Summary
Premature aging of both CD4+ regulatory T (Treg) and CD4+ responder‐T (Tresp) cells in patients with end‐stage renal disease (ESRD) is expected to affect the success of later kidney transplantation. Both T‐cell populations are released from the thymus as inducible T‐cell co‐stimulator‐positive (ICOS +) and ICOS − recent thymic emigrant (RTE) Treg/Tresp cells, which differ primarily in their proliferative capacities. In this study, we analysed the effect of ESRD and subsequent renal replacement therapies on the differentiation of ICOS + and ICOS − RTE Treg/Tresp cells into ICOS + CD31− or ICOS − CD31− memory Treg/Tresp cells and examined whether diverging pathways affected the suppressive activity of ICOS + and ICOS − Treg cells in co‐culture with autologous Tresp cells. Compared with healthy controls, we found an increased differentiation of ICOS + RTE Treg/Tresp cells and ICOS − RTE Treg cells through CD31+ memory Treg/Tresp cells into CD31− memory Treg/Tresp cells in ESRD and dialysis patients. In contrast, ICOS − RTE Tresp cells showed an increased differentiation via ICOS − mature naive (MN) Tresp cells into CD31− memory Tresp cells. Thereby, the ratio of ICOS + Treg/ICOS + Tresp cells was not changed, whereas that of ICOS − Treg/ICOS − Tresp cells was significantly increased. This differentiation preserved the suppressive activity of both Treg populations in ESRD and partly in dialysis patients. After transplantation, the increased differentiation of ICOS + and ICOS − RTE Tresp cells proceeded, whereas that of ICOS + RTE Treg cells ceased and that of ICOS − RTE Treg cells switched to an increased differentiation via ICOS − MN Treg cells. Consequently, the ratios of ICOS + Treg/ICOS + Tresp cells and of ICOS − Treg/ICOS − Tresp cells decreased significantly, reducing the suppressive activity of Treg cells markedly. Our data reveal that an increased tolerance‐inducing differentiation of ICOS + and ICOS − Treg cells preserves the functional activity of Treg cells in ESRD patients, but this cannot be maintained during long‐term renal replacement therapy.
Keywords: end‐stage renal disease, immunosenescence, regulatory T cells, renal replacement therapy, T‐cell differentiation
Abbreviations
- ESRD
end‐stage renal disease
- ICOS
inducible T‐cell co‐stimulator
- MN
mature naive
- RTE
recent thymic emigrant
- Treg
regulatory T
- Tresp
responder T
Introduction
Chronic kidney disease is characterized by a progressive loss of renal function. The final stage, classified as end‐stage renal disease (ESRD), requires replacement therapies, which cannot completely restore renal function. Consequently, the accumulation of uraemic toxins favours the occurrence of immunological disorders,1, 2, 3 with the T‐cell compartment being most affected.4, 5, 6, 7, 8, 9, 10, 11 A lower thymic output and therefore an increased differentiation and premature aging of the T‐cells are already documented.12, 13, 14, 15 Thereby, both responder T (Tresp) and regulatory T (Treg) cells,16 which normally suppress the function of activated Tresp cells, are affected. Kidney transplantation is the preferred method of renal replacement therapy, as, compared with dialysis, it reduces mortality and improves long‐term survival.17, 18 In particular, it was suggested that chronic inflammation was improved by restoring kidney function.19 However, with increasing time after transplantation, most transplant recipients revert to renal insufficiency.20 As the advanced aging of the T cells cannot be reversed, a further impairment of the T‐cell compartment has to be expected.21 .
Currently, the effects of renal insufficiency and its recovery on the differentiation and functionality of T cells are not sufficiently understood. Different subsets of Treg and Tresp cells are likely to be influenced differently. The most prominent naturally occurring Treg cell populations differ concerning their expression of the inducible T‐cell co‐stimulator (ICOS) molecule, which is already expressed in the thymus. Their induction by dendritic cells, proliferation and survival were shown to be regulated differentially.22, 23 In addition to its expression on Treg cells, the ICOS molecule is also expressed on Tresp cells and it was shown that both ICOS+ Treg/Tresp cells and ICOS− Treg/Tresp cells are released from the thymus as CD45RA+ CD31+ recent thymic emigrant (RTE) Treg/Tresp cells.24 Normally, RTE T cells undergo peripheral post‐thymic proliferation to form a long‐living CD45RA+ CD31− mature naive (MN) T‐cell population, which has the capacity to maintain the naive T‐cell pool throughout adult life.25, 26 After stimulation with appropriate antigens, both RTE and MN T cells differentiate into memory T cells.27 Recently, we demonstrated that different differentiation pathways affect the suppressive activity of Treg cells on autologous Tresp cells differentially and therefore had a decisive role for the maintenance of Treg cell function during both pregnancy and renal insufficiency.28, 29
In this study, we examined the influence of renal insufficiency and the subsequent renal replacement therapies on the differentiation of ICOS+ and ICOS− Treg/Tresp cells. We show that different pathways have an influence on the composition of the total CD4+ T helper cell pool with these Treg/Tresp subsets and especially affect the ratios of ICOS+ Treg cells to ICOS+ Tresp cells or of ICOS− Treg cells to ICOS− Tresp cells. Our data reveal that tolerance‐inducing differentiation pathways of both ICOS+ and ICOS− Treg cells preserve the functional activity of Treg cells in patients with ESRD, but this cannot be maintained during renal replacement therapy.
Materials and methods
Patient collectives
For quantitative analysis, peripheral blood samples were collected from 156 healthy volunteers, 49 ESRD patients before initiation of dialysis therapy, 61 dialysis patients and 196 renal transplant recipients. The blood samples from ESRD patients were taken a few days before the start of the dialysis therapy. Blood samples from dialysis patients were obtained at the beginning of the repeated dialysis treatment. These patients did not show recent illness (within the previous 3 months) or significant anaemia and had not received any immunosuppressive drugs. Blood samples from stable kidney transplant patients (inconspicuous creatinine levels and glomerular filtration rate, no clinical signs of renal failure) were collected during routine control checks. Kidney transplant recipients were subdivided into two different groups, one group containing 103 patients who had undergone kidney transplantation < 2 years before participation in the study and one group consisting of 93 recipients > 2 years post transplantation. Exclusion criteria included infection‐induced graft failure, post‐renal obstruction or drug‐induced renal failure. For functional analysis, we collected 10 healthy volunteers, 10 ESRD patients, 10 dialysis patients and 22 kidney transplant recipients (n (< 2 years) = 11 and n (> 2 years) = 11).
Clinical characteristics of all participants are summarized in Table 1. The study was approved by the Regional Ethics Committee. All participants were fully informed of the aim of the study and written informed consent was obtained from all participants.
Table 1.
Clinical characteristics of patients
| Healthy Controls n = 156 | ESRD‐Patients n = 49 | Dialysis‐Patients n = 61 | Transplant recipients < 2 years post‐NTX n = 103 | Transplant recipients > 2 years post‐NTX n = 93 | |
|---|---|---|---|---|---|
| Female sex, n (%) | 96 (62%) | 17 (35%) | 19 (31%) | 38 (37%) | 40 (43%) |
| Age (years) | 42 (18–86) | 53 (20–87) | 58 (25–88) | 50 (20–75) | 48 (20–79) |
| Primary disease, n (%) | |||||
| Diabetes | 5 (10%) | 8 (13%) | 8 (8%) | 4 (4%) | |
| Hypertension | 3 (6%) | 3 (5%) | 5 (5%) | 2 (2%) | |
| GN/vasculitis | 19 (39%) | 17 (28%) | 47 (45%) | 40 (43%) | |
| Interstitial nephritis | 3 (6%) | 1 (2%) | 5 (5%) | 9 (10%) | |
| Polycystic kidney disease | 8 (17%) | 4 (6%) | 20 (19%) | 12 (13%) | |
| Renal malformations | 2 (4%) | 10 (16%) | 4 (4%) | 8 (9%) | |
| Nephrectomy after renal carcinoma | 0 | 3 (5%) | 0 | 1 (1%) | |
| Cardio‐renal syndrome | 2 (4%) | 1 (2%) | 0 | 0 | |
| Obstructive uropathy | 2 (4%) | 3 (5%) | 4 (4%) | 7 (8%) | |
| Others | 3 (6%) | 5 (8%) | 4 (4%) | 6 (6%) | |
| Unknown | 2 (4%) | 6 (10%) | 6 (6%) | 4 (4%) | |
| Dialysis method | – | – | – | ||
| HD, n (%) | 41 (67%) | ||||
| CAPD, n (%) | 20 (33%) | ||||
| Time on dialysis (y) | – | – | 2·26 (0·01–31·0) | 2·33 (0–14·3) | 2·00 (0–14·3) |
| Deceased donor kidney, n (%) | – | – | – | 54 (52%) | 45 (48%) |
| Time since transplantation (years) | – | – | – | 0·25 (0·01–1·96) | 6·67 (2·03–37·7) |
| Induction therapy | |||||
| Basiliximab | 100 (97%) | 55 (59%) | |||
| ATG | 3 (3%) | 8 (9%) | |||
| None | 0 | 30 (32%) | |||
| Immunosuppression | |||||
| Tac + MPA + Steroid | – | – | – | 39 (34%) | 28 (34%) |
| CsA + MPA + Steroid | 45 (35%) | 23 (35%) | |||
| mTOR‐inh. + MPA + Steroid | 3 (10%) | 16 (10%) | |||
| Belatacept + others | 8 (6%) | 3 (6%) | |||
| Others | 8 (15%) | 22 (15%) | |||
| None | 0 | 1 (0%) | |||
| Creatinine (mg/dl) at Treg measurement | < 1·0 | 5·64 (2·0–14·6) | 6·87 (1·9–15·8) | 1·51 (0·76–4·75) | 1·44 (0·73–3·45) |
| Urea (mg/dl) at Treg measurement | < 40 | 166 (55–283) | 123 (50–212) | 51 (16–132) | 58 (15–161) |
| CKD‐EPI GFR (ml/min/1·73 m2) at Treg measurement | > 90 | 9·4 (2·7–22·7) | 6·8 (2·8–36·5) | 48·1 (13·1–88·3) | 50·3 (17·6–107·4) |
The data are presented as their median values together with their range (minimum‐maximum).
ATG, antithymocyte globulin; CKD‐EPI GFR, chronic kidney disease epidemiology collaboration estimated glomerular filtration rate; CsA, cyclosporin; GN, glomerulonephritis; HD, haemodialysis; MPA, mycophenolic acid; mTOR‐inh., mechanistic target of rapamycin‐inhibitor; NTX, renal transplantation; PD, peritoneal dialysis; Tac, tacrolimus.
Fluorescence‐activated cell sorter staining
Venous blood samples (9 ml) from all participants were collected into EDTA‐containing tubes. Whole peripheral blood mononuclear cells were isolated by Lymphodex (Inno‐Train Diagnostik GMBH, Kronberg, Germany) gradient centrifugation and analysed by six‐colour flow cytometric analysis. Briefly, peripheral blood mononuclear cells (8 × 106 cells) were surface‐stained with 10 μl peridinin chlorophyll protein‐conjugated anti‐CD4 (BD Bioscience, Heidelberg, Germany), 5 μl phycoerythrin‐Cy7‐conjugated anti‐CD127 (eBioscience, Frankfurt, Germany), 5 μl Alexa Fluor 647‐conjugated anti‐CD31 (BD Bioscience), 5 μl allophycocyanin (APC‐H7)‐conjugated anti‐CD45RA (BD Bioscience) and 20 μl phycoerythrin‐conjugated anti‐CD278 (ICOS) (BD Bioscience) mouse monoclonal antibodies. Subsequently, intracellular staining was performed for the detection of FoxP3 using a fluorescein isothiocyanate‐labelled anti‐human FoxP3 staining set (clone PCH101; eBioscience), according to the manufacturer's instructions. Negative control samples were incubated with isotype‐matched antibodies. Dead cells were excluded by forward‐ and side‐scatter characteristics. Cells were analysed on a FACS Canto cytometer (BD Bioscience). Statistical analysis was based on at least 100 000 gated CD4+ T cells.
Positive selection of CD4+ CD127low+/− CD25+ Treg cells
Whole peripheral blood mononuclear cells were isolated from 45 ml EDTA‐blood samples by Lymphodex (Inno‐Train Diagnostik GMBH, Kronberg, Germany) gradient centrifugation. CD4+ CD127low+/− CD25+ Treg cells were purified using the Regulatory‐T‐Cell‐Isolation‐Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. First, CD4+ CD127low+/− T cells were isolated by magnetic depletion of non‐CD4+ CD127high+ T cells. In the second step, the CD4+ CD127low+/− CD25+ Treg cells were isolated by positive selection over two consecutive columns. The CD4+ CD127low+/− CD25− T cells were obtained in the flow‐through fraction and used as responder T cells. The CD4+ CD127low+/− CD25+ Treg cells were subsequently retrieved from the columns.
Sorting and functional testing of the different Treg cell subsets
For the sorting of the isolated CD4+ CD127low+/− CD25+ Treg cells into ICOS+ and ICOS− Treg cells, cells were stained with 5 μl APC‐H7‐conjugated anti‐CD45RA and 2 μl phycoerythin‐conjugated anti‐CD278 (ICOS) mouse monoclonal antibodies (BD Bioscience). In all experiments, dead cells were excluded while the remaining CD4+ CD127low+/− CD25+ Treg cells were sorted using a FACS‐Vantage SE‐Sorter or a FACS‐Aria II‐Sorter (BD Bioscience). To analyse the suppressive activity of the isolated ICOS+ Treg and ICOS− Treg populations, 2 × 104 responder T cells were co‐cultured with the purified Treg subsets at ratios of 1 : 2 ‐ 1 : 256 in 96‐well v‐shaped bottom plates. Suppression assays were performed in a final volume of 100 μl/well of X‐VIVO15 medium (Lonza, Verviers, Belgium). For T‐cell stimulation, the medium was supplemented with 1 μg/ml anti‐CD3 and 2 μg/ml anti‐CD28 antibodies (eBioscience). As controls, CD4+ CD127low+/− CD25+ Treg cells and Tresp cells alone were cultured both with and without any stimulus. Cells were incubated at 37° in 5% CO2. After four days, 1 μCi [3H]thymidine (Hartmann Analytic, Braunschweig, Germany) was added to the cultures and cells were further incubated for 16 hr. Then, the cells were harvested and 3H incorporation was measured by scintillation counting. Depending on the number of the separated cells, the suppression assays were performed as single or multiple determinations. To compare the suppressive activity of the different Treg cell subsets between the different patient groups the maximum suppressive activity (ratio of Treg cells to Tresp cells 1 : 2) and the minimum ratio of Treg cells to Tresp cells at which a suppression of at least 15% could be achieved was calculated.30
Statistical analysis
Linear regression was used to evaluate the changes with age in the percentages of RTE, MN, CD31+ memory and CD31− memory Treg/Tresp cells within the total ICOS+ and ICOS− Treg/Tresp pool using separate models for all patient groups. The same approach was used for evaluating the influence of age on the composition of the total CD4+ helper cell pool with ICOS+ and ICOS− Treg/Tresp cells as well as for analysing the changes of the ratio of ICOS+ Treg/ICOS+ Tresp cells or ICOS− Treg/ICOS− Tresp cells with age. Differences between the different patient groups concerning the above listed Treg/Tresp subsets, were examined using multiple regression analysis adjusted for the age variable (centred on the mean), wherein an interaction term of the age and the patient group was included.
Comparison of the suppressive activity of purified ICOS+ Treg cells and ICOS− Treg cells between the different age‐matched participant groups was performed using the non‐parametric Wilcoxon–Mann–Whitney U‐test. P < 0·05 was considered significant. For all tests, the software package bias for windows (version 10·06) was used.
Results
Renal replacement therapy restores thymic output of highly proliferative ICOS+ RTE Treg cells, but not of ICOS+ RTE Tresp cells
In this study, we used flow cytometric analysis for comparative investigation of the differentiation of ICOS+ and ICOS− RTE T cells in healthy volunteers, patients with ESRD and patients undergoing subsequent renal replacement therapies. Due to the potentially significant influence of the immunosuppressive therapy in patients undergoing transplantation, we examined both short‐term and long‐term transplant patients. Since the most significant differences in T cell differentiation were observed in patients transplanted less than or more than 2 years prior to the study, we chose a 2‐year cut‐off period. To examine, whether there were differences in the differentiation pathways of ICOS+ and ICOS− Treg/Tresp cells between groups, we calculated the composition of the total ICOS+ and ICOS− Treg/Tresp cell pool with CD45RA+ CD31+ RTE, CD45RA+ CD31− MN, CD45RA− CD31+ memory and CD45RA− CD31− memory Treg/Tresp cells (in the following abbreviated as RTE‐, MN‐, CD31+ memory and CD31− memory Treg/Tresp cells). Figure 1(a–h) shows the gating strategy that was used in all experiments.
Figure 1.
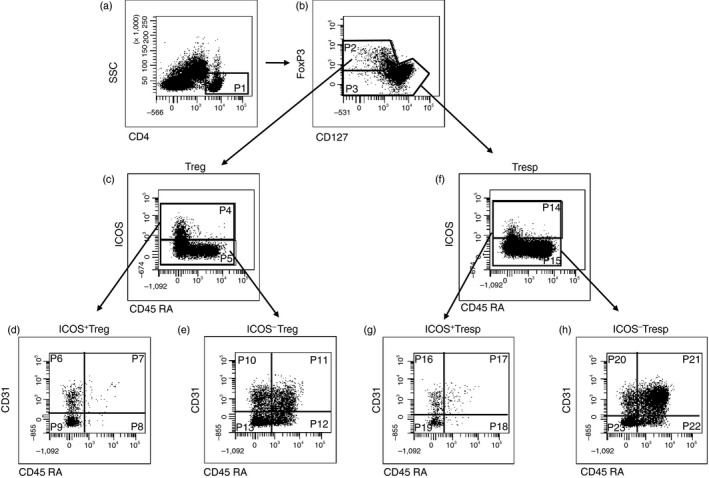
Gating strategy for six‐colour‐flow‐cytometric detection of recent thymic emigrant (RTE), mature naive (MN), CD31+ memory and CD31– memory T cells within total inducible T‐cell co‐stimulator‐positive (ICOS+) and ICOS− regulatory T (Treg)/responder T (Tresp) cells. At first, CD4+ T cells (P1) were gated by fluorescence intensity of CD4 versus side light scatter (SSC) (a). The CD4+ CD127low+/– FoxP3+ Treg cells (P2) and the CD4+ CD127+ FoxP3– Tresp cells (P3) were gated by fluorescence intensity of FoxP3 versus CD127 (b). The ICOS+ (P4) and ICOS− Treg cells (P5) were gated by fluorescence intensity of CD45RA versus ICOS (c). The percentages of RTE Treg cells (P7, P11), MN Treg cells (P8, P12), CD31+ memory Treg cells (P6, P10) and CD31– memory Treg cells (P9, P13) were estimated by analysing the total ICOS+ (P4) and ICOS− Treg pool (P5) for its expression of CD45RA and CD31 (d, e). Moreover, the ICOS+ (P14) and ICOS− Tresp cells (P15) were gated by fluorescence intensity of CD45RA versus ICOS (f). The percentages of RTE Tresp cells (P17, P21), MN Tresp cells (P18, P22), CD31+ memory Tresp cells (P16, P20) and CD31– memory Tresp cells (P19, P23) were estimated by analysing the total ICOS+ (P14) and ICOS− Tresp pool (P15) for its expression of CD45RA and CD31 (g, h).
Figure 2 shows the composition of the total ICOS+ Treg/Tresp pools with ICOS+ RTE, MN, CD31+ memory and CD31− memory T cells in all participants during course of life. Compared with healthy volunteers, we found that the composition of the total ICOS+ Treg/Tresp pool in ESRD patients changed in the way that the percentages of RTE Treg/Tresp, MN Treg/Tresp and CD31+ memory Treg/Tresp cells decreased, whereas the percentages of CD31− memory Treg/Tresp cells increased significantly (Fig. 2a,c). These findings suggest that renal insufficiency is associated with a severe impairment of ICOS+ RTE Treg/Tresp release from the thymus and that the already distributed ICOS+ RTE Treg/Tresp cells increasingly differentiate via highly proliferative ICOS+ CD31+ memory Treg/Tresp cells into ICOS+ CD31− memory Treg/Tresp cells (Fig. 2b,d). Only minor differences were found between men and women (see Supplementary material, Fig. S1). After the initiation of dialysis therapy, the percentages of the naive ICOS+ RTE and/or MN Treg/Tresp cells rose again while the percentages of ICOS+ CD31− memory Treg/Tresp cells decreased markedly. However, compared with healthy volunteers, the percentages of ICOS+ RTE Treg/Tresp cells and particularly of ICOS+ CD31+ memory Tresp cells remained significantly reduced in dialysis patients, whereas the percentages of ICOS+ CD31− memory Treg/Tresp cells were still increased (Fig. 2e,g). These findings propose that ICOS+ RTE Treg cells still show an increased differentiation into ICOS+ CD31− memory Treg cells, presumably through both pathways (Fig. 2f), whereas ICOS+ RTE Tresp cells differentiate increasingly through CD31+ memory Tresp cells into ICOS+ CD31− memory Tresp cells (Fig. 2h). After transplantation, the percentages of naive ICOS+ RTE Treg/Tresp, ICOS+ MN Treg/Tresp and ICOS+ CD31+ memory Treg cells further increased, while the percentages of ICOS+ CD31− memory Treg/Tresp cells decreased strongly. Presumably, thymic function is largely restored after transplantation, as the percentages of naive RTE Treg/Tresp cells are significantly increased or at least equal to those of healthy volunteers (Fig. 2i,k). Consequently, the increased differentiation of ICOS+ RTE Treg cells into ICOS+ CD31− memory Treg cells ceases and is also weakened for ICOS+ RTE Tresp cells (Fig. 2j,l). Surprisingly, 2 years after transplantation, a new intensified differentiation of naive RTE Treg/Tresp cells through ICOS+ CD31+ memory Treg/Tresp cells into ICOS+ CD31− memory Treg/Tresp cells could be observed (Fig. 2m–p). This especially applies to ICOS+ RTE Tresp cells, as the composition of the ICOS+ Tresp pool remains characteristically altered in long‐term transplant patients compared with healthy volunteers (Fig. 2o,p).
Figure 2.
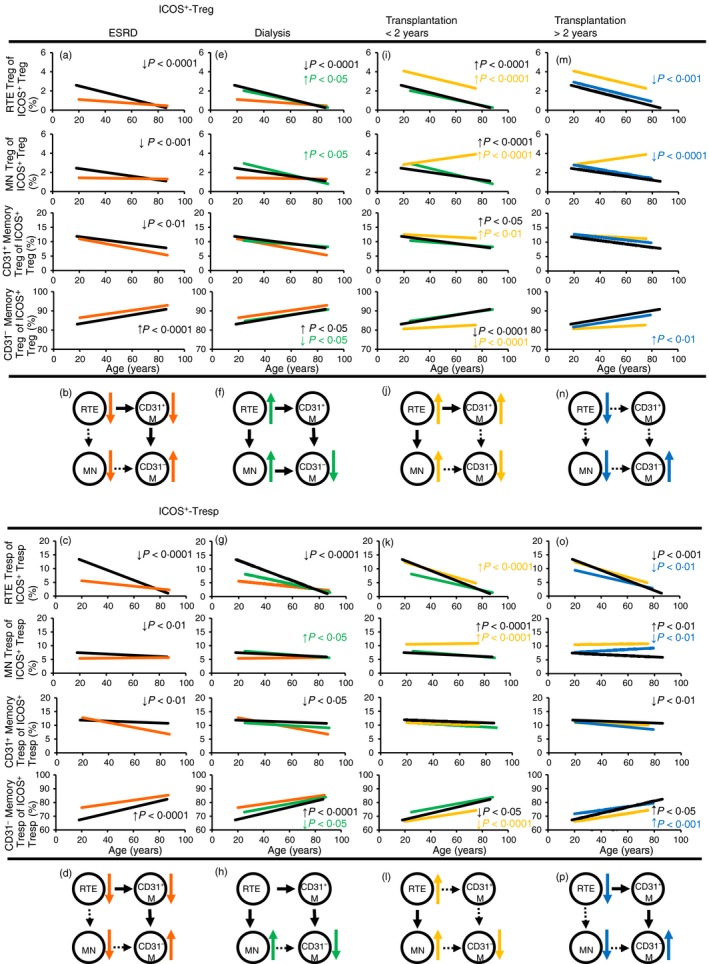
Differentiation of inducible T‐cell co‐stimulator‐positive (ICOS+) regulatory T (Treg)/responder T (Tresp) cells. The percentage of recent thymic emigrant (RTE), mature naive (MN), CD31+ memory and CD31– memory Treg/Tresp cells within total ICOS+ Treg and ICOS+ Tresp cells was estimated in healthy volunteers ( ) compared with end‐stage renal disease (ESRD) patients (
) compared with end‐stage renal disease (ESRD) patients ( ) (a, c), ESRD patients compared with dialysis patients (
) (a, c), ESRD patients compared with dialysis patients ( ) (e, g), dialysis patients compared with transplant recipients 2 years after transplantation (
) (e, g), dialysis patients compared with transplant recipients 2 years after transplantation ( ) (i, k) and transplant recipients < 2 years compared with transplant recipients > 2 years post transplantation (
) (i, k) and transplant recipients < 2 years compared with transplant recipients > 2 years post transplantation ( ) (m, o). The figures present the regression lines concerning the changes in the percentages of the individual Treg/Tresp subsets with increasing age. Significant differences between the study groups and healthy volunteers are marked by black P‐values. Significant differences between the different study groups are marked in the corresponding colours. A significant decrease/increase is marked by an arrow (
) (m, o). The figures present the regression lines concerning the changes in the percentages of the individual Treg/Tresp subsets with increasing age. Significant differences between the study groups and healthy volunteers are marked by black P‐values. Significant differences between the different study groups are marked in the corresponding colours. A significant decrease/increase is marked by an arrow ( ). The proposed differentiation pathway of ICOS+ RTE Treg/Tresp cells is shown in (b) and (d) for ESRD patients, in (f) and (h) for dialysis patients, in (j) and (l) for patients transplanted for < 2 years and in (n) and (p) for patients transplanted for > 2 years.
). The proposed differentiation pathway of ICOS+ RTE Treg/Tresp cells is shown in (b) and (d) for ESRD patients, in (f) and (h) for dialysis patients, in (j) and (l) for patients transplanted for < 2 years and in (n) and (p) for patients transplanted for > 2 years.
Severely diminished release of ICOS− RTE T cells from the thymus in ESRD patients cannot be restored during renal replacement therapy
Similar to ICOS+ RTE Treg/Tresp cells, ICOS− RTE Treg/Tresp cells also revealed an impaired thymic output and an increased differentiation into ICOS− CD31− memory Treg/Tresp cells in ESRD patients compared with healthy volunteers. Although ICOS− RTE Treg cells differentiated through ICOS− CD31+ memory Treg cells into ICOS− CD31− memory Treg cells (Fig. 3a,b), the ICOS− RTE Tresp cells differentiated through both ICOS− MN and ICOS− CD31+ memory Tresp cells into ICOS− CD31− memory Tresp cells (Fig. 3c,d). Differences between men and women were not detected (see Supplementary material, Fig. S1). After initiation of dialysis, ICOS− MN Treg cells accumulate again within total ICOS− Treg cells, whereas ICOS− CD31− memory Treg cells decrease significantly (Fig. 3e). However, compared with healthy volunteers, an increased differentiation of ICOS− RTE Treg cells via ICOS− CD31+ memory Treg cells is still detectable in dialysis patients (Fig. 3f). Compared with ESRD patients, no significant changes concerning the composition of the ICOS− Tresp pool could be shown for dialysis patients (Fig. 3g). Yet, compared with healthy volunteers, the percentage of ICOS− RTE Tresp cells was significantly decreased, that of ICOS− CD31+ memory Tresp cells did not change and those of ICOS− MN Tresp cells and ICOS− CD31− memory Tresp cells were increased (Fig. 3g). These findings propose an enhanced differentiation of ICOS− RTE Tresp cells via ICOS− MN Tresp cells into ICOS− CD31− memory Tresp cells in dialysis patients (Fig. 3h). After transplantation, both the percentages of ICOS− RTE Treg/Tresp cells increase, whereas those of ICOS− CD31− memory Treg/Tresp cells decrease significantly, indicating that the increased differentiation of ICOS− RTE Treg cells via ICOS− CD31+ memory Treg cells, or ICOS− RTE Tresp cells via ICOS− MN Tresp cells is attenuated (Fig. 3i–l). However, in spite of the redistribution of ICOS− RTE Treg/Tresp cells after transplantation, their percentages never reach those of healthy volunteers. Two years after transplantation, the percentages of ICOS− MN Treg/Tresp cells decrease significantly, whereas the percentages of both CD31+ and CD31− memory Treg/Tresp cells increase strongly (Fig. 3m,o), indicating that RTE Treg/Tresp cells produce less proliferative CD31+ memory Treg/Tresp cells. Consequently, a strengthened differentiation of MN Treg/Tresp cells occurs (Fig. 3n,p).
Figure 3.
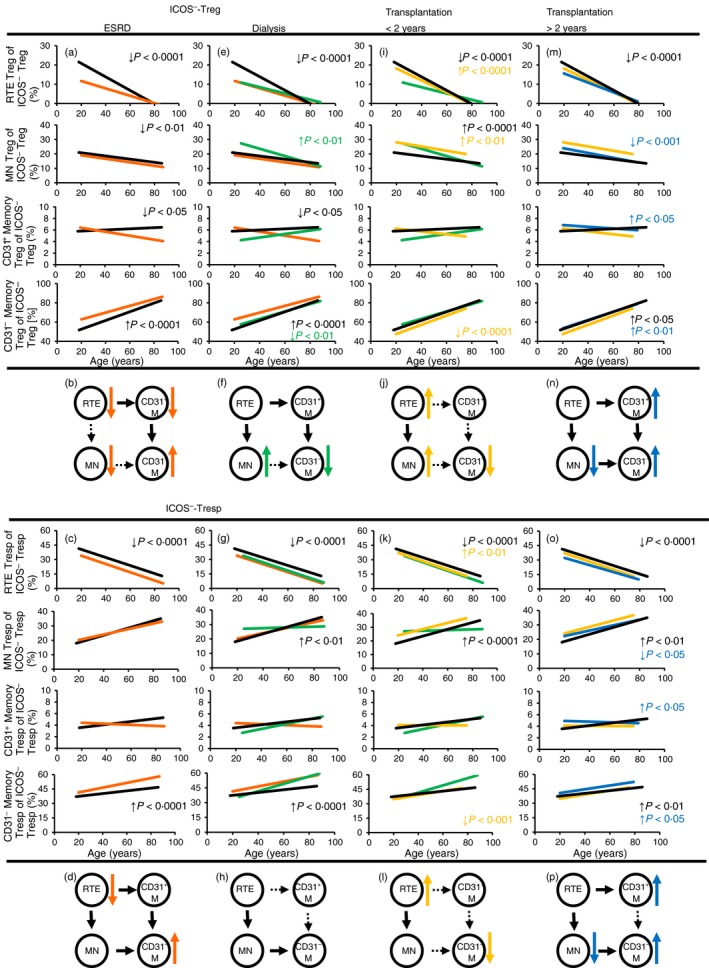
Differentiation of inducible T‐cell co‐stimulator‐negative (ICOS−) regulatory T (Treg)/responder T (Tresp) cells. The percentage of recent thymic emigrant (RTE), mature naive (MN), CD31+ memory and CD31− memory Treg/Tresp cells within total ICOS− Treg and ICOS− Tresp cells was estimated in healthy volunteers ( ) compared with end‐stage renal disease (ESRD) patients (
) compared with end‐stage renal disease (ESRD) patients ( ) (a, c), ESRD patients compared with dialysis patients (
) (a, c), ESRD patients compared with dialysis patients ( ) (e, g), dialysis patients compared with transplant recipients 2 years after transplantation (
) (e, g), dialysis patients compared with transplant recipients 2 years after transplantation ( ) (i, k) and transplant recipients < 2 years compared with transplant recipients > 2 years post transplantation (
) (i, k) and transplant recipients < 2 years compared with transplant recipients > 2 years post transplantation ( ) (m, o). The figures present the regression lines concerning the changes in the percentages of the individual Treg/Tresp subsets with increasing age. Significant differences between the study groups and healthy volunteers are marked by black P‐values. Significant differences between the different study groups are marked in the corresponding colours. A significant decrease/increase is marked by an arrow (
) (m, o). The figures present the regression lines concerning the changes in the percentages of the individual Treg/Tresp subsets with increasing age. Significant differences between the study groups and healthy volunteers are marked by black P‐values. Significant differences between the different study groups are marked in the corresponding colours. A significant decrease/increase is marked by an arrow (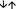 ). The proposed differentiation pathway of ICOS− RTE Treg/Tresp cells is shown in (b) and (d) for ESRD patients, in (f) and (h) for dialysis patients, in (j) and (l) for patients transplanted for < 2 years and in (n) and (p) for patients transplanted for > 2 years.
). The proposed differentiation pathway of ICOS− RTE Treg/Tresp cells is shown in (b) and (d) for ESRD patients, in (f) and (h) for dialysis patients, in (j) and (l) for patients transplanted for < 2 years and in (n) and (p) for patients transplanted for > 2 years.
In summary, our results propose that ESRD leads to considerably diminished release of RTE T cells causing their increased differentiation. In contrast to ICOS+ RTE T cells, the redistribution of ICOS− RTE T cells cannot be sufficiently achieved after transplantation. This especially affects the differentiation pathway of ICOS− RTE Treg cells, which switch their differentiation via CD31+ memory Treg cells to an alternative differentiation via MN Treg cells. The differentiation pathways of ICOS+ RTE Treg/Tresp cells and ICOS− RTE Tresp cells seem to be maintained throughout renal insufficiency and subsequent renal replacement therapies.
Intensified T‐cell differentiation in ESRD and dialysis patients improves the ratio of ICOS– Treg/ICOS– Tresp cells, but this cannot be maintained during renal replacement therapy
To elucidate the influence on the changing Treg/Tresp differentiation on the ratios of ICOS+ Treg to ICOS+ Tresp cells or ICOS− Treg to ICOS− Tresp cells in patients with ESRD and in patients undergoing renal replacement therapy, we estimated the percentages of all four Treg/Tresp subsets within their total CD4+ T helper cell pool compared with healthy volunteers. For ICOS+ Treg/Tresp cells we found a significant decrease in ESRD patients, whereby the ratio of ICOS+ Treg to ICOS+ Tresp cells was not influenced (Fig. 4a). After initiation of dialysis therapy, the percentages of ICOS+ Treg/Tresp cells increased again without affecting their ratio (Fig. 4b). After transplantation, the percentages of ICOS+ Treg/Tresp cells decreased substantially, changing the ratio of ICOS+ Treg cells to ICOS+ Tresp cells in favour of ICOS+ Tresp cells (Fig. 4c). These conditions were maintained even after > 2 years after transplantation (Fig. 4d).
Figure 4.
Composition of the CD4+ T helper cell pool with inducible T‐cell co‐stimulator‐positive (ICOS+) and ICOS− regulatory T (Treg)/responder T (Tresp) cells and ratio of ICOS+ and ICOS− Treg to Tresp cells. The percentages of ICOS+ Treg/Tresp cells and ICOS− Treg/Tresp cells of the total CD4+ T helper cell pool as well as the ratios of ICOS+ Treg to ICOS+ Tresp cells and ICOS− Treg to ICOS− Tresp cells were estimated for healthy volunteers ( ) compared with end‐stage renal disease (ESRD) patients (
) compared with end‐stage renal disease (ESRD) patients ( ) (a, e), compared with dialysis patients (
) (a, e), compared with dialysis patients ( ) (b, f), compared with patients who had undergone transplantation < 2 years ago (
) (b, f), compared with patients who had undergone transplantation < 2 years ago ( ) (c, g) and compared with transplant recipients > 2 years post transplantation (
) (c, g) and compared with transplant recipients > 2 years post transplantation ( ) (d, h). The figures present the regression lines concerning the changes in the percentages of the individual Treg/Tresp subsets with increasing age. Significant differences between the study groups and healthy volunteers are marked by black P‐values. Significant differences in the ratios of Treg to Tresp cells were marked in red P‐values. A significant decrease/increase is marked by an arrow (
) (d, h). The figures present the regression lines concerning the changes in the percentages of the individual Treg/Tresp subsets with increasing age. Significant differences between the study groups and healthy volunteers are marked by black P‐values. Significant differences in the ratios of Treg to Tresp cells were marked in red P‐values. A significant decrease/increase is marked by an arrow (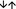 ).
).
For ICOS− Treg/Tresp cells of patients with ESRD, we found an increase of ICOS− Treg cells, whereas ICOS− Tresp cells showed no significant difference, resulting in a significant increase in the ratio of ICOS− Treg to ICOS− Tresp cells (Fig. 4e). After the initiation of dialysis therapy these conditions were maintained (Fig. 4f). However, after transplantation, ICOS− Treg cells decreased sharply so that no significant difference could be detected compared with healthy volunteers. In contrast, ICOS− Tresp cells increased so that the positive ratio of ICOS− Treg cells to ICOS− Tresp cells could not be sustained (Fig. 4g). Another 2 years after transplantation, ICOS− Treg cells were strongly decreased compared with healthy volunteers while ICOS− Tresp cells were significantly increased. Consequently, the ratio of ICOS− Treg cells to ICOS− Tresp cells decreased considerably in these long‐term transplant patients (Fig. 4h).
The suppressive activity of Treg cells is maintained in ESRD patients, but can hardly be preserved during renal replacement therapy
To examine whether there were differences in the suppressive activity of separated ICOS+ and ICOS− Treg cells, the total CD4+ CD127low+/− CD25+ Treg pool of 10 healthy volunteers (mean age 48 ± 9 years), 10 ESRD patients (mean age 50 ± 12 years), 10 dialysis patients (mean age 50 ± 14 years), 11 transplant recipients who were transplanted for > 2 years (mean age 49 ± 10 years) and 11 transplant recipients who were transplanted for < 2 years (mean age 52 ± 13 years) was isolated by magnetic‐activated‐cell‐sorting and sorted into both Treg cell subsets (Fig. 5a). Subsequently, the isolated ICOS+ and ICOS− Treg cells were analysed separately for their suppressive capacity. Compared with healthy volunteers, we found that the suppressive activity of both Treg subsets was maintained in ESRD patients. In dialysis patients, the suppressive activity of both Treg subsets was significantly reduced. For ICOS− Treg cells, a significantly decreased suppressive activity was also revealed for both transplant patient groups (Fig. 5b,c). The suppressive activity of ICOS+ Treg cells could not be determined for transplant patients, due to an extremely low number of ICOS+ Treg cells in these patients.
Figure 5.
Suppressive activity of inducible T‐cell co‐stimulator‐positive (ICOS+) regulatory T (Treg) cells and ICOS− Treg cells. Total CD4+ CD127low+/− CD25+ Treg cells were isolated by magnetic‐activated‐cell‐sorting, stained with anti‐CD45RA and anti‐CD278 monoclonal antibodies and sorted into ICOS− Treg (P1) and ICOS+ Treg (P2) cells (a). The suppressive activity of the different Treg cell subsets was estimated by suppression assays. Blood samples were obtained from age‐matched healthy volunteers (n = 10), end‐stage renal disease (ESRD) patients (n = 10), dialysis patients (n = 10) and transplant recipients < 2 years (n = 11) and > 2 years (n = 11) post transplantation. The figure shows the individual and median values of the maximum suppressive activity (Treg/Tresp = 1/2) (b) and of the ratio of Treg to Tresp cells up to which the purified ICOS+ Treg ( ) and ICOS− Treg (
) and ICOS− Treg ( ) cells could be diluted to achieve a minimum suppressive activity of at least 15% (c). Compared with healthy volunteers, the suppressive activity of both Treg subsets is maintained in ESRD patients, but is significantly decreased in dialysis and transplant recipients.
) cells could be diluted to achieve a minimum suppressive activity of at least 15% (c). Compared with healthy volunteers, the suppressive activity of both Treg subsets is maintained in ESRD patients, but is significantly decreased in dialysis and transplant recipients.
Discussion
The mechanisms leading to altered T‐cell differentiation in ESRD patients are poorly understood. Meanwhile, these alterations, which correspond to a prematurely differentiated T‐cell system, are expected to have consequences for the success of later kidney transplantation.31 The most obvious symptom in ESRD patients is T‐cell lymphopenia, due to a selective loss of circulating naive T cells and memory T cells attributed to increased activation‐induced apoptosis.6, 32, 33 Currently it is not known whether Treg and Tresp cells are equally affected. In this study, we show that the suppressive Treg cell function is initially completely preserved in ESRD patients, but that it is lost during the subsequent long‐term renal replacement procedures. With regard to the fact that ESRD affects the thymic output of RTE Treg cells and RTE Tresp cells, we examined whether there were differences in the differentiation pathways of already distributed highly proliferative ICOS+ RTE Treg/Tresp cells and less proliferative ICOS− RTE Treg/Tresp cells, which could explain this phenomenon. Figure 6 summarizes the changes in the differentiation of these different T‐cell subsets from the stage of ESRD to transplantation. In ESRD patients, we found that all RTE Treg/Tresp subsets differentiated strongly via CD31+ memory Treg/Tresp cells into CD31− memory Treg/Tresp cells with the exception of ICOS− RTE Tresp cells, which differentiated intensely through both ICOS− MN Tresp cells and CD31+ memory Tresp cells into ICOS− CD31− memory Tresp cells. As the differentiation via CD31+ memory Treg/Tresp cells presumably produces apoptotically more stable CD31− memory Treg/Tresp cells than the differentiation via MN Treg/Tresp cells, the ratio of ICOS+ Treg cells to ICOS+ Tresp cells does not change, whereas that of ICOS− Treg cells to ICOS− Tresp cells changes in favour of ICOS− Treg cells in ESRD patients (Fig. 6a). Consequently, the suppressive activity can be maintained for both ICOS+ and ICOS− Treg cells. In dialysis patients, the improvement of renal insufficiency increased the thymic output, but could still not be fully restored. Therefore, compared with ESRD patients, similar differentiation in a weakened form may prevail in dialysis patients (Fig. 6b). Yet, continued renal insufficiency may provoke additional differentiation of ICOS+ RTE Treg cells via ICOS+ MN Treg cells. On the other hand, the redistribution of ICOS− RTE Tresp cells in dialysis patients diminished their differentiation via ICOS− CD31+ memory Tresp cells. As recently shown, the age of dialysis patients, which additionally influences thymic distribution, may also have a decisive impact on Treg cell function.29 After transplantation, the improvement of renal function reconstituted the thymic output of ICOS+ RTE Treg/Tresp cells to normal levels or even exaggerated levels. Hence, the increased differentiation of ICOS+ RTE Treg/Tresp cells into ICOS+ CD31− memory Tresp cells ceased. Nevertheless, the ratio of ICOS+ Treg to ICOS+ Tresp cells was shifted in favour of ICOS+ Tresp cells (Fig. 6c). The improvement of renal function after transplantation also reinforced the thymic output of ICOS− RTE Treg/Tresp cells; however, compared with healthy controls, it remained significantly reduced. It seems that the intensified opposing differentiation of ICOS− RTE Treg cells and ICOS− RTE Tresp cells is attenuated after transplantation, consequently degrading the ratio of ICOS− Treg to ICOS− Tresp cells (Fig. 6c). Over time, renal function cannot be fully sustained in transplant patients and may cause a renewed reduction of the thymic output, especially of ICOS+ RTE Tresp cells and ICOS− RTE Treg/Tresp cells. By that, the differentiation of ICOS+ RTE Tresp cells via ICOS+ CD31+ memory Tresp cells and that of ICOS− RTE Tresp cells via ICOS− MN Tresp cells may be reinforced. However, the exhaustion of ICOS− RTE Treg differentiation may result in an increased alternative differentiation of ICOS− MN Treg cells into ICOS− CD31− memory Treg cells. This means that the ratios of ICOS+ Treg to ICOS+ Tresp cells and also that of ICOS− Treg to ICOS− Tresp cells changes in favour of ICOS+ and ICOS− Tresp cells (Fig. 6d). Therefore, it has to be assumed that the suppressive activity of ICOS+ Treg cells can probably not be preserved in transplant patients. Unfortunately, immunosuppressive drugs especially affect the highly proliferative ICOS+ Treg/Tresp cells, rendering it impossible to isolate sufficient amounts of ICOS+ Treg cells from transplant recipients for functional analysis. The suppressive activity of ICOS− Treg cells was also significantly decreased in both short‐ and long‐term transplant patients.
Figure 6.

Differentiation pathways of inducible T‐cell co‐stimulator‐positive (ICOS+) and ICOS− recent thymic emigrant (RTE) regulatory T (Treg)/responder T (Tresp) cells during renal replacement therapy. The privileged differentiation pathway (red arrows) of ICOS+ and ICOS− RTE Treg/Tresp cells into ICOS+ and ICOS− CD31− memory Treg/Tresp cells is shown for end‐stage renal disease (ESRD) patients (a), dialysis patients (b) and patients who underwent kidney transplantation < 2 years (c) or > 2 years (d) previously. Decreasing percentages of CD31+ memory T cells propose an increased differentiation via this subset, whereas increasing percentages of mature naive (MN) T cells propose an increased differentiation via MNs. Changes in the ratios of ICOS+ Treg to ICOS+ Tresp cells or ICOS− Treg to ICOS− Tresp cells are marked by ( ). Over the period from ESRD to long‐term transplantation, the increased differentiation of ICOS+ RTE Treg cells ceases, whereas that of ICOS+ Tresp cells is maintained, changing the ratio of ICOS+ Treg to ICOS+ Tresp cells in favour of ICOS+ Tresp cells. In addition, the differentiation of ICOS− RTE Treg cells via ICOS− CD31+ memory Treg cells cannot be maintained over time and switches to a differentiation via ICOS− MN Treg cells. Consequently, as ICOS− RTE Tresp cells maintain their differentiation via ICOS− MN Tresp cells over the period from ESRD to long‐term transplantation, the ratio of ICOS− Treg cells to ICOS− Tresp cells is changed in favour of ICOS− Tresp cells.
). Over the period from ESRD to long‐term transplantation, the increased differentiation of ICOS+ RTE Treg cells ceases, whereas that of ICOS+ Tresp cells is maintained, changing the ratio of ICOS+ Treg to ICOS+ Tresp cells in favour of ICOS+ Tresp cells. In addition, the differentiation of ICOS− RTE Treg cells via ICOS− CD31+ memory Treg cells cannot be maintained over time and switches to a differentiation via ICOS− MN Treg cells. Consequently, as ICOS− RTE Tresp cells maintain their differentiation via ICOS− MN Tresp cells over the period from ESRD to long‐term transplantation, the ratio of ICOS− Treg cells to ICOS− Tresp cells is changed in favour of ICOS− Tresp cells.
Our data reveal that the maintenance of the increased differentiation of RTE Treg cells via CD31+ memory Treg cells preserves the suppressive function of both ICOS+ and ICOS− Treg cells in ESRD patients and presumably also in young, but not in elderly, dialysis patients.29 Particularly, the switching in the differentiation of ICOS− RTE Treg cells via ICOS− MN Treg cells in long‐term transplant patients may be responsible for the loss of ICOS− Treg activity in these patients. This may be due to the recurrence of renal insufficiency in long‐term transplant patients, which is frequently described in the literature.20 However, our data did not show significant worsening of kidney function in long‐term transplant patients compared with short‐term transplant patients. Therefore, it will be of great importance to clarify whether such mechanisms may be involved in graft rejection, which is associated with a more severe loss of renal function and also of Treg cell function.34 Our data indicate that a further reduction in the ratios of ICOS+/ICOS− Treg cells to ICOS+/ICOS− Tresp cells could be involved in graft rejection. In view of the fact that the ICOS molecule is highly expressed on follicular helper T cells and follicular regulatory T cells, which control the germinal centre B‐cell response to antigens,35 it might be assumed that the balance between ICOS+ Treg cells and ICOS+ Tresp cells could be particularly involved in humoral rejection processes. Of course, a clear limitation to our approach is that only ICOS was used to distinguish Treg/Tresp subsets, whereas further characterization by the expression of the transcription factor Bcl‐6 or chemokine receptor CXCR5 for Tfh cells was not performed. Nevertheless, our data suggest that unfavourable ratios of ICOS+ Treg/ICOS+ Tresp cells or ICOS− Treg/ICOS− Tresp cells may represent potential indicators for impaired immune homeostasis after transplantation. Further studies also confirm that especially the balance between Treg and Tresp cells is essential for maintaining immune homeostasis and that it is disturbed in diseases with involvement of the immune system, such as autoimmunity and cancer.36, 37
Meanwhile, it is widely accepted that renal insufficiency is closely related to premature T‐cell senescence, even in children.38 Recent studies also confirmed that neither frequencies nor the suppressive function of Treg cells were impaired in ESRD patients before and after the initiation of dialysis.39 However, similar to our studies, Treg frequencies and Treg function were shown to be reduced in long‐term kidney transplant patients.40 Our findings indicate that, similar to aging41 or pregnancy,24, 28 renal insufficiency may provoke diminished thymic output of RTE Treg/Tresp cells and therefore accelerated peripheral differentiation of these cells. The increased peripheral Treg cell differentiation via initially tolerance‐inducing pathways preserves the Treg function, as for example in old age,29 during pregnancy24, 28, 42 and presumably also in ESRD patients. However, in patients who had been exposed to renal replacement therapies for many years, these conditions cannot be sustained. In these patients, the switching in the differentiation of ICOS− RTE Treg cells via ICOS− MN Treg cells produces ICOS− memory Treg cells with diminished functional activity. Further studies may be necessary to clarify whether the differentiation of previously accumulated MN Treg cells ensures the maintenance of the memory Treg pool, or whether a different population of RTE Treg cells with greater proliferation capacity is released from the thymus or produced in the bone marrow. In addition, the most poorly matured PTK7+ RTE Tresp cells, which normally decrease with age, may accumulate with age as so‐called old‐aged ‘veteran PTK7+ RTE Tresp cells’ in conditions of diminished thymic output and therefore may also have an influence on Treg cell function.43, 44 Further interesting T‐cell populations, such as stem memory T cells, central memory T cells, effector memory T cells and terminally differentiated effector T cells were not the subject of our research and therefore may limit the informative value of our investigations.45 Further studies, detecting the characteristic markers of stem memory T cells and central memory T cells, on MN and CD31+ memory cells may be necessary to identify preferred differentiation pathways of such cells under special clinical conditions.
In summary, our data show for the first time how the CD4+ T helper cell pool changes over the years of renal replacement therapy and propose that timely transplantation of ESRD patients may significantly reduce the risk of rejection (Fig. 7). Possibly, CD8+ T cells may also be affected by years of kidney failure. Further investigations regarding the differentiation of CD8+ RTE Treg/Tresp cells may be necessary to assess the influence of immunosenescence on the responsiveness of the entire T‐cell system. From a clinical perspective, there are now numerous indications that the waiting time on dialysis is the strongest risk factor for poor renal transplant outcome, and that pre‐emptive transplantation is associated with superior graft survival compared with pre‐transplant dialysis.46, 47, 48
Figure 7.

Effect of inducible T‐cell co‐stimulator‐positive (ICOS+) and ICOS− recent thymic emigrant (RTE) regulatory T (Treg)/ responder T (Tresp) cell differentiation on the ratios of total ICOS+ Treg/ICOS+ Tresp cells and of ICOS− Treg/ICOS− Tresp cells within total CD4+ T helper cells. Premature aging of both Treg and Tresp cells in end‐stage renal disease (ESRD) patients is expected to affect the success of later kidney transplantation. Changing differentiation pathways of inducible co‐stimulatory ICOS+ and ICOS− RTE Treg/Tresp cells in ESRD (a) dialysis (b) short‐term (c) and long‐term (d) transplant patients are responsible for changes in the ratios of total ICOS+ Treg/ICOS+ Tresp cells and of ICOS−Treg/ICOS− Tresp cells within total CD4+ T helper cells. These ratios were found to influence the suppressive activity of autologous ICOS+ and ICOS− Treg cells in these patients.
Disclosure
None of the authors have any financial conflicts of interest related to this manuscript.
Author contribution
MS, AL and AS designed the study; MS and AL performed the study; LU, FK, CM, VE, AH, CMT, SM, KM, CS and MZ contributed important methods and patients. MS, AL and AS analysed the data and wrote the manuscript. All authors contributed to the final version of the manuscript and approved it.
Supporting information
Figure S1. Differentiation of inducible T‐cell co‐stimulatory‐positive (ICOS+) and ICOS− regulatory T (Treg)/responder T (Tresp) cells in healthy volunteers and patients with end‐stage renal disease (ESRD), separately for men and women.
Acknowledgements
The authors would like to thank the nursing staff of the Department of Medicine I (Nephrology) (University of Heidelberg, Heidelberg, Germany) for arranging the collection of the blood samples. In addition, we would like to thank Helmut Simon, Sabine Bönisch‐Schmidt and Iris Arnold for excellent technical assistance. For professional help with FACS sorting of the Treg cell subsets we would like to thank Sven Rüffer (Institute of Immunology, University of Heidelberg, Heidelberg, Germany) and Panagiotis Gitsioudisi (Department of Medicine V, University of Heidelberg, Heidelberg, Germany). This work was supported by the Dietmar Hopp Foundation, Sankt Leon‐Rot, Germany.
References
- 1. Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J et al Indoxyl sulfate (IS)‐mediated immune dysfunction provokes endothelial damage in patients with end‐stage renal disease (ESRD). Sci Rep 2017; 7:3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. Uremia impairs monocyte and monocyte‐derived dendritic cell function in hemodialysis patients. Kidney Int 2007; 72:1138–48. [DOI] [PubMed] [Google Scholar]
- 3. Cohen G, Horl WH. Immune dysfunction in uremia – an update. Toxins 2012; 4:962–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Betjes MG. Immune cell dysfunction and inflammation in end‐stage renal disease. Nat Rev Nephrol 2013; 9:255–65. [DOI] [PubMed] [Google Scholar]
- 5. Moser B, Roth G, Brunner M, Lilaj T, Deicher R, Wolner E et al Aberrant T cell activation and heightened apoptotic turnover in end‐stage renal failure patients: a comparative evaluation between non‐dialysis, haemodialysis, and peritoneal dialysis. Biochem Biophys Res Comm 2003; 308:581–5. [DOI] [PubMed] [Google Scholar]
- 6. Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naive and central memory T‐cell lymphopenia in end‐stage renal disease. Kidney Int 2006; 70:371–6. [DOI] [PubMed] [Google Scholar]
- 7. Litjens NH, Huisman M, van den Dorpel M, Betjes MG. Impaired immune responses and antigen‐specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol 2008; 19:1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Betjes MG, de Wit EE, Weimar W, Litjens NH. Circulating pro‐inflammatory CD4posCD28null T cells are independently associated with cardiovascular disease in ESRD patients. Nephrol Dial Transplant 2010; 25:3640–6. [DOI] [PubMed] [Google Scholar]
- 9. Betjes MG, Meijers RW, de Wit LE, Litjens NH. A killer on the road: circulating CD4+CD28null T cells as cardiovascular risk factor in ESRD patients. J Nephrol 2012; 25:183–91. [DOI] [PubMed] [Google Scholar]
- 10. Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M et al Inflammation and Progression of CKD: the CRIC Study. Clin J Am Soc Nephrol 2016; 11:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pecoits‐Filho R, Heimburger O, Barany P, Suliman M, Fehrman‐Ekholm I, Lindholm B et al Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 2003; 41:1212–8. [DOI] [PubMed] [Google Scholar]
- 12. Meijers RW, Litjens NH, de Wit EA, Langerak AW, van der Spek A, Baan CC et al Uremia causes premature ageing of the T cell compartment in end‐stage renal disease patients. Immun Ageing 2012; 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH. Premature aging of circulating T cells in patients with end‐stage renal disease. Kidney Int 2011; 80:208–17. [DOI] [PubMed] [Google Scholar]
- 14. Betjes MG, Meijers RW, Litjens NH. Loss of renal function causes premature aging of the immune system. Blood Purif 2013; 36:173–8. [DOI] [PubMed] [Google Scholar]
- 15. Meijers RW, Betjes MG, Baan CC, Litjens NH. T‐cell ageing in end‐stage renal disease patients: assessment and clinical relevance. World J Nephrol 2014; 3:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hendrikx TK, van Gurp EA, Mol WM, Schoordijk W, Sewgobind VD, Ijzermans JN et al End‐stage renal failure and regulatory activities of CD4+ CD25bright+ FoxP3+ T‐cells. Nephrol Dial Transplant 2009; 24:1969–78. [DOI] [PubMed] [Google Scholar]
- 17. Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol 2005; 16:1859–65. [DOI] [PubMed] [Google Scholar]
- 18. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–30. [DOI] [PubMed] [Google Scholar]
- 19. Simmons EM, Langone A, Sezer MT, Vella JP, Recupero P, Morrow JD et al Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end‐stage renal disease patients. Transplantation 2005; 79:914–9. [DOI] [PubMed] [Google Scholar]
- 20. Karthikeyan V, Karpinski J, Nair RC, Knoll G. The burden of chronic kidney disease in renal transplant recipients. Am J Transplant 2004; 4:262–9. [DOI] [PubMed] [Google Scholar]
- 21. Meijers RW, Litjens NH, de Wit EA, Langerak AW, Baan CC, Betjes MG. Uremia‐associated immunological aging is stably imprinted in the T‐cell system and not reversed by kidney transplantation. Transpl Int 2014; 27:1272–84. [DOI] [PubMed] [Google Scholar]
- 22. Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L et al Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 2008; 28:870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Shen S, Gorentla BK, Gao J, Zhong XP. Murine regulatory T cells contain hyperproliferative and death‐prone subsets with differential ICOS expression. J Immunol 2012; 188:1698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagner MI, Jost M, Spratte J, Schaier M, Mahnke K, Meuer S et al Differentiation of ICOS+ and ICOS– recent thymic emigrant regulatory T cells (RTE T regs) during normal pregnancy, pre‐eclampsia and HELLP syndrome. Clin Exp Immunol 2016; 183:129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A et al Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med 2002; 195:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R et al Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol 2008; 180:1499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohler S, Thiel A. Life after the thymus: CD31+ and CD31– human naive CD4+ T‐cell subsets. Blood 2009; 113:769–74. [DOI] [PubMed] [Google Scholar]
- 28. Wagner MI, Mai C, Schmitt E, Mahnke K, Meuer S, Eckstein V et al The role of recent thymic emigrant‐regulatory T‐cell (RTE‐Treg) differentiation during pregnancy. Immunol Cell Biol 2015; 93:858–67. [DOI] [PubMed] [Google Scholar]
- 29. Schaier M, Leick A, Uhlmann L, Kalble F, Eckstein V, Ho A et al The role of age‐related T cell differentiation in patients with renal replacement therapy. Immunol Cell Biol 2017; 95:895–905. [DOI] [PubMed] [Google Scholar]
- 30. Steinborn A, Schmitt E, Kisielewicz A, Rechenberg S, Seissler N, Mahnke K et al Pregnancy‐associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol 2012; 167:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winterberg PD, Ford ML. The effect of chronic kidney disease on T cell alloimmunity. Curr Opin Organ Transplant 2017; 22:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end‐stage renal disease. J Am Soc Nephrol 2002; 13:204–12. [DOI] [PubMed] [Google Scholar]
- 33. Litjens NH, van Druningen CJ, Betjes MG. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol 2006; 118:83–91. [DOI] [PubMed] [Google Scholar]
- 34. Schaier M, Seissler N, Schmitt E, Meuer S, Hug F, Zeier M et al DRhigh+CD45RA–‐Tregs potentially affect the suppressive activity of the total Treg pool in renal transplant patients. PLoS ONE 2012; 7:e34208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A p85α‐osteopontin axis couples the receptor ICOS to sustained Bcl‐6 expression by follicular helper and regulatory T cells. Nat Immunol 2015; 16:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benito‐Miguel M, Garcia‐Carmona Y, Balsa A, Perez de Ayala C, Cobo‐Ibanez T, Martin‐Mola E et al A dual action of rheumatoid arthritis synovial fibroblast IL‐15 expression on the equilibrium between CD4+ CD25+ regulatory T cells and CD4+ CD25– responder T cells. J Immunol 2009; 183:8268–79. [DOI] [PubMed] [Google Scholar]
- 37. Bhattacharya K, Chandra S, Mandal C. Critical stoichiometric ratio of CD4+ CD25+ FoxP3+ regulatory T cells and CD4+ CD25– responder T cells influence immunosuppression in patients with B‐cell acute lymphoblastic leukaemia. Immunology 2014; 142:124–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. George RP, Mehta AK, Perez SD, Winterberg P, Cheeseman J, Johnson B et al Premature T cell senescence in pediatric CKD. J Am Soc Nephrol 2017; 28:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Litjens NH, Boer K, Zuijderwijk JM, Klepper M, Peeters AM, Verschoor W et al Natural regulatory T cells from patients with end‐stage renal disease can be used for large‐scale generation of highly suppressive alloantigen‐specific Tregs. Kidney Int 2017; 91:1203–13. [DOI] [PubMed] [Google Scholar]
- 40. Alvarez Salazar EK, Cortes‐Hernandez A, Aleman‐Muench GR, Alberu J, Rodriguez‐Aguilera JR, Recillas‐Targa F et al Methylation of FOXP3 TSDR underlies the impaired suppressive function of Tregs from long‐term belatacept‐treated kidney transplant patients. Front Immunol 2017; 8:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palmer DB. The effect of age on thymic function. Front Immunol 2013; 4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalble F, Mai C, Wagner M, Schober L, Schaier M, Zeier M et al Aberrant ICOS+ T cell differentiation in women with spontaneous preterm labor. Am J Reprod Immunol 2016; 76:415–25. [DOI] [PubMed] [Google Scholar]
- 43. Bains I, Yates AJ, Callard RE. Heterogeneity in thymic emigrants: implications for thymectomy and immunosenescence. PLoS ONE 2013; 8:e49554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haines CJ, Giffon TD, Lu LS, Lu X, Tessier‐Lavigne M, Ross DT et al Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med 2009; 206:275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med 2017; 23:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meier‐Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation 2002; 74:1377–81. [DOI] [PubMed] [Google Scholar]
- 47. Meier‐Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM et al Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–7. [DOI] [PubMed] [Google Scholar]
- 48. Haller MC, Kainz A, Baer H, Oberbauer R. Dialysis vintage and outcomes after kidney transplantation: a retrospective cohort study. Clin J Am Soc Nephrol 2017; 12:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Differentiation of inducible T‐cell co‐stimulatory‐positive (ICOS+) and ICOS− regulatory T (Treg)/responder T (Tresp) cells in healthy volunteers and patients with end‐stage renal disease (ESRD), separately for men and women.


