Abstract
Predicting the prognosis of unresectable pancreatic ductal adenocarcinoma (PDAC) is useful in determining the appropriate management strategy. The present study aimed to investigate the association between PDAC prognosis and inflammation-based markers, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio, prognostic nutritional index, modified Glasgow prognostic score (mGPS) and controlling nutritional status score. A total of 72 patients with unresectable PDAC who received chemotherapy were included. Inflammation-based markers were measured prior to treatment. The median progression-free survival (PFS) and overall survival (OS) were 117 days (range, 10–781 days) and 244 days (range 43–781 days), respectively. The cut-off value of continuous variables that predicted the median OS (244 days) was calcualted. Univariate analysis of PFS showed that disease stage, first-line chemotherapy regimen, carcinoembryonic antigen (CEA), NLR, platelet-to-lymphocyte ratio (PLR), mGPS and controlling nutritional status (CONUT) scores were associated with PFS. Among them, stage, first-line chemotherapy regimen, CEA, NLR and mGPS were independent prognostic factors for PFS in multivariate analysis. Univariate analysis of OS showed that stage, first-line chemotherapy regimen, CA19-9, NLR, PLR, prognostic nutritional index (PNI), mGPS and CONUT score were associated wtih OS. Among them, first-line chemotherapy and mGPS were independent prognostic factors for OS according to multivariate analysis. Univariate and multivariate analyses revealed that a NLR ≥4.0 and mGPS 2 were independent prognostic factors for PFS. For OS, mGPS 2 was an independent prognostic factor. In conclusion, mGPS was the most useful marker in predicting the prognosis of patients with unresectable PDAC who received chemotherapy.
Keywords: pancreatic ductal adenocarcinoma, chemotherapy, inflammation-based markers, modified Glasgow prognostic score, predictive factor
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies worldwide (1). Most PDACs are unresectable at the time of diagnosis and are treated with palliative chemotherapy. Gemcitabine monotherapy was the standard treatment for PDAC until 2011; however, combination chemotherapy regimens such as FOLFIRINOX and gemcitabine plus nab-paclitaxel therapy have become standard treatments in recent years and have improved the prognosis of PDAC patients. However, it is sometimes difficult to determine the most appropriate regimen for each PDAC patient. The prediction of patient prognosis is useful for providing proper treatment.
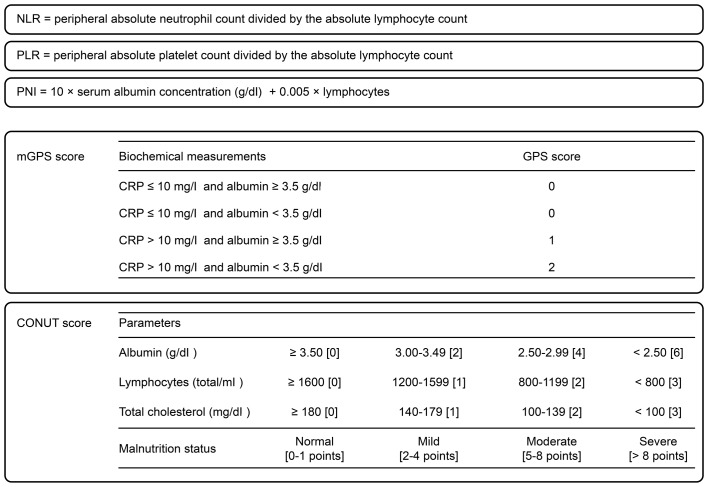
In advanced cancers, inflammation is associated with tumor proliferative activity. Nutritional state is also important, because patients with advanced cancer tend to become malnourished due to cachexia. Malnutrition leads to poor prognosis and decreased quality of life. Some inflammatory and nutritional scores, such as neutrophil-to-lymphocyte ratio (NLR) (2–4), platelet-to-lymphocyte ratio (PLR) (5), prognostic nutritional index (PNI) (6–8), modified Glasgow prognostic score (mGPS) (9,10), and controlling nutritional status (CONUT) score (11,12), have been reported to be useful in estimating the prognosis of patients with advanced cancers. The definition of each score is shown in Fig. 1. We previously reported that NLR is useful for predicting the outcome of gemcitabine therapy in unresectable PDAC (13). However, which marker is the most reliable for predicting the prognosis of PDAC patients treated with chemotherapy, including several combination regimens, remains unknown.
Figure 1.
Definition of inflammatory and nutrition scores.
The present study aimed to detect the most useful marker for predicting the prognosis of unresectable PDAC patients treated with chemotherapy.
Materials and methods
This retrospective observational study reviewed the data of unresectable PDAC patients, including those with locally advanced and metastatic disease, who underwent palliative chemotherapy between September 2006 and August 2016 at Fukushima Medical University Hospital (Fukushima, Japan). All patients were pathologically diagnosed with PDAC, and patients with rare primary pancreatic neoplasms, including acinar cell carcinoma or neuroendocrine carcinoma, were excluded. The chemotherapy regimen (FOLFIRINOX, gemcitabine plus nab-paclitaxel therapy: GnP, gemcitabine, or S-1) was decided by each patient's physician. All clinico-pathological data were measured prior to treatment.
The progression-free survival (PFS) and overall survival (OS) times were calculated from the date of histological diagnosis to the date of disease progression and mortality by any cause, respectively. We applied receiver operating characteristic (ROC) curve analysis to determine ideal cut-off values to predict poor prognosis for the following continuous variables: age, tumor diameter, carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9), NLR, PLR, PNI and CONUT score. The association of each clinico-pathological parameter (age, gender, tumor diameter, disease stage (UICC7th), first-line chemotherapy, NLR, PLR, PNI, mGPS and CONUT score) with PFS and OS time was investigated. Survival analysis was performed using the Kaplan-Meier method with log-rank tests in univariate analysis. Multivariate Cox regression analysis was performed to determine the effect of clinico-pathological variables on survival time. The data were analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, version 3.2.1) that was a modified version of the R commander (version 2.1–7). Differences with a P-value <0.05 were considered statistically significant (14). This study was approved by the Institutional Review Board of Fukushima Medical University.
Results
We included 72 PDAC patients who met the inclusion criteria described above in the analysis. The characteristics and laboratory data of the 72 patients are shown in Table I. The median PFS and OS was 117 days (range, 10–781 days) and 244 days (range, 43–781 days), respectively.
Table I.
Patient characteristics.
| Category | Value |
|---|---|
| Age, yearsa | 63 (42–85) |
| Sexb | |
| Male | 40 (55.6%) |
| Female | 32 (44.4%) |
| Tumor diameter, mma | 30 (7–106) |
| Stageb | |
| Locally advanced | 20 (27.8%) |
| Metastatic | 52 (72.2%) |
| First-line regimenb | |
| GEM + nab-PTX | 16 (22.2%) |
| FOLFIRINOX | 10 (13.9%) |
| GEM | 44 (61.1%) |
| S-1 | 2 (2.8%) |
| CEA (ng/ml)a | 4.2 (1.2–1738.0) |
| CA19-9 (U/ml)a | 1326.1 (0.3–603000.2) |
| NLRa | 3.2 (1.2–11.6) |
| PLRa | 175.0 (63.9–547.1) |
| PNIa | 45.5 (32.4–60.7) |
| mGPSb | |
| 0 | 52 (72.2%) |
| 1 | 11 (15.3%) |
| 2 | 9 (12.5%) |
| CONUT scoreb | |
| 0–1 | 18 (27.3%) |
| 2–4 | 39 (59.1%) |
| 5–8 | 8 (12.1%) |
| 8< | 1 (1.5%) |
Data presented as the mean (range)
Data presented as the number of patients (%). GEM, gemcitabine; nab-PTX, nab-paclitaxel; CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; mGPS, modified Glasgow prognostic score; CONUT, controlling nutritional status.
We calculated the cut-off value of continuous variables that predicted the median OS (244 days) using ROC analysis. The cut-off value of laboratory data and inflammatory markers are shown in Table II.
Table II.
Diagnostic yield of each marker at the optimal cut-off values.
| Variable | Cut-off value | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| Age, years | <64 | 52.8 | 63.9 | 55.0 |
| Size, mm | <28 | 41.7 | 66.7 | 53.2 |
| CEA, ng/ml | <5.0 | 63.9 | 44.4 | 49.5 |
| CA19-9, U/ml | <1772 | 61.1 | 50.0 | 52.0 |
| NLR | <4.0 | 75.0 | 55.6 | 63.3 |
| PLR | <182.0 | 63.9 | 58.3 | 60.1 |
| PNI | ≥45.2 | 63.9 | 55.6 | 54.2 |
| CONUT score | <3 | 70.6 | 43.8 | 61.7 |
CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; mGPS, modified Glasgow prognostic score; CONUT, controlling nutritional status.
Univariate analysis of PFS showed that disease stage (locally advanced vs. metastatic, P<0.001), first-line chemotherapy regimen (combination therapy vs. monotherapy, P<0.001), CEA (<5.0 vs. ≥5.0 ng/ml, P=0.043), NLR (<4.0 vs. ≥4.0, P<0.001), PLR (182.0< vs. ≥182.0, P=0.044), mGPS (0, 1 vs. 2, P<0.001) and CONUT score (3< vs. ≥3, P=0.034) were related to PFS. Among them, stage (P=0.036), first-line chemotherapy regimen (P<0.001), CEA (P=0.024), NLR (P<0.001) and mGPS (P=0.005) were independent prognostic factors for PFS in multivariate analysis (Table III).
Table III.
Univariate and multivariate analyses to detect independent prognostic factors for PFS.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | No. | Median survival, days | P-value | HR (95% CI) | P-value |
| Age, years | 0.302 | ||||
| <64 | 32 | 136 | |||
| ≥64 | 40 | 108 | |||
| Sex | 0.625 | ||||
| Male | 40 | 123 | |||
| Female | 32 | 118 | |||
| Diameter, mm | 0.465 | ||||
| <28 | 27 | 134 | |||
| ≥28 | 45 | 117 | |||
| Stage | <0.001 | 2.36 (1.06–5.06) | 0.036 | ||
| Locally advanced | 20 | 244 | |||
| Metastatic | 52 | 94 | |||
| First-line regimen | <0.001 | 0.25 (0.12–0.53) | <0.001 | ||
| Combination therapy | 24 | 244 | |||
| Monotherapy | 48 | 92 | |||
| CEA, ng/ml | 0.043 | 2.24 (1.11–4.53) | 0.024 | ||
| <5.0 | 43 | 136 | |||
| ≥5.0 | 29 | 115 | |||
| CA19-9, U/ml | 0.106 | 0.89 (0.42–1.88) | 0.765 | ||
| <1,772 | 40 | 147 | |||
| ≥1,772 | 32 | 108 | |||
| NLR | <0.001 | 6.93 (2.77–17.35) | <0.001 | ||
| <4.0 | 43 | 203 | |||
| ≥4.0 | 29 | 77 | |||
| PLR | 0.044 | 0.47 (0.22–1.00) | 0.050 | ||
| <182.0 | 38 | 156 | |||
| ≥182.0 | 34 | 92 | |||
| PNI | 0.113 | 1.09 (0.55–2.18) | 0.807 | ||
| <45.2 | 39 | 112 | |||
| ≥45.2 | 33 | 126 | |||
| mGPS | <0.001 | 5.00 (1.64–15.23) | 0.005 | ||
| 0, 1 | 63 | 134 | |||
| 2 | 9 | 33 | |||
| CONUT score | 0.034 | 0.87 (0.41–1.84) | 0.722 | ||
| <3 | 42 | 156 | |||
| ≥3 | 24 | 89 | |||
PFS, progression-free survival; HR, hazard ratio; CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; mGPS, modified Glasgow prognostic score; CONUT, controlling nutritional status.
Univariate analysis of OS showed that the stage (P<0.001), first-line chemotherapy regimen (P<0.001), CA19-9 (P=0.040), NLR (P<0.001), PLR (P=0.004), PNI (<45.2 vs. ≥45.2, P=0.013), mGPS (P<0.001) and CONUT score (P=0.016) were related to OS. Among them, first-line chemotherapy (P=0.007) and mGPS (P<0.001) were independent prognostic factors for OS according to multivariate analysis (Table IV).
Table IV.
Univariate and multivariate analyses to detect independent prognostic factors for OS.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | No. | Median survival, days | P-value | HR (95% CI) | P-value |
| Age, years | 0.073 | ||||
| <64 | 32 | 292 | |||
| ≥64 | 40 | 271 | |||
| Sex | 0.801 | ||||
| Male | 40 | 270 | |||
| Female | 32 | 292 | |||
| Diameter, mm | 0.399 | ||||
| <28 | 27 | 391 | |||
| ≥28 | 45 | 251 | |||
| Stage | <0.001 | 2.31 (0.91–5.90) | 0.078 | ||
| Locally advanced | 20 | 641 | |||
| Metastatic | 52 | 249 | |||
| First-line regimen | <0.001 | 0.25 (0.09–0.69) | 0.007 | ||
| Combination therapy | 24 | 641 | |||
| Monotherapy | 48 | 241 | |||
| CEA, ng/ml | 0.268 | 1.19 (0.52–2.73) | 0.680 | ||
| <5.0 | 43 | 292 | |||
| ≥5.0 | 29 | 251 | |||
| CA19-9, U/ml | 0.040 | 1.67 (0.68–4.11) | 0.263 | ||
| <1,772 | 40 | 376 | |||
| ≥1,772 | 32 | 247 | |||
| NLR | <0.001 | 2.18 (0.88–5.38) | 0.092 | ||
| <4.0 | 43 | 392 | |||
| ≥4.0 | 29 | 206 | |||
| PLR | 0.004 | 0.99 (0.47–2.10) | 0.984 | ||
| <182.0 | 38 | 391 | |||
| ≥182.0 | 34 | 236 | |||
| PNI | 0.013 | 0.52 (0.23–1.19) | 0.123 | ||
| <45.2 | 39 | 249 | |||
| ≥45.2 | 33 | 376 | |||
| mGPS | <0.001 | 26.16 (5.22–131.10) | <0.001 | ||
| 0, 1 | 63 | 342 | |||
| 2 | 9 | 97 | |||
| CONUT score | 0.016 | 0.69 (0.29–1.67) | 0.413 | ||
| <3 | 42 | 368 | |||
| ≥3 | 24 | 249 | |||
OS, overall survival; HR, hazard ratio; CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; mGPS, modified Glasgow prognostic score; CONUT, controlling nutritional status.
The Kaplan-Meier analysis of mGPS revealed that the survival time of patients with mGPS 2 was significantly poorer than that of patients with mGPS 0 and 1 (Figs. 2 and 3).
Figure 2.
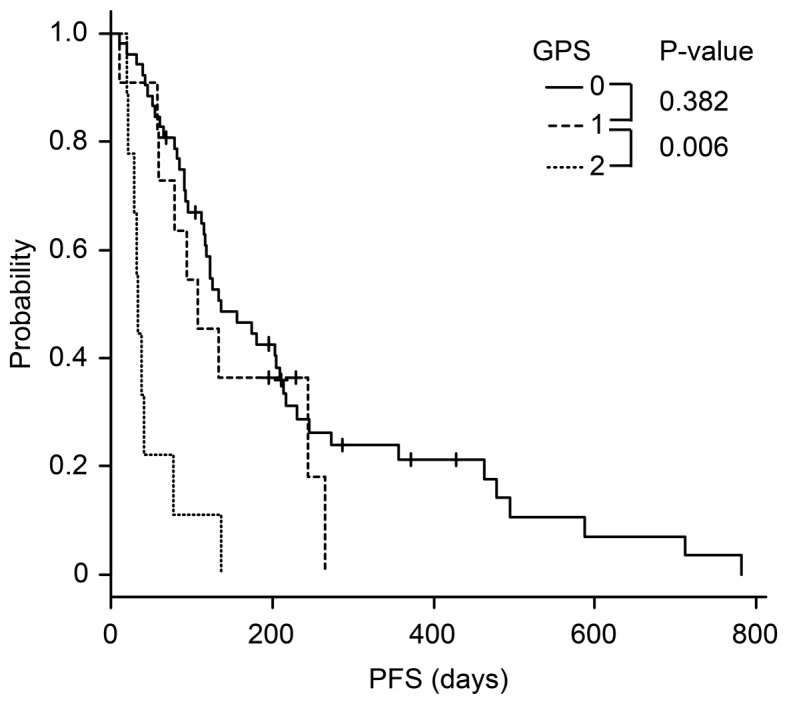
Kaplan-Meier analysis of progression-free survival according to mGPS. The PFS time of patients with mGPS 2 was significantly poorer than that of patients with mGPS 0 and 1.
Figure 3.
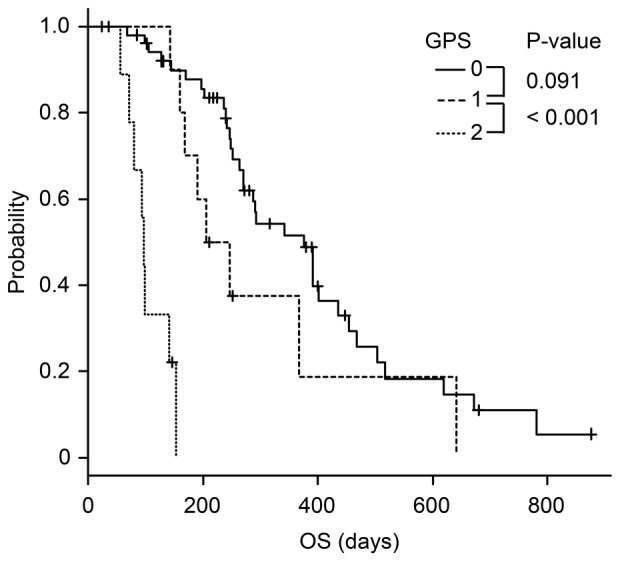
Kaplan-Meier analysis of overall survival according to mGPS. The OS of patients with mGPS 2 was significantly poorer than that of patients with mGPS 0 and 1.
Discussion
We aimed to clarify the best inflammation-based marker to predict the prognosis of unresectable PDAC patients treated with chemotherapy. The results showed that mGPS was the most reliable inflammation-related marker to predict PFS and OS. To the best of our knowledge, this study is the first to clarify the most useful inflammatory marker to predict prognosis in cases of unresectable PDAC treated with palliative chemotherapy.
Several studies have assessed the association between inflammation-based markers and the prognosis of resectable and unresectable PDAC patients. In patients with resectable PDAC, preoperative NLR (15), PLR (16,17), GPS (18) and PNI 45 (7) were reported to be factors in early recurrence and poor prognosis after surgical resection. In unresectable PDAC, previous reports showed that patients with a high NLR had a shorter OS time (13,19). In terms of GPS, a retrospective study intended for PDAC patients treated with gemcitabine showed that GPS could predict poor prognosis (20). PNI has been shown to be related to prognosis and a systemic inflammatory response (i.e., NLR, PLR and serum TNF-α level) in PDAC (8).
There are many reports concerning the relation between each inflammation-based marker and the prognosis of PDAC, but there are few reports comparing the utility of several inflammation-based markers. Yamada et al (21) conducted a retrospective study to clarify which score could best reflect survival in resected pancreatic cancer patients and found that GPS was superior to the other markers. Additionally, a systematic review summarized past reports about PDAC and inflammatory markers and concluded that GPS and NLR were useful in predicting prognosis (22); however, unlike our study, this study included patients with various stages and treatments (e.g., surgical resection and chemotherapy).
mGPS, which most accurately reflected the prognosis of unresectable PDAC patients in our study, is based on C-reactive protein (CRP) and serum albumin levels. CRP elevation indicates systemic inflammation. Because the cytokines produced by tumors increase the inflammatory response, CRP is considered to reflect growth activity in tumors. Furthermore, the serum albumin level indicates patient nourishment state and is related to performance status. Unresectable PDAC patients often fall into an undernourished state because of a decrease in pancreatic exocrine function and cancer cachexia. This undernourished state worsens the prognosis by reducing the patient's quality of life and making the continuation of chemotherapy more difficult. In the original GPS, hypoalbuminemia (Alb <3.5 g/dl) or elevated C-reactive protein (CRP >10 mg/l) is defined as a score of 1 (23). The modified GPS, which defined hypoalbuminemia without elevated CRP as a score of 0, was proposed because the GPS score of 1 was most commonly due to elevated CRP (24). In this study, mGPS 2 patients had a significantly poorer prognosis than those with scores of 0 or 1 because of the high-grade malignancy and cachexic state of the tumors. Patients with a score of 1 tended to have a poorer prognosis than patients with a score of 0, but the difference was not significant. This result indicates that nourishment state plays a very important role in determining the prognosis of PDAC. Naturally, serum CRP levels can increase as a result of an infection rather than tumor activity. Therefore, we used blood data collected in the afebrile state just before the initiation of chemotherapy, but some cases might be complicated by occult cholangitis.
A limitation of this study was that it was a single-center study with a limited number of patients. The results should be validated in a large population across multiple clinical sites. In addition, the chemotherapy regimen was determined at the physician's discretion, which may have resulted in bias. Studies focused only on patients treated with the single regimen or on those treated with multidrug therapy are expected.
In conclusion, mGPS appears to be an independent prognostic factor for predicting the PFS and OS of unresectable PDAC patients. Predicting prognosis before the start of therapy may be helpful in choosing the optimal treatment.
Acknowledgements
We thank all of the staff members of the Department of Gastroenterology of Fukushima Medical University, the Department of Endoscopy of Fukushima Medical University Hospital, and the gastroenterology ward of Fukushima Medical University Hospital. We also thank American Journal Experts for their English editing services.
Glossary
Abbreviations
- PDAC
pancreatic ductal adenocarcinoma
- NLR
neutrophil-to-lymphocyte ratio
- PLR
platelet-to-lymphocyte ratio
- PNI
prognostic nutritional index
- mGPS
modified Glasgow prognostic score
- CONUT
controlling nutritional status
- PFS
progression-free survival
- OS
overall survival
- CEA
carcinoembryonic antigen
- CA19-9
cancer antigen 19-9
- CRP
C-reactive protein
Funding
Supported by Department of Gastroenterology, Fukushima Medical University, School of Medicine.
Availability of data and materials
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HA and RS designed and performed the research; HA, RS and HO analyzed the data; HA, RS and HO wrote the manuscript; TH, TT, MS, NK, HT, KW, JN, HK, MT, YS and HI provided clinical advice; TH and HO supervised the report.
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of the Fukushima Medical University Hospital. Patients were not required to provide informed consent for this study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Patient consent for publication
Patients were not required to provide informed consent for this study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent. For full disclosure, the details of the study are published on the home page of Fukushima Medical University.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. (In English, Slovak) [PubMed] [Google Scholar]
- 3.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 4.An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 5.Aliustaoglu M, Bilici A, Seker M, Dane F, Gocun M, Konya V, Ustaalioglu BB, Gumus M. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology. 2010;57:640–645. [PubMed] [Google Scholar]
- 6.Lowe EF, Stein M, Woolley T, Waycaster M, Scroggins B, Acuff R, Smith JT, Lefemine AA. Prognostic nutritional index: Its usefulness as a predictor of clinical course. J Am Coll Nutr. 1983;2:231–240. doi: 10.1080/07315724.1983.10719927. [DOI] [PubMed] [Google Scholar]
- 7.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 8.Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41:1508–1514. doi: 10.1016/j.ejso.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Glen P, Jamieson NB, McMillan DC, Carter R, Imrie CW, McKay CJ. Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology. 2006;6:450–453. doi: 10.1159/000094562. [DOI] [PubMed] [Google Scholar]
- 10.Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, Tanaka T, Ishihara M, Yogi T, Tsutsumi H, Fujiyoshi T, et al. Evaluation of modified glasgow prognostic score for pancreatic cancer: A retrospective cohort study. Pancreas. 2016;45:211–217. doi: 10.1097/MPA.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 11.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 12.Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa T, et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10:e0132488. doi: 10.1371/journal.pone.0132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki R, Takagi T, Hikichi T, Konno N, Sugimoto M, Watanabe KO, Nakamura J, Waragai Y, Kikuchi H, Takasumi M, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett. 2016;11:3441–3445. doi: 10.3892/ol.2016.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868–872. doi: 10.1007/s00268-011-0984-z. [DOI] [PubMed] [Google Scholar]
- 16.Song W, Tian C, Wang K, Zhang RJ, Zou SB. Preoperative platelet lymphocyte ratio as independent predictors of prognosis in pancreatic cancer: A systematic review and meta-analysis. PLoS One. 2017;12:e0178762. doi: 10.1371/journal.pone.0178762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirai Y, Shiba H, Sakamoto T, Horiuchi T, Haruki K, Fujiwara Y, Futagawa Y, Ohashi T, Yanaga K. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158:360–365. doi: 10.1016/j.surg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 18.Morinaga S, Murakawa M, Katayama Y, Yamaoku K, Aoyama T, Kanazawa A, Higuchi A, Shiozawa M, Kobayashi S, Ueno M, Morimoto M. Glasgow prognostic score predicts clinical outcomes in patients with pancreatic cancer undergoing adjuvant gemcitabine monotherapy after curative surgery. Anticancer Res. 2015;35:4865–4870. [PubMed] [Google Scholar]
- 19.Xue P, Kanai M, Mori Y, Nishimura T, Uza N, Kodama Y, Kawaguchi Y, Takaori K, Matsumoto S, Uemoto S, Chiba T. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3:406–415. doi: 10.1002/cam4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimoda M, Katoh M, Kita J, Sawada T, Kubota K. The glasgow prognostic score is a good predictor of treatment outcome in patients with unresectable pancreatic cancer. Chemotherapy. 2010;56:501–506. doi: 10.1159/000321014. [DOI] [PubMed] [Google Scholar]
- 21.Yamada S, Fujii T, Yabusaki N, Murotani K, Iwata N, Kanda M, Tanaka C, Nakayama G, Sugimoto H, Koike M, et al. Clinical implication of inflammation-based prognostic score in pancreatic cancer: Glasgow prognostic score is the most reliable parameter. Medicine (Baltimore) 2016;95:e3582. doi: 10.1097/MD.0000000000003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: A systematic review. Hepatobiliary Pancreat Dis Int. 2014;13:474–481. doi: 10.1016/S1499-3872(14)60284-8. [DOI] [PubMed] [Google Scholar]
- 23.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, Scott HR. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92:1834–1836. doi: 10.1038/sj.bjc.6602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.