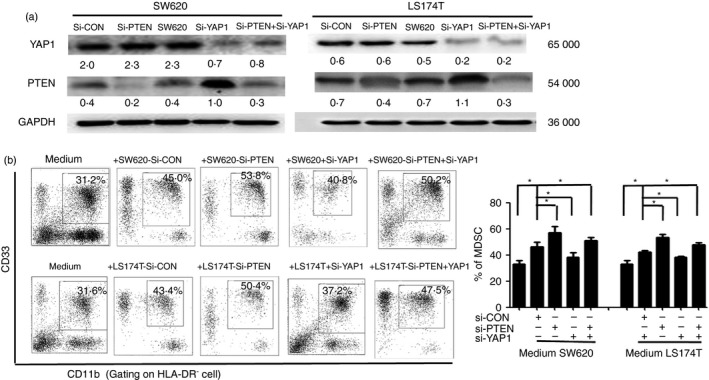
Blockage of Yes‐associated protein 1 (YAP1) or phosphatase and tensin homologue (PTEN) alters the CRC cell‐mediated induction of functional myeloid‐derived suppressor cells (MDSCs) in vitro. (a) The expression of YAP1 and PTEN was knocked down in LS174T and SW620 cells by small interfering RNA (siRNA) targeting YAP1 or PTEN. (b) FACS density plots and statistical analysis showed the percentages of the MDSC population induced by SW620, LS174T, SW620‐siYAP1, LS174T‐siYAP1, SW620‐siPTEN, LS174T‐siPTEN, SW620‐siYAP1 + siPTEN or LS174T‐siYAP1 + siPTEN cell, and the CD33+ cells in medium alone were included as a control. Data are representative of three independent experiments. *P < 0·05 compared with the control treatment.
To further investigate the molecular mechanisms of YAP1‐ and PTEN‐mediated CRC‐induced MDSC expansion, we determined that the secretion of GM‐CSF was decreased in SW620‐siYAP1 and LS174T‐siYAP1 cells but increased in SW620‐siPTEN and LS174T‐siPTEN cells or SW620‐siYAP1, SW620 + verteporfin, LS174T‐siYAP1 or LS74T+verteporfin cells once PTEN was knocked down (Fig. 5a, and see Supplementary material, Fig. S1B). Importantly, the expression of molecules related to MDSC differentiation, including P‐AKT, P‐p65 and COX‐2, was increased in SW620‐siPTEN and LS174T‐siPTEN cells but decreased in SW620‐siYAP1 and LS174T‐siYAP1 cells or verteporfin‐treated cells (Fig. 5b, and see Supplementary material, Fig. S1C). These observations indicated that the activation of YAP1 could suppress PTEN expression in CRC, which could potentially affect CRC‐derived MDSC expansion by regulating the activation of the P‐AKT, P‐p65 and COX‐2 pathways to produce the cytokine GM‐CSF (Fig. 5c).
Figure 5.
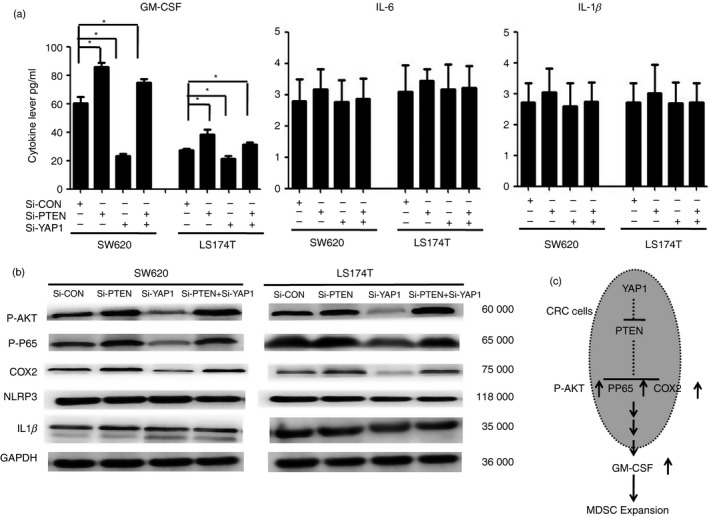
Blockage of Yes‐associated protein 1 (YAP1) or phosphatase and tensin homologue (PTEN) alters granulocyte–macrophage colony‐stimulating factor (GM‐CSF) secretion and is associated with the activation of P‐AKT, P‐p65 and COX‐2. (a) ELISA array for the levels of GM‐CSF, interleukin‐6 (IL‐6) and IL‐1β in the supernatants of LS174T and SW620 cell cultures with or without the presence of small interfering RNA (siRNA) ‐PTEN, siRNA‐YAP1 or siRNA‐PTEN +siRNA‐YAP1. The data are derived from three independent experiments. (b) Western blot analysis showed that P‐AKT, P‐p65, and COX‐2 expression, but not NLRP3 and IL‐1β expression, was increased with the blockage of YAP1 or PTEN. Representative data are shown; the experiment was repeated five times. (c) The signalling pathway of YAP1 and PTEN regulates tumour‐associated myeloid‐derived suppressor cell (MDSC) expansion in CRC.
Discussion
In our previous study, we found that the MDSC population was expanded in the peripheral blood and tumour tissues of CRC patients; in turn, these MDSCs inhibited T‐cell proliferation and promoted CRC cell growth in vitro.14 In the present study, we further demonstrated that the density of CD33+ MDSCs was correlated with the expression of YAP1 and PTEN in the CRC microenvironment; furthermore, all these factors were predictors for the DFS and OS of CRC patients. Importantly, the CD33+ MDSC density is an independent factor of poorer CRC patient survival. The associations between ectopic YAP1 and PTEN expression and MDSC induction were further determined using an in vitro analysis, which revealed an association with the activation of the MDSC‐related molecules COX‐2, P‐AKT and P‐p65 by the up‐regulation of YAP1 and the subsequently induced down‐regulation of PTEN, resulting in the secretion of GM‐CSF from CRC‐derived cells. Overall, these findings suggest that aside from their direct oncogenic role in CRC, YAP1 and PTEN are involved in tumour development through promoting pro‐tumorigenic MDSC expansion.
The MDSC expansion and the resulting suppression of anti‐tumour immunity is a general phenomenon in some cancer types, such as CRC.14, 18 However, the phenotype and induction of MDSCs varies extensively among different cancer types, and the molecular mechanism of MDSC induction has remained largely unclear until now. Here, we first demonstrated three associations among CD33+ MDSC, YAP1 and PTEN levels in clinical specimens from CRC patients: the number of CD33+ MDSCs was positively associated with the level of YAP1 (P = 0·005) but negatively associated with the level of PTEN (P = 0·040), and the level of YAP1 was negatively correlated with that of PTEN (P = 0·042) in the CRC microenvironment. We further demonstrated a strong, positive correlation between tumour‐infiltrating CD33+ MDSC and YAP1 levels and advanced clinicopathological features, including the Duke stage, histological grade and metastatic status (P < 0·05), all of which are signatures of a poor clinical outcome. However, a negative correlation was found between the PTEN level and advanced clinicopathological features, including the Duke stage and histological grade (P < 0·05). Hence, CD33+ MDSCs and YAP1 were predictors for reduced DFS and OS (P < 0·05), whereas PTEN was a predictor for favourable DFS and OS (P < 0·05). However, after adjusting the Cox model to include histological grade, metastatic status and CD33+ MDSC, YAP1 and PTEN levels, only the CD33+ MDSC level was an independent predictor in CRC patients. These data indicate that tumour YAP1 and PTEN expression might mediate CRC tumorigenesis in association with the induction of MDSCs in the tumour microenvironment. To date, the contributions of MDSCs, YAP1 and PTEN to tumour prognosis in CRC have not been verified; hence, this report is the first to describe the correlations among MDSCs, YAP1 and PTEN and their prognostic values in CRC patients.
YAP1 is activated in CRC‐derived cells and CRC and is correlated with CRC cell growth and proliferation,19 and low PTEN expression has been identified in CRC tissues and CRC‐derived cell lines and is also associated with tumour progression.8, 9 The down‐regulation of PTEN in CRC tissues is reported to be induced by PTEN gene mutation or deletion.20 However, a recent study demonstrated that YAP1 down‐regulates PTEN expression by inducing microRNA 29 (miR29) to inhibit PTEN translation.21 Here, we found that the levels of YAP1 and PTEN expression in CRC‐derived cells reflected the induction of functional CRC‐mediated MDSCs in vitro. Furthermore, our data showed that YAP1 and PTEN are involved in CRC‐derived MDSC induction, and YAP1 promotes CRC‐derived MDSC induction by suppressing PTEN expression. In melanoma patients, the loss of PTEN in tumour cells is linked to resistance to T‐cell‐based immunotherapies and inferior outcomes of anti‐PD‐1 immunotherapy, which are associated with immunosuppression in the tumour microenvironment.22, 23, 24 In a murine model, miRs, including miR‐155, miR‐21 and miR‐494, can inhibit MDSC expansion and activity by targeting PTEN.15, 25, 26 Importantly, recent studies have revealed that YAP1 is activated in prostate cancer and drives MDSC recruitment through heterotypic CXCL5‐CXCR2 signalling.15 In addition, YAP1 suppresses PTEN expression by inducing miR‐29 to inhibit PTEN translation, which is associated with the regulation of cell growth and proliferation.27
In the present study, we first identified that YAP1 can modulate tumour‐associated MDSC induction through suppressing PTEN expression to activate P‐AKT, P‐p65 and COX‐2 and subsequently induce the secretion of GM‐CSF, which is associated with the expansion of MDSCs in CRC (Fig. 5c). In addition to their direct contributions to oncogenesis in CRC, ectopic YAP1 and PTEN expression may lead to immunosuppression by inducing CRC‐derived MDSC expansion. These findings indicate, for the first time, a novel molecular mechanism for MDSC expansion in CRC and may guide the future development of therapeutic strategies for CRC patients.
Disclosures
The authors declare that they have no conflicts of interest to disclose.
Supporting information
Figure S1. Blockade of Yes‐associated protein 1 by verteporfin inhibitor increased colorectal cancer‐derived myeloid‐derived suppressor cell induction through the up‐regulation of phosphatase and tensin homologue expression.
Table S1. General data of patients with colorectal cancer.
Acknowledgements
This work was supported by grants from the General Programme (Grant Nos. 81372442 and 81172164, JL) of the National Natural Science Foundation of China and the Guangdong Province Science Foundation (Grant No. 2014A020212066, JL).
Contributor Information
Xiao‐shi Zhang, Email: zhaoxsh@sysucc.org.cn.
Gang Ma, Email: magang@sysucc.org.cn.
Jiang Li, Email: lijiang2@mail.sysu.edu.cn.
References
- 1. Pietrzyk L. Biomarkers discovery for colorectal cancer: a review on tumor endothelial markers as perspective candidates. Dis Markers 2016; 2016:4912405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Zeng H, Zhang S. The incidence and mortality of major cancers in China, 2012. Chin J Cancer 2016; 35:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer 2012; 106:1713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson PM, Labonte MJ, Lenz HJ. Molecular markers in the treatment of metastatic colorectal cancer. Cancer J 2010; 16:262–72. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Wang G, Yang Y, Mei Z, Liang Z, Cui A et al Increased TEAD4 expression and nuclear localization in colorectal cancer promote epithelial–mesenchymal transition and metastasis in a YAP‐independent manner. Oncogene 2016; 35:2789–800. [DOI] [PubMed] [Google Scholar]
- 6. Liang K, Zhou G, Zhang Q, Li J, Zhang C. Expression of hippo pathway in colorectal cancer. Saudi J Gastroenterol 2014; 20:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konsavage WM Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/β‐catenin signaling regulates Yes‐associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem 2012; 287:11730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shacham S, Kauffman M, Sandanayaka V, Draetta G, Shechter S, Williams J et al Preclinical development of small‐molecule CRM1 inhibitors as novel therapy for the treatment of colorectal cancer (CRC). J Clin Oncol 2011; 29:430. [Google Scholar]
- 9. Abubaker J, Bavi P, Al‐Harbi S, Ibrahim M, Siraj AK, Al‐Sanea N et al Clinicopathological analysis of colorectal cancers with PIK3CA mutations in Middle Eastern population. Oncogene 2008; 27:3539–45. [DOI] [PubMed] [Google Scholar]
- 10. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghiringhelli F, Apetoh L, Housseau F, Kroemer G, Zitvogel L. Links between innate and cognate tumor immunity. Curr Opin Immunol 2007; 19:224–31. [DOI] [PubMed] [Google Scholar]
- 12. Marvel D, Gabrilovich DI. Myeloid‐derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015; 125:3356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer‐induced immunosuppressive cells. Cancer Res 2014; 74:2663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. OuYang LY, Wu XJ, Ye SB, Zhang RX, Li ZL, Liao W et al Tumor‐induced myeloid‐derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J Transl Med 2015; 13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S et al Targeting YAP‐dependent MDSC infiltration impairs tumor progression. Cancer Discov 2016; 6:80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Q, Zhang L, Li L, Wang Z, Ying J, Fan Y et al Interferon regulatory factor 8 functions as a tumor suppressor in renal cell carcinoma and its promoter methylation is associated with patient poor prognosis. Cancer Lett 2014; 354:227–34. [DOI] [PubMed] [Google Scholar]
- 17. Li ZL, Ye SB, OuYang LY, Zhang H, Chen YS, He J et al COX‐2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid‐derived suppressor cells. Oncoimmunology 2015; 4:e1044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G et al CCL2 promotes colorectal carcinogenesis by enhancing polymorphonuclear myeloid‐derived suppressor cell population and function. Cell Rep 2015; 12:244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang H, Ramakrishnan SK, Triner D, Centofanti B, Maitra D, Gyorffy B et al Tumor‐selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci Signal 2015; 8:ra98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M et al Crucial role of p53‐dependent cellular senescence in suppression of PTEN‐deficient tumorigenesis. Nature 2005; 436:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N et al YAP mediates crosstalk between the Hippo and PI3K‐TOR pathways by suppressing PTEN via miR‐29. Nat Cell Biol 2012; 14:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Homet Moreno B, Zaretsky JM, Garcia‐Diaz A, Tsoi J, Parisi G, Robert L et al Response to programmed cell death‐1 blockade in a murine melanoma syngeneic model requires costimulation, CD4, and CD8 T cells. Cancer Immunol Res 2016; 4:845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT et al Loss of PTEN promotes resistance to T cell‐mediated immunotherapy. Cancer Discov 2016; 6:202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D et al Effects of MAPK and PI3K pathways on PD‐L1 expression in melanoma. Clin Cancer Res 2014; 20:3446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Zhang J, Diao W, Wang D, Wei Y, Zhang CY et al MicroRNA‐155 and MicroRNA‐21 promote the expansion of functional myeloid‐derived suppressor cells. J Immunol 2014; 192:1034–43. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F et al MicroRNA‐494 is required for the accumulation and functions of tumor‐expanded myeloid‐derived suppressor cells via targeting of PTEN. J Immunol 2012; 188:5500–10. [DOI] [PubMed] [Google Scholar]
- 27. McCarthy N. Signalling: YAP, PTEN and miR‐29 size each other up. Nat Rev Cancer 2013; 13:4–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Blockade of Yes‐associated protein 1 by verteporfin inhibitor increased colorectal cancer‐derived myeloid‐derived suppressor cell induction through the up‐regulation of phosphatase and tensin homologue expression.
Table S1. General data of patients with colorectal cancer.


