Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumour in adults. Identification of accessible and cost-effective prognostic factors may better guide adjuvant treatment-related decisions. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are markers of host inflammatory response, and their increase has recently been shown to be a poor prognostic factor in several malignancies. The aim of the present study was to investigate the prognostic value of preoperative NLR and PLR in GBM patients. Between 2012 and 2017, 104 patients who had undergone surgery for GBM were considered for adjuvant therapy in our institution. Of those, 80 patients with evaluable pre-corticosteroid full blood count results were identified and included in the final analysis. The Eastern Cooperative Oncology Group performance status, localization, radiochemotherapy and second-line systemic therapy were found to be independent prognostic indicators for progression-free and overall survival. The median overall survival was 13.2 months. Patients with NLR <4 had a better median overall survival of 10.7 vs. 7.8 months in patients with NLR >4; however, this difference was not statistically significant (P>0.05). Overall survival also did not differ significantly between patients with low and those with high PLR values (10.2 vs. 15.2 months, respectively; P=0.105). In conclusion, the results of the present study suggest that pre-treatment NLR and PLR do not have prognostic value in GBM patients; however, large-scale trials are required to confirm these findings.
Keywords: glioblastoma, glioblastoma multiforme, neutrophil-to-lymphocyte ratio
Introduction
Glioblastoma multiforme (GBM) is the most common malignant brain tumour in adults (1). The current standard of first-line therapy is maximal safe resection followed by radiation therapy concurrent with temozolomide and subsequent adjuvant temozolomide chemotherapy (2). However, almost all patients experience relapse after adjuvant therapy and overall survival (OS) is dismal, despite the best available treatment modalities (3). New adjuvant treatment strategies, better patient selection and personalized therapy are crucial for improving clinical outcomes.
Clinical factors such as age at presentation, tumour location, Karnofsky performance status, extent of surgery and isocitrate dehydrogenase (IDH) status are well-known prognostic factors for GBM (4). Identification of more accessible and cost-effective prognostic factors may better guide adjuvant treatment decisions. The neutrophil-to-lymphocyte ratio (NLR) is a marker of host inflammatory response, and its elevation has recently been shown to be a poor prognostic factor in a number of malignancies, including colon, prostate, lung and bladder cancer (5–9). The platelet-to-lymphocyte ratio (PLR) is another inflammatory marker, although it has been less extensively investigated as a prognostic factor in cancer patients compared with NLR.
A limited number of studies have evaluated the role of NLR and PLR in GBM prognosis and survival, but the results are controversial. Some studies found that higher NLR values were associated with worse OS at the time of first diagnosis or prior to second surgery, whereas one study demonstrated that lower NLR values were associated with better prognosis only in IDH wild-type patients. In addition, other studies did not observe any association between NLR values and clinical outcome.
A total of 3 studies evaluated the prognostic effect of PLR in GBM patients: One of those studies reported a negative prognostic effect of increased PLR values, whereas the remaining 2 studies did not identify any association between PLR and OS.
The aim of the present study was to evaluate the prognostic value of NLR and PLR for OS in a cohort of patients with GBM.
Patients and methods
Patient information
In this retrospective, single-centre study, a total of 80 patients who were diagnosed between January 2012 and June 2017 at the Departments of Radiation Oncology and Medical Oncology of Samsun Training Hospital (Samsun, Turkey) were evaluated. The Stupp protocol (primary radiotherapy with a total of 60 Gy with concomitant temozolamide and subsequent temozolamide) was used for all patients as the postoperative radiochemotherapy regimen. The protocol of the present study was approved by the local Ethics Committee.
Demographic data, clinicopathological data and treatment parameters (i.e., extent of surgical resection, radiotherapy and use of chemotherapy) were obtained from medical records. Data on patient death were obtained from the National Electronic Death Registration System, Turkey.
Patients with complete blood count results before receiving corticosteroid therapy or surgery were included in the study. NLR was calculated by dividing the neutrophil count by the lymphocyte count, and the PLR was defined as the absolute platelet count divided by the absolute lymphocyte count.
Statistical analysis
Progression-free survival (PFS) was calculated as the time interval between the date of the initial surgery and the detection of tumour progression documented on magnetic resonance imaging or the date of death. The time interval between the date of diagnosis and the date of death, or of the last follow-up for surviving patients, was defined as the OS. Kaplan-Meier curves were used to calculate OS and PFS. Patients who were alive at the last visit were censored in the analysis. Univariate and multivariate analyses were performed using the Cox proportional hazards model to evaluate the effect of variables on PFS and OS. Both the NLR and PLR were evaluated as continuous or dichotomous variables. A cut-off value of 4 for NLR was selected according to previous trials, as it had been shown to correlate with clinical outcomes in GBM patients. P<0.05 was considered to indicate statistically significant differences. All analyses were performed using SPSS version 23 software (IBM SPSS, Armonk, NY, USA).
Results
Study population
Between 2012 and 2017, 104 patients with GBM were assessed for consideration of adjuvant therapy at our institution, among whom 80 patients with evaluable pre-corticosteroid full blood count results were identified and included in the present study. Of the 80 patients, 39 (48.7%) were male and 41 (51.3%) were female, with a mean age of 56.8±13.1 years. The median tumour diameter was 42.3±14.8 mm. The majority of the patients (85%) had received concurrent chemoradiotherapy after surgery. In addition, the majority of the patients (72.5%) subsequently received adjuvant temozolomide. Gross total excision was achieved in over half of the patients (52.5%). The most common tumour localization was the temporal lobe (27.5%). The median follow-up time was 12 months (range, 3–55 months).
The mean pre-treatment neutrophil, platelet and lymphocyte counts were 7.9±3.7×109/l (range, 2.4–21.6×109/l), 259.1±65.7×109/l (range, 133–462×109/l), and 1.7±0.7×109/l (range, 0.5–3.6×109/l), respectively.
The mean pre-treatment NLR was 6.3±5.5 (median, 4.39; range, 1.03–30.29), and the mean pre-treatment PLR was 182.9±95.4 (median, 163.1; range, 56.8–607.9). Baseline demographic data are presented in Table I.
Table I.
Demographic and clinical characteristics.
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Female | 41 | 51.3 |
| Male | 39 | 48.7 |
| Age (years), mean ± SD | 80 | 56.8±13.1 |
| Tumour location | ||
| Temporal | 22 | 27.5 |
| Parietal | 13 | 16.3 |
| Frontal | 13 | 16.3 |
| Frontoparietal | 9 | 11.3 |
| Parietooccipital | 10 | 12.5 |
| Frontotemporal | 5 | 6.3 |
| Occipital | 3 | 3.8 |
| Other | 5 | 6.4 |
| Primary/secondary | ||
| Primary | 77 | 96.3 |
| Secondary | 3 | 3.8 |
| Hemisphere | ||
| Right | 32 | 40 |
| Left | 45 | 56.3 |
| Midline | 3 | 3.8 |
| Type of operation | ||
| Total | 42 | 52.5 |
| Subtotal | 30 | 37.5 |
| Biopsy | 7 | 8.8 |
| Unknown | 1 | 1.3 |
| Adjuvant treatment | ||
| Chemoradiotherapy | 68 | 85 |
| Radiotherapy | 5 | 6.3 |
| No treatment | 3 | 3.7 |
| Unknown | 2 | 2.5 |
| Chemoradiotherapy + Cyberknife | 2 | 2.5 |
| Temozolamide | ||
| Yes | 58 | 72.5 |
| No | 11 | 13.8 |
| Unknown | 11 | 13.8 |
| Preoperative NLR, mean ± SD | 80 | 6.3±5.5 |
| Preoperative PLR, mean ± SD | 80 | 182.9±95.4 |
SD, standard deviation; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
PFS
The median PFS was 9.1 months. On univariate analysis, PFS did not differ significantly according to sex, laterality, or pre-treatment neutrophil, lymphocyte and platelet counts. However, age <65 years, Eastern Cooperative Oncology Group (ECOG) performance status 1 and 2, administration of concurrent radiotherapy compared with radiotherapy alone, administration of second- and third-line systemic therapy, and frontal compared with occipital tumour localization were considered as prognostic factors. PFS did not differ significantly between patients with higher (>4) and those with lower (<4) NLR values.
On multivariate analysis, ECOG performance status, localization, radiochemotherapy and second-line systemic therapy remained as independent prognostic indicators (Table II).
Table II.
Univariate and multivariate analysis for progression-free survival.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age | 0.508 | 0.300–0.859 | 0.012a | 0.845 | 0.421–1.695 | 0.635 |
| Sex | 0.869 | 0.546–1.384 | 0.555 | |||
| ECOG PS | ||||||
| 1–2 | ||||||
| 3–4 | 0.220 | 0.130–0.371 | 0.000a | 6.629 | 3.096–14.194 | 0.000a |
| Localization | ||||||
| Frontal | ||||||
| Parietal/temporal | 1.293 | 0.764–2.189 | 0.339 | 1.926 | 1.057–3.510 | 0.032a |
| Occipital | 5.936 | 1.632–21.596 | 0.007a | 3.965 | 0.829–18.950 | 0.084 |
| Other | 3.579 | 1.306–9.807 | 0.013a | 2.320 | 0.782–6.884 | 0.129 |
| Laterality | ||||||
| Right | ||||||
| Left | 0.719 | 0.440–1.173 | 0.187 | |||
| Midline | 1.590 | 0.480–5.261 | 0.448 | |||
| Type of operation | ||||||
| Biopsy | ||||||
| Subtotal | 0.803 | 0.307–2.106 | 0.656 | |||
| Gross total | 0.408 | 0.157–1.064 | 0.067 | |||
| Radiotherapy | ||||||
| Concurrent | ||||||
| Alone | 3.007 | 1.187–7.620 | 0.020a | 4.201 | 1.292–13.657 | 0.017a |
| Temozolamide | ||||||
| Yes | ||||||
| No | 1.366 | 0.706–2.645 | 0.355 | |||
| Second-/third-line systemic therapy | ||||||
| Yes | ||||||
| No | 0.331 | 0.196–0.558 | 0.000a | 0.292 | 0.161–0.527 | 0.000a |
| NLR | ||||||
| >4 | ||||||
| <4 | 1.374 | 0.858–2.202 | 0.186 | |||
| PLR | ||||||
| >135 | ||||||
| <135 | 0.724 | 0.444–1.182 | 0.197 | |||
| Pre-treatment neutrophils | 1.000 | 1.000–1.000 | 0.07 | |||
| Pre-treatment lymphocytes | 1.000 | 1.000–1.001 | 0.236 | |||
| Pre-treatment platelets | 1.000 | 1.000–1.000 | 0.556 | |||
| Pre-treatment NLR | 1.015 | 0.968–1.065 | 0.532 | |||
| Tumour size | 0.993 | 0.972–1.014 | 0.525 | |||
Statistically significant. HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
OS
A total of 53 patients had succumbed to the disease by the time of the analysis. The OS was 13.2 months in the entire study population. Univariate and multivariate analyses demonstrated that patients with ECOG 1–2, and those receiving concurrent chemoradiotherapy or additional systemic therapy, had longer OS. On multivariate analysis, frontal localization was also a significant predictor of survival (Table III).
Table III.
Univariate and multivariate analysis for overall survival.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age | 1.416 | 0.807–2.485 | 0.225 | |||
| Sex | 0.847 | 0.486–1.478 | 0.559 | |||
| ECOG PS | ||||||
| 1–2 | ||||||
| 3–4 | 2.647 | 1.505–4.658 | 0.001a | 2.526 | 1.256–5.080 | 0.009a |
| Localization | ||||||
| Frontal | ||||||
| Parietal/temporal | 1.197 | 0.652–2.197 | 0.562 | 2.006 | 0.963–4.179 | 0.063a |
| Occipital | 4.042 | 0.872–18.733 | 0.074 | 5.432 | 1.034–28.533 | 0.046a |
| Other | 8.331 | 2.078–33.403 | 0.003a | 2.394 | 0.513–11.178 | 0.267 |
| Laterality | ||||||
| Right | ||||||
| Left | 0.747 | 0.416–1.338 | 0.326 | |||
| Midline | 6.154 | 0.742–51.067 | 0.092 | |||
| Operation type | ||||||
| Biopsy | ||||||
| Subtotal | 0.672 | 0.198–2.278 | 0.524 | |||
| Gross total | 0.395 | 0.116–1.345 | 0.137 | |||
| Radiotherapy | ||||||
| Concurrent | ||||||
| Alone | 6.752 | 2.164–21.072 | 0.001a | 7.127 | 1.768–28.740 | 0.06a |
| Temozolamide | ||||||
| Yes | ||||||
| No | 1.864 | 0.901–3.855 | 0.093 | |||
| Second-/third-line systemic therapy | ||||||
| Yes | ||||||
| No | 0.176 | 0.091–0.341 | 0.000a | 0.085 | 0.036–0.205 | 0.000a |
| NLR | ||||||
| >4 | ||||||
| <4 | 1.258 | 0.727–2.179 | 0.412 | |||
| PLR | ||||||
| >135 | ||||||
| <135 | 0.649 | 0.365–1.125 | 0.121 | |||
| Pre-treatment neutrophils | 1.000 | 1.000–1.000 | 0.074 | |||
| Pre-treatment lymphocytes | 1.000 | 1.000–1.001 | 0.236 | |||
| Pre-treatment platelets | 1.000 | 1.000–1.000 | 0.556 | |||
| Pre-treatment NLR | 1.015 | 0.968–1.065 | 0.532 | |||
| Tumour size | 0.993 | 0.972–1.014 | 0.525 | |||
Statistically significant. HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
NLR and PLR
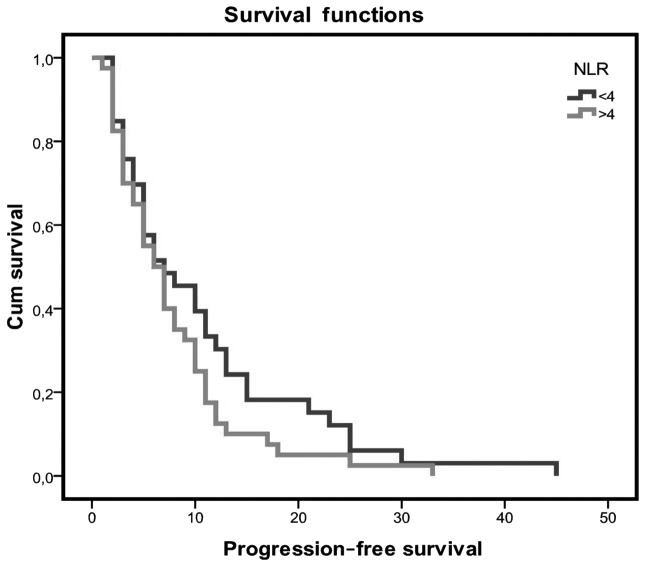
Patients with an NLR value of <4 had a longer PFS when compared with patients with higher NLR values (10.7 vs. 7.8 months, respectively), but the difference was not statistically significant (Fig. 1). The PFS for patients with low PLR values was 7.4 months as compared with 10.02 months for those with high PLR values, but the difference was not statistically significant (P=0.166).
Figure 1.
Kaplan-Meier curves for progression-free survival. NLR, neutrophil-to-lymphocyte ratio.
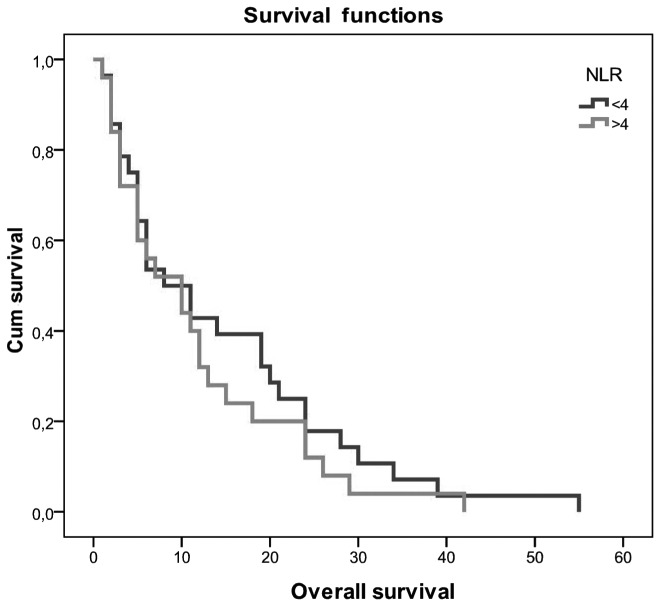
Patients with NLR <4 had a better OS of 14.5 vs. 11.6 months in patients with NLR >4. The difference was not statistically significant (P>0.05; Fig. 2). OS did not differ significantly between patients with low and those with high PLR values (10.2 vs. 15.2 months, respectively; P=0.105).
Figure 2.
Kaplan-Meier curves for overall survival. NLR, neutrophil-to-lymphocyte ratio.
Discussion
The results of the present study demonstrated that patients with lower NLR values exhibited a longer OS compared with patients with higher NLR values, but the difference was not statistically significant. PLR was not prognostic for clinical outcome. Although NLR is an easily available and cost-effective test, its clinical use in patients with GBM is associated with some challenges. NLR should be calculated prior to steroid therapy and surgery, as both these interventions may increase the neutrophil count and lead to misinterpretation of the value (10). NLR may also be affected by various factors, such as hypertension, autoimmune diseases, cardiovascular diseases and insulin resistance. In addition, the optimal cut-off value has not been established.
The identification of prognostic factors is crucial for GBM patients and may guide clinical treatment. Several studies have demonstrated an association between inflammatory status and cancer development. Pre-treatment neutrophil, lymphocyte and platelet counts are indicators of cancer-associated inflammation. High neutrophil count has been demonstrated to be an independent negative prognostic marker for recurrence and survival in gastric cancer, metastatic renal cell carcinoma, metastatic melanoma and advanced non-small-cell lung cancer (11). NLR is considered to reflect the balance between activation of the inflammatory pathway and the anti-tumour immune function (12).
As a marker of systemic inflammation, the prognostic significance of pre-treatment NLR in GBM patients remains unclear. NLR values <4 were shown to be significantly correlated with a better OS for GBM patients in 3 retrospective studies (10,13,14). By contrast, 2 retrospective studies did not identify any association between NLR values and OS, in accordance with our results. Mason et al evaluated NLR values in postoperative GBM patients who mostly received corticosteroids, which affect the NLR values, and used a cut-off value of 7.5 (15). Lopes et al also did not observe any correlation of NLR with OS, but they reported a shorter OS in patients with an NLR value >7 who completed the Stupp protocol (16).
McNamara et al assessed the prognostic value of NLR prior to second surgery in GBM patients and demonstrated that low NLR values were associated with longer OS after the second surgery compared with high NLR values (17).
PLR is also described as a prognostic factor in different cancer types; however, it is less extensively investigated compared with NLR in GBM patients. A total of 3 retrospective studies evaluated the prognostic value of PLR in GBM patients: Wang et al reported that PLR had independent prognostic value in GBM patients (10). Conversely, the other 2 trials could not find any association between PLR and clinical outcome in GBM patients (14,16).
Of the known prognostic factors in our study population, ECOG performance status, localization, combined therapy and second-line systemic treatment were identified as prognostic factors for clinical outcome.
There were certain limitations to the present study. First, this was a retrospective study and the sample size was not sufficient to reach statistical significance for survival due to worse disease outcomes; the results should be confirmed in a prospective study. Second, the O6-methylguanine-DNA methyltransferase methylation status of the patients was not known. Moreover, the post-progression salvage treatments were heterogeneous. Finally, cardiovascular diseases, infection, or drug treatments may also have affected the neutrophil and lymphocyte counts; however, we could not detect all confounders for patients with such medical history.
In conclusion, pre-treatment NLR and PLR values were not associated with prognosis in GBM patients and do not appear to be useful markers for predicting prognosis in GBM patients. There remains an unmet need to identify better prognostic factors for GBM patients.
Acknowledgements
The authors would like to thank Ferhat Yıldız for the statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
OY made substantial contributions to conception and design, and acquisition of data, and interpretation of data; EO made substantial contributions to conception and acquisition of data; OO made substantial contributions to acquisition of data; YK involved in drafting the manuscript and revising it critically for important intellectual content.
Ethics approval and consent to participate
Samsun Research and Training Hospital approval no. 2017/14.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no potential competing interests to disclose.
References
- 1.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Kawano H, Hirano H, Yonezawa H, Yunoue S, Yatsushiro K, Ogita M, Hiraki Y, Uchida H, Habu M, Fujio S, et al. Improvement in treatment results of glioblastoma over the last three decades and beneficial factors. Br J Neurosurg. 2015;29:206–212. doi: 10.3109/02688697.2014.967750. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa Y, Sasaki H, Ohara K, Ezaki T, Toda M, Ohira T, Kawase T, Yoshida K. Clinical and molecular prognostic factors for long-term survival of the patients with glioblastomas in a single-institutional consecutive cohort. World Neurosurg. 2017;106:165–173. doi: 10.1016/j.wneu.2017.06.126. [DOI] [PubMed] [Google Scholar]
- 5.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir Y, Akin ML, Sucullu I, Balta AZ, Yucel E. Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pac J Cancer Prev. 2014;15:2647–2650. doi: 10.7314/APJCP.2014.15.6.2647. [DOI] [PubMed] [Google Scholar]
- 7.Buttigliero C, Pisano C, Tucci M, Vignani F, Bertaglia V, Iaconis D, Guglielmini P, Numico G, Scagliotti GV, Di Maio M. Prognostic impact of pretreatment neutrophil-to-lymphocyte ratio in castration-resistant prostate cancer patients treated with first-line docetaxel. Acta Oncol. 2017;56:555–562. doi: 10.1080/0284186X.2016.1260772. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Ad V, Palmer J, Li L, Lai Y, Lu B, Myers RE, Ye Z, Axelrod R, Johnson JM, Werner-Wasik M, et al. Neutrophil to lymphocyte ratio associated with prognosis of lung cancer. Clin Transl Oncol. 2017;19:711–717. doi: 10.1007/s12094-016-1593-y. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, Du P, Yang Y. The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: A systematic review and meta-analysis. Int J Clin Oncol. 2017;22:817–825. doi: 10.1007/s10147-017-1171-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang PF, Song HW, Cai HQ, Kong LW, Yao K, Jiang T, Li SW, Yan CX. Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget. 2017;8:50117–50123. doi: 10.18632/oncotarget.15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–146. doi: 10.1016/j.critrevonc.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Faria SS, Fernandes PC, Jr, Silva MJ, Lima VC, Fontes W, Freitas-Junior R, Eterovic AK, Forget P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience. 2016;10:702. doi: 10.3332/ecancer.2016.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bambury RM, Teo MY, Power DG, Yusuf A, Murray S, Battley JE, Drake C, O'Dea P, Bermingham N, Keohane C, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114:149–154. doi: 10.1007/s11060-013-1164-9. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. doi: 10.1186/s12885-015-1629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason M, Maurice C, McNamara MG, Tieu MT, Lwin Z, Millar BA, Menard C, Laperriere N, Milosevic M, Atenafu EG, et al. Neutrophil-lymphocyte ratio dynamics during concurrent chemo-radiotherapy for glioblastoma is an independent predictor for overall survival. J Neurooncol. 2017;132:463–471. doi: 10.1007/s11060-017-2395-y. [DOI] [PubMed] [Google Scholar]
- 16.Lopes M, Carvalho B, Vaz R, Linhares P. Influence of neutrophil-lymphocyte ratio in prognosis of glioblastoma multiforme. J Neurooncol. 2018;136:173–180. doi: 10.1007/s11060-017-2641-3. [DOI] [PubMed] [Google Scholar]
- 17.McNamara MG, Lwin Z, Jiang H, Templeton AJ, Zadeh G, Bernstein M, Chung C, Millar BA, Laperriere N, Mason WP. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. 2014;117:147–152. doi: 10.1007/s11060-014-1366-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.