Abstract
Background
Type 2 diabetes mellitus (T2D), rapidly increasing to epidemic proportions, globally escalates cardiovascular disease risk. Although intensive interventions and comprehensive management of environmental risks factors for T2D are associated with reduced cardiovascular disease, such approaches are limited for individuals with high genetic T2D risk. In this study we investigated possible associations of ACE2 polymorphisms and cardiovascular risks in Uygur patients with T2D.
Methods
275 Uygur T2D patients and 272 non-diabetic Uygur individuals were enrolled as study participants. 14 ACE2 polymorphisms were genotyped by Matrix-assisted laser desorption ionization time-of-flight mass spectrometry.
Results
ACE2 SNP rs1978124, rs2048683, rs2074192, rs233575, rs4240157, rs4646156, rs4646188 and rs879922 were associated with T2D (all P < 0.05). The 8 diabetic risk related ACE2 SNPs were further associated with diabetic related cardiovascular complications or events but exhibited heterogeneity as fellows: firstly, almost all diabetic risk related ACE2 SNPs (all P < 0.05) were associated with increased SBP except rs1978124 and rs2074192, while rs2074192, rs4646188 and rs879922 were associated elevated DBP (all P < 0.05). Secondly, SNP rs4646188 was not correlated with any type of dyslipidemia (TRIG, HDL-C, LDL-C or CHOL), and the other 7 diabetic risk related loci were at least correlated with one type of dyslipidemia (all P < 0.05). In particular, rs879922 were simultaneously correlated with four type of dyslipidemia (all P < 0.05). Thirdly, ACE2 SNP rs2074192 and rs879922 were associated with carotid arteriosclerosis stenosis (CAS) ≥ 50% (both P < 0.05). Fourthly, ACE2 SNP rs2074192, rs4240157, rs4646188 and 879922 were associated with increased MAU (all P < 0.05). In addition, ACE2 SNP rs2048683, rs4240157, rs4646156, rs4646188 and rs879922 were linked to heavier LVMI (all P < 0.05), but only rs4240157, rs4646156 and rs4646188 were associated with lower LVEF (all P < 0.05).
Conclusion
ACE2 SNP rs879922 may be a common genetic loci and optimal genetic susceptibility marker for T2D and T2D related cardiovascular risks in Uygurs.
Electronic supplementary material
The online version of this article (10.1186/s12933-018-0771-3) contains supplementary material, which is available to authorized users.
Keywords: Association, ACE2 polymorphism, Cardiovascular risk, Type 2 diabetes mellitus, Uygur
Background
Type 2 diabetes mellitus (T2D) is a clinical syndrome characterized by increased blood glucose, attributable to both genetic and environmental risk factors. T2D is one of the most serious challenges to global public health [1]. Cardiovascular disease is recognized as the leading cause in all-cause mortality of T2D, and the earlier onset of T2D, the more complication (e.g., increased blood pressure, dyslipidemia, arteriosclerosis cardiovascular disease (ASCVD), heart failure (HF), etc.) [2], the higher risk of cardiovascular death [3]. Thus a major goal of T2D prevention and control is to reduce the risk of cardiovascular events. In China the latest overall prevalence of T2D among adults is 10.9%, the age of onset is the trend of younger, and the number of T2D patients has been ranked the first in the world [4]. Under the circumstances, addressing the challenge of preventing and treating T2D in China should focus on decreasing cardiovascular events by intensive interventions and comprehensive management of cardiovascular risk factors in diabetic patients [5]. Although comprehensive management of those multiple modifiable risk factors (e.g., unhealthy diet, lack of physical activity, smoking, obesity, and dyslipidemia, etc.) is significant associated with lower blood sugar, lower blood pressure, lower LDL-C and lower incidence of T2D related cardiovascular events (e.g., ASCVD) [6], the cardiovascular benefits could be weaken or offset by metabolic memory effect from long-term elevated blood sugar and high genetic risk because it is impossible to modify the genetic structure [7]. Genetic background notwithstanding, recent studies examining the genetic basis of diabetes risk have revealed overall genetic risk factors and genetic loci leading to beta cell dysfunction, the major cause of T2D in China [8]. Thus, early identification (or) screening populations at high risk of T2D and T2D related cardiovascular complications, especially early-onset T2D, could provide a possible strategy for early prevention of diabetes.
Numerous candidate genes have been thus far been implicated in susceptibility to T2D, but few studies have focused on genes related to the renin-angiotensin-aldosterone system (RAAS). Angiotensin converting enzyme 2 (ACE2) is a key RAAS enzyme and a recently recognized target for the prevention and treatment of T2D. The gene encoding ACE2 maps to chromosome Xp22, spans 39.98 kbp of genomic DNA and consists of 20 introns and 18 exons. The ACE2 gene encodes a type I membrane-bound glycoprotein of 805 amino acids. Functional domains include a C-terminal transmembrane anchoring region, an N-terminal signal peptide and an HEXXH zinc binding metalloprotease motif. The ACE2 gene exhibits a high degree of genetic polymorphism and some polymorphisms are associated with T2D. There is also a high degree of genetic heterogeneity among ACE2 polymorphisms linked to T2D and not all variants exhibit association with T2D risk. Among Europeans, single nucleotide polymorphisms (SNPs) were not associated with T2D or T2D with diabetic nephropathy among persons of British (rs1978124, rs2074192, rs4646188 and rs2023802) or Finnish (rs2285666, rs2048684, rs879922, rs714205 and rs5978731) descent [9, 10]. Among persons of Australian descent rs2074192, rs4240157 and rs4646188 exhibited higher T2D with hypertension risk, rs1978124 was associated with risk of T2D related left ventricular remodeling [11].
However, the association of ACE2 SNPs with T2D and T2D related cardiovascular complications (e.g., hypertension and dyslipidemia) or events (e.g., ASCVD) in Chinese population are rarely reported. Theoretically, there may be common genetic basis between them [8, 12, 13] manifesting the characteristics of ethnic-specific genetic pleiotropy among cardio-metabolic traits [14]. In this study we investigated possible associations between ACE2 gene variation and cardiovascular risk in Uygur patients with type 2 diabetes mellitus.
Materials and methods
Study participants
This study was reviewed and approved by the Ethics Committee of Guangzhou First People’s Hospital, South China University of Technology. A total of 275 consecutive patients with T2D and 272 non-diabetes subjects from the south Xinjiang region were enrolled in the study from 2012 to 2017. All participants were long resident in the region and were from multi-generation resident families. All participants shared the same high- salt, sugar and fat diet. The newly T2D patients were diagnosed according to the criteria of the American Diabetic Association (ADA) guidelines of 1997 or World Health Organization (WHO) National diabetic group criteria of 2006 as follows: a single raised glucose reading with symptoms (polyuria, polydipsia, polyphagia and weight loss), otherwise raised values on two occasions, of either fasting plasma glucose (FPG) 7.0 mmol/L or with an oral glucose tolerance test (OGTT), 2 h after the oral dose a plasma glucose 11.1 mmol/L [15]. Blood pressure was measured in the seated position after 10 min of rest using a mercury sphygmomanometer by experienced and certified examiners, and measured in the brachial artery 3 times at 5-min intervals in at least two separate visits to the health care office. The mean of the last 2 measurements per visit was recorded as representative of clinic BP. All biochemistry tests were performed by standard methods in the Chemical Laboratory. Bilateral carotid and cardiac ultrasonic scanning was performed on admission to the study according to the measurement of degree of stenosis used in the North American Symptomatic Carotid Endarterectomy Trial [16] and the recommendations for chamber quantification from the American Society of Echocardiography [17], respectively.
Genotyping assay
Genomic DNA was extracted from whole blood using the Maxwell RSC Whole blood DNA kit (Promega, Madison, WI), quantified using NanoDrop-1000 (ThermoFisher, Waltham, MA) and diluted to 10 ng/μL concentration. 14 ACE2 SNPs (rs1978124, rs2048683, rs2074192, rs2235306, rs2285666, rs233575, rs4240157, rs4646142, rs4646155, rs4646156, rs4646188, rs4830542, rs6632677 and rs879922) were identified based on existing literature and human genome sequence databases. Primers for ACE2 SNPs were designed based upon sequence information from GenBank using Primer 5.0 (Whitehead Institute Cambridge, Massachusetts, USA) and Operon’s Oligo software 7.60 (OperonTechnologies Inc., Alameda, California, USA). Primers are shown in Additional file 1: Table S1. ACE2 SNPs were analyzed using the Sequenom MassARRAY system according to previously described methods [18]. Genotyping accuracy was determined by genotype concordance between duplicate samples and was 100% for each SNP.
Statistical analysis
The Hardy–Weinberg equilibrium was assessed for the control (non-diabetic) participants as shown in Additional file 1: Table S2. Analysis was performed using SPSS version 20 (SPSS, Chicago, IL) and PASS version 15 (Statistical Solution Ltd, Cork, Ireland). Categorical variables (nationality, gender, smoking, drinking, T2D and T2D complicated with CAS ≥ 50% were presented as frequencies. The relationship between each ACE2 SNP and those categorical variables were assessed using the Chi square test. The Odds ratio (OR) between control genotype and high T2D risk genotype for each ACE2 SNP among categorical variables was evaluated using binary logistic regression. Considering the possible false positive risk to the final result, Bonferroni adjustment was applied to adjust the P value obtained in multi-logit regression. Continuous variables (age, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), blood biochemical index, left ventricular mass index (LVMI) and left ventricular ejection fraction (LVEF)) were presented as mean ± SD. Significant differences for continuous variables were analyzed by two way ANOVA, One way ANOVA or independent-sample t-test according to our research design. The least significant difference (LSD) test was further used to assess differences for two subgroups after variance analysis, to show distinct differences with homogeneous variance, while the Games-Howell test was used for heterogeneous variance. A P value less than 0.05 was considered statistically significant. All probabilities are two-tailed.
Result
Characteristics of the study participants
Among Uygur participants, diabetic and non-diabetic subjects showed significant differences in SBP, DBP, BMI, triglyceride (TRIG), high-density lipoprotein cholesterol (HDL-C), Lipoprotein A, FBG, glycosylated hemoglobin (HbA1C), blood uric acid (UA), blood electrolytes (sodium and potassium), microalbuminuria (MAU), left heart remodeling (LVMI and LVEF) and the activation of RAAS (renin and Angiotensin I/II (ANG I/II)) (all P < 0.05) but not in gender, age, smoking, drinking, DBP, total cholesterol (CHOL), low-density lipoprotein cholesterol (LDL-C), lipoprotein A, the ratio of ApoA1/Apo B, renal function (Cr, BUN), liver function (ALT, AST, Alb) and high-sensitivity C-reactive protein (P > 0.05) (see Additional file 1: Table S3).
Association of ACE2 SNPs and T2D
As shown in Table 1, ACE2 SNPs rs1978124 (P < 0.001), rs2048683 (P < 0.001), rs2074192 (P < 0.001), rs233575 (P < 0.001), rs4240157 (P < 0.001), rs4646156 (P < 0.001), rs4646188 (P < 0.001) and rs879922 (P = 0.005) were significantly associated with T2D except rs2235306, rs2285666, rs4646142, rs4830542 and rs6632677 (all P > 0.05, see Additional file 1: Table S4).
Table 1.
Association of ACE2 SNPs with T2D in study participants
| ACE2 SNPs | Non-diabetic (N/%) | Diabetic (N/%) | OR (95% CI)a | P-value | |
|---|---|---|---|---|---|
| rs1978124 | |||||
| CC | 194 (71.3) | 137 (49.8) | 1.00 | ||
| TT + CT | 78 (28.7) | 138 (50.2) | 2.24 (1.53–3.26) | < 0.001 | |
| rs2048683 | |||||
| GG | 222 (81.6) | 171 (62.2) | 1.00 | ||
| TT + GT | 50 (18.4) | 104 (37.8) | 3.07 (2.02–4.68) | < 0.001 | |
| rs2074192 | |||||
| CC | 104 (38.2) | 136 (49.5) | 2.12 (1.43–3.13) | < 0.001 | |
| TT + CT | 168 (61.8) | 139 (50.5) | 1.00 | ||
| rs233575 | |||||
| CC + CT | 58 (21.3) | 112 (40.7) | 3.00 (1.99–4.51) | < 0.001 | |
| TT | 214 (78.7) | 163 (59.3) | 1.00 | ||
| rs4240157 | |||||
| CC + CT | 64 (23.5) | 124 (45.1) | 2.75 (1.87–4.04) | < 0.001 | |
| TT | 208 (76.5) | 151 (54.9) | 1.00 | ||
| rs4646156 | |||||
| AA + AT | 54 (19.9) | 104 (37.8) | 2.71 (1.79–4.10) | < 0.001 | |
| TT | 218 (80.1) | 171 (62.2) | 1.00 | ||
| rs4646188 | |||||
| CC + CT | 102 (37.5) | 64 (23.3) | 1.00 | ||
| TT | 170 (62.5) | 211 (76.7) | 2.18 (1.47–3.22) | < 0.001 | |
| rs879922 | |||||
| CC + CG | 128 (47.1) | 176 (64.0) | 1.78 (1.19–2.65) | 0.005 | |
| GG | 144 (52.9) | 271 (36.0) | 1.00 | ||
aAfter adjustment for nationality, gender, age, smoking and BMI
Association of T2D risk related ACE2 SNPs with elevated blood pressure
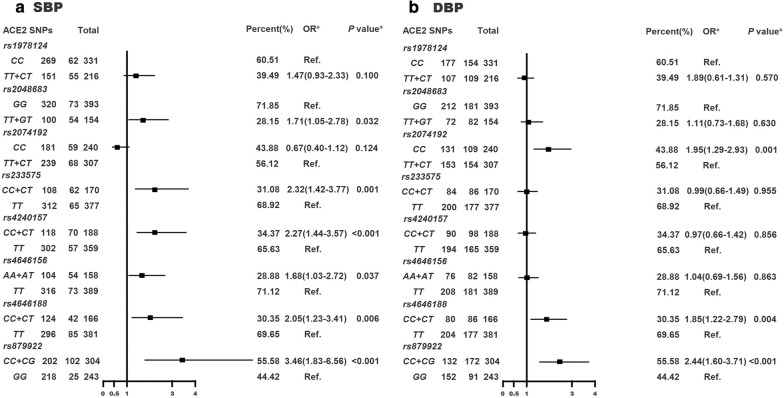
As shown in Fig. 1, T2D related ACE2 SNP rs4646188 and rs879922 were associated with increased SBP (P = 0.006 and < 0.001) and DBP (P = 0.004 and < 0.001) while rs1978124 was not (both P > 0.05). rs2048683 (P = 0.032), rs233575 (P = 0.001), rs4240157 (P < 0.001) and rs4646156 (P = 0.037) were only correlated with increased SBP while rs2074192 (P = 0.001) was only correlated with elevated DBP.
Fig. 1.
Association of T2D risk related ACE2 SNPs with elevated systolic (a) and diastolic (b) blood pressure in study participants. *After adjustment for gender, age, smoking, BMI and T2D
Association of T2D risk related ACE2 SNPs with dyslipidemia
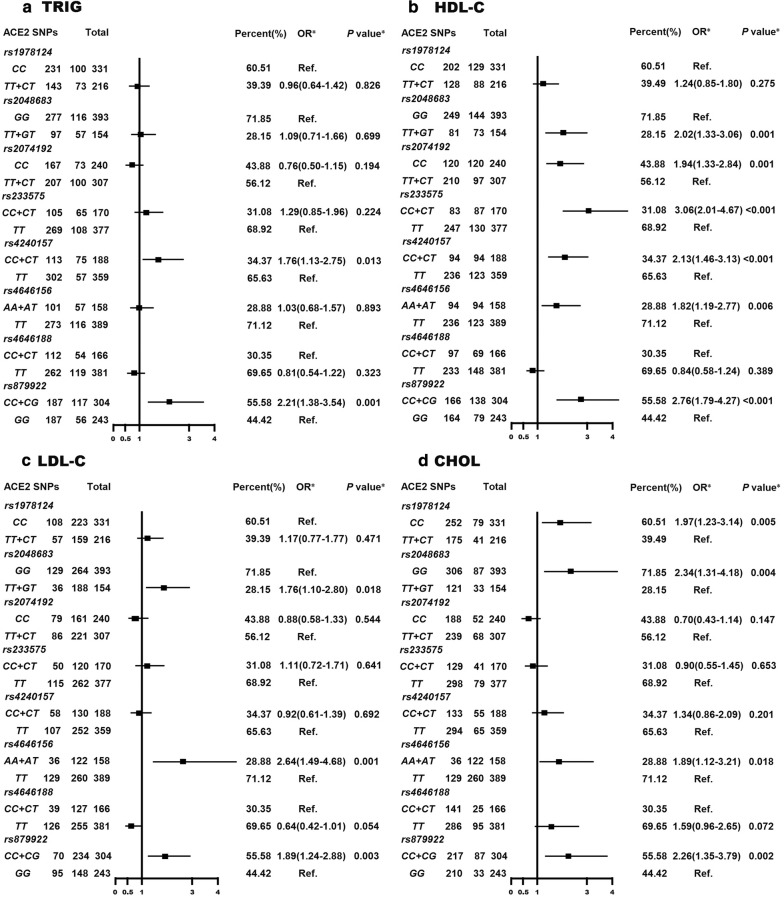
As shown in Fig. 2, T2D risk related ACE2 SNP rs1978124 and rs4646188 were not correlated with any type of dyslipidemia (TRIG, HDL-C, LDL-C, CHOL), rs1978124, rs2074192 and rs233575 were correlated with one type of dyslipidemia (CHOL or HDL-C, all P < 0.05), rs4240157 was correlated with two type of dyslipidemia (TRIG and HDL-C, both P < 0.05), rs2058683 and rs4646156 were correlated with three type of dyslipidemia (HDL-C, LDL-C and CHOL, all P < 0.05) and rs879922 correlated with four type of dyslipidemia (TRIG, HDL-C, LDL-C and CHOL, all P < 0.05).
Fig. 2.
Association of T2D risk related ACE2 SNPs with dyslipidemia in study participants. a TRIG; b HDL-C; c LDL-C; d CHOL. *After adjustment for gender, age, BMI and T2D
Association of T2D risk related ACE2 SNPs with CAS ≥ 50%
As shown in Table 2, ACE2 SNPs rs2074192 (P = 0.045) and rs879922 (P = 0.022) were associated with T2D complicated by CAS ≥ 50%.
Table 2.
Association of T2D risk related ACE2 SNPs with CAS ≥ 50% in study participants
| ACE2 SNPs | Non-CAS ≥ 50% (N/%) | CAS ≥ 50% (N/%) | OR (95% CI)* | P-value* | |
|---|---|---|---|---|---|
| rs2074192 | |||||
| CC | 190 (40.4) | 50 (64.9) | 1.97 (1.01–3.82) | 0.045 | |
| TT + CT | 280 (59.6) | 27 (35.1) | 1.00 | ||
| rs879922 | |||||
| CC + CG | 244 (51.9) | 60 (77.9) | 2.30 (1.12–4.68) | 0.022 | |
| GG | 226 (48.1) | 17 (22.1) | 1.00 | ||
*After adjustment for T2D, smoking, age, BMI, DBP, LDL-C and Ang II
Association of T2D risk related ACE2 SNPs with MAU
As shown in Table 3, T2D patients with the high diabetic risk genotype of rs2074192 (CC, P = 0.038), rs4240157 (CC + CT, P < 0.001), rs4646188 (TT, P < 0.001) and rs879922 (CC + CG, P = 0.049) were associated with increased MAU level.
Table 3.
Association of T2D risk related ACE2 SNPs and MAU in study participants
| ACE2 SNPs | ACR (mg/g) | |||
|---|---|---|---|---|
| Non-diabetic | Diabetic | P-value | ||
| rs2074192 | ||||
| CC | 90.2 ± 28.6 | 98.7 ± 26.5 | 0.019 | |
| TT + CT | 87.5 ± 22.1 | 92.3 ± 26.7 | 0.088 | |
| P-value | 0.404 | 0.048 | ||
| rs4240157 | ||||
| CC + CT | 85.8 ± 25.6 | 104.7 ± 29.7 | < 0.001 | |
| TT | 89.3 ± 24.5 | 87.9 ± 21.2 | 0.552 | |
| P-value | 0.324 | < 0.001 | ||
| rs4646188 | ||||
| CC + CT | 85.3 ± 23.1 | 78.8 ± 18.7 | 0.059 | |
| TT | 90.4 ± 25.6 | 100.5 ± 26.7 | < 0.001 | |
| P-value | 0.100 | < 0.001 | ||
| rs879922 | ||||
| CC + CG | 85.9 ± 22.6 | 100.7 ± 29.7 | < 0.001 | |
| GG | 90.9 ± 26.4 | 86.2 ± 16.8 | 0.089 | |
| P-value | 0.091 | < 0.001 | ||
ACR urinary albumin-to-creatinine ratio
Association of T2D risk related ACE2 SNPs with left heart remodeling
As shown in Table 4, T2D patients with the high diabetic risk genotype of rs2048683 (TT + CT, P = 0.007), rs4240157 (CC + CT, P = 0.008), rs4646156 (AA + AT, P = 0.003), rs4646188 (TT, P = 0.010) and rs879922 (CC + CG, P < 0.001) were linked to heavier LVMI, but only rs4240157 (P = 0.020), rs4646156 (P = 0.043), rs4646188 (P = 0.018) were further associated with lower LVEF.
Table 4.
Association of T2D risk related ACE2 SNPs with left heart remodeling
| ACE2 SNPs | LVMI (g/m2) | LVEF (%) | |||||
|---|---|---|---|---|---|---|---|
| Non-diabetic | Diabetic | P-value | Non-diabetic | Diabetic | P-value | ||
| rs1978124 | |||||||
| CC | 86.5 ± 14.8 | 92.3 ± 15.4 | 0.001 | 62.3 ± 5.9 | 58.6 ± 7.6 | < 0.001 | |
| TT + CT | 88.5 ± 16.7 | 95.2 ± 17.3 | 0.006 | 61.7 ± 7.3 | 57.7 ± 8.0 | < 0.001 | |
| P-value | 0.347 | 0.142 | 0.579 | 0.336 | |||
| rs2048683 | |||||||
| GG | 86.6 ± 14.5 | 91.9 ± 16.2 | 0.001 | 62.0 ± 6.0 | 59.0 ± 7.3 | < 0.001 | |
| TT + GT | 89.4 ± 18.6 | 96.8 ± 16.3 | 0.013 | 62.5 ± 7.7 | 56.7 ± 8.4 | < 0.001 | |
| P-value | 0.310 | 0.015 | 0.698 | 0.020 | |||
| rs2074192 | |||||||
| CC | 85.8 ± 15.4 | 93.3 ± 13.1 | < 0.001 | 62.4 ± 6.1 | 57.7 ± 7.0 | < 0.001 | |
| TT + CT | 87.9 ± 15.3 | 94.2 ± 19.1 | 0.002 | 62.0 ± 6.5 | 58.5 ± 8.5 | < 0.001 | |
| P-value | 0.273 | 0.652 | 0.627 | 0.412 | |||
| rs233575 | |||||||
| CC + CT | 85.9 ± 14.1 | 96.8 ± 15.3 | < 0.001 | 62.8 ± 7.0 | 56.9 ± 8.3 | < 0.001 | |
| TT | 87.4 ± 15.7 | 91.7 ± 16.8 | 0.012 | 61.9 ± 6.1 | 58.9 ± 7.4 | < 0.001 | |
| P-value | 0.513 | 0.011 | 0.382 | 0.041 | |||
| rs4240157 | |||||||
| CC + CT | 86.6 ± 13.9 | 97.7 ± 16.1 | < 0.001 | 62.4 ± 6.6 | 56.5 ± 8.5 | < 0.001 | |
| TT | 87.2 ± 15.8 | 90.5 ± 15.9 | 0.054 | 62.0 ± 6.3 | 59.4 ± 7.0 | < 0.001 | |
| P-value | 0.766 | < 0.001 | 0.698 | 0.003 | |||
| rs4646156 | |||||||
| AA + AT | 90.2 ± 18.1 | 96.8 ± 16.3 | 0.020 | 62.1 ± 7.5 | 56.7 ± 8.4 | < 0.001 | |
| TT | 86.3 ± 14.5 | 91.9 ± 16.2 | < 0.001 | 62.1 ± 6.0 | 59.0 ± 7.3 | < 0.001 | |
| P-value | 0.152 | 0.015 | 0.967 | 0.020 | |||
| rs4646188 | |||||||
| CC + CT | 86.4 ± 16.6 | 88.1 ± 12.0 | 0.487 | 62.0 ± 6.1 | 61.1 ± 8.2 | 0.432 | |
| TT | 87.5 ± 14.6 | 95.5 ± 17.1 | < 0.001 | 62.2 ± 6.5 | 57.2 ± 7.5 | < 0.001 | |
| P-value | 0.575 | < 0.001 | 0.798 | 0.001 | |||
| rs879922 | |||||||
| CC + CG | 89.0 ± 15.0 | 96.9 ± 16.9 | < 0.001 | 62.2 ± 6.4 | 57.5 ± 8.9 | < 0.001 | |
| GG | 85.4 ± 15.5 | 88.2 ± 13.9 | 0.147 | 62.0 ± 6.3 | 59.3 ± 5.4 | 0.001 | |
| P-value | 0.058 | < 0.001 | 0.758 | 0.039 | |||
Discussion
T2D is rapidly developing into an epidemic and dramatically increases the global cardiovascular events that have become a serious public health problem especially in developing countries (e.g., China) [19]. During the last decade in China, the prevalence of T2D has continuously increased, and followed by an increased risk of cardiovascular morbidity and mortality, including hypertension, dyslipidemia, macrovascular (e.g. ASCVD) and microvascular (e.g. MAU) complications [20]. T2D prevalence also differs among different groups, differentially affecting Han and different non-Han populations [4], especially in the minority areas (e.g., Xinjiang region). The prevalence of T2D in the Uygur population of Xinjiang is particularly high in both urban and rural environments due to unhealthy lifestyle related factors (such as high BMI, unhealthy diet, and low physical activity) and an aging population [4], while at the same time diabetes related cardiovascular risk is extremely high [21]. Thus, early identification and assessing populations at high risk of T2D and T2D related cardiovascular diseases are the key steps in diabetes prevention and control.
ACE2 gene polymorphisms and risk of diabetes
This study investigated possible associations between ACE2 polymorphisms and T2D in the Uygur population of the Xinjiang region of China. We found that patients carrying the genotypes of rs879922 (CC + CG) had a moderate risk to develop T2D while rs1978124 (TT + CT), rs2048683 (TT + GT), rs2074192 (CC), rs233575 (CC + CT), rs4240157 (CC + CT), rs4646156 (AA + AT) and rs4646188 (TT) had a high risk. This is the first report showing that SNP rs4830542 were not associated with T2D (see Additional file 1: Table S4). We also found that ACE2 SNP rs2285666 was not linked to T2D in Uygur participants, which is also true for persons of European descent [9]. Indeed, HbA1c is an important biological marker for predicting cardiovascular events in T2D patients [22]. We also found that the levels of HbA1C in T2D Uygur patients with the high diabetic risk genotypes of the three loci (rs2074192, rs4240157 and rs879922) increased significantly (see Additional file 1: Table S10), suggesting that those would be at higher potentially risk of cardiovascular events.
ACE2 gene polymorphisms and diabetic related cardiovascular risk
Essential hypertension (EH) is recognized as the leading cause in global death of vascular disease [23]. Although the BP targets in diabetic hypertensive individuals are controversial, it was common practice to aim for BP targets lower than 130/80 mmHg in most diabetic patients [24], which will help reduce cardiovascular events (e.g., at least stroke) [39]. In this study we found that 7 diabetic risk related ACE2 SNPs were associated with increased SBP or DBP with exception of rs1978124. Our results are partial consistent with a study in a diabetic Australian Caucasian population which reported association of four ACE2 SNPs (rs4646188, rs4240157, rs2074192 and rs1978124) with hypertension. We found that rs4646188 was associated with high risk of SBP ≥130 mmHg and moderate risk of DBP ≥80 mmHg, rs4240157 and was only associated with high risk of increased SBP while rs2074192 was only associated with moderate risk of increased DBP. Framingham Study showed that a 1.5–2.0 fold increased risk of cardiovascular events when SBP increased from 130 mmHg to 139 mmHg [25]. These results suggest that those elevated SBP risk related loci may be genetic factors contributing to T2D related cardiovascular events risk in Uygurs.
Beside hypertension, dyslipidemia is another important risk factor for increased prevalence of cardiovascular events in T2D [23]. Our study found that abnormal blood lipid metabolism was significantly higher in diabetic patients compared to non-diabetic subjects and was characterized by high triglycerides and low HDL-C but normal levels of LDL-C and total cholesterol, which are consistent with previously reported characteristics of the lipid spectrum in the Chinese T2D patient population [26]. In previous studies SNP rs2285666 was not linked to dyslipidemia in T2D patients, but its relationship with various subtypes of dyslipidemia is not shown [27]. Especially, LDL-C is the most important risk factor for cardiovascular events (e.g., ASCVD) in T2D patients. Also, significantly increased cardiovascular events are associated with LDL-C levels above 1.8 mmol/L. To the best of our knowledge, this is the first more comprehensive study to investigate the association of ACE2 gene polymorphism with dyslipidemia in T2D patients. We found that 3 ACE2 SNPs (rs2048683, rs4646156 and rs879922) were correlated with increased risk of LDL-C ≥1.8 mmol/L in Uygur T2D patients, and rs879922 was significantly associated with four type of dyslipidemia. The high TRIG level and low HDL-C level are also powerful independent predictors of cardiovascular events independent of LDL-C levels [28]. ACE2 SNP rs4240157 and rs879922 were associated with high TRIG level, and almost all diabetic risk related ACE2 SNPs were associated with low HDL-C level except rs1978124 and rs4646188, but rs1978124 was correlations with high CHOL level as well as the other 3 SNPs (rs2048683, rs4646156 and rs879922). Remarkably, although there were no significant differences on LDL-C and CHOL levels between non-diabetic and diabetic individuals, but we found that T2D patients with the high diabetic risk or control genotypes of 4 diabetic risk related ACE2 SNPs (rs1978124, rs2048683, rs4646156 and rs879922) had a higher risk to develop high levels of LDL-C (≥ 1.8 mmol/L) and CHOL (≥ 5.2 mmol/L) adjusted for gender, age, BMI, T2D and ACE2 SNPs, which further suggests that the 4 ACE2 SNPs may be potential influential factors of dyslipidemia in diabetics. However, the mechanism behind this remains unclear, it is speculated that it may be related to a body-size dependent manner [29] and the BMI level was indeed statistically different between non-diabetic and diabetic between non-diabetic and diabetic individuals in our study. These results suggest that ACE2 SNPs correlations with elevated risk of dyslipidemia were obvious heterogeneity, and rs879922 was associated with four type of dyslipidemia suggesting it may be a genetic factor contributing to T2D with dyslipidemia in Uygurs.
Unfortunately, despite diabetics received standard hypoglycemic therapy, hypotensive therapy and lipid-lowering therapy, there is still a significant increase residual risk of macrovascular (e.g., ASCVD) and microvessels (e.g., retinopathy, MAU, etc.) complications, that is not only related to atherogenic dyslipidemia (high TRIG and low HDL-C level as described above) [30] but also at least partly related to the genetic background of individuals [31]. It’s well known that there is a common genetic basis for dyslipidemia and ASCVD [8]. Our results showed that 2 SNPs (rs2285666 and rs4646142) was not associated with T2D (see Additional file 1: Table S4) but exhibited association with T2D with moderate risk of atherogenic dyslipidemia (see Additional file 1: Table S6, S7), which were consistent with previously reported association of the loci with ASCVD (e.g., coronary heart disease [32], ischemic stroke [33]) in T2D patients as well as cardiovascular death in European females [34]. Similar effects also existed in between rs10911021 and CAD in T2D [35] as well as between CLOCK polymorphism and stroke in T2D patients [36]. According to the 2014 NLA recommendations [37], carotid arteriosclerosis stenosis (CAS) ≥ 50% is defined as a new type of ASCVD. In our study we newly found that both diabetic and atherogenic dyslipidemia risk related ACE2 SNPs (rs2074192 and rs879922) were respectively linked to moderate and high risk of CAS ≥ 50%, which previously reported that both loci had nothing to do with the recurrence risk of stroke [38] but were associated with sudden cardiac arrest [39] and retinopathy [40]. On the other hand, MAU is another key biomarker of microvascular complications in T2D and is one of the most valuable factors for predicting cardiovascular events in T2D [41]. We found that the 2 carotid arteriosclerosis risk related SNPs were also linked to increased MAU level besides rs4240157 and rs4646188. In addition, rs1978124 was correlations with elevated CHOL risk, and also exhibited association with death in European patients with acute coronary syndromes [42].
T2D is acknowledged as a key risk factor for atherosclerosis but is not yet fully recognized as an important independent risk factor for HF [43]. T2D complicated with HF is very common and associated with increased risk for all-cause and cardiovascular mortality and HF hospitalization. Nevertheless, diabetes associated HF, especially HF with preserved ejection fraction (HFpEF) frequently goes unrecognized. Importantly, there are as yet no effective treatment strategies to reduce all-cause death in patients with HFpEF, which highlights the importance of early identification of damage indices of HF in clinical practice [44]. LVMI and LVEF are important early damage biomarkers reflecting left heart remodeling. We found that 5 SNPs (rs2048683, rs4240157, rs4646156, rs4646188 and rs879922) were associated with increased LVMI and 3 SNPs (rs4240157, rs4646156 and rs4646188) were associated with lower LVEF. This is the first report showing that SNP rs2048683 and rs4646188 were associated with increased LVMI. With exception of rs233575, our findings coincides with the observation by Lieb et al. [45] who reported 4 ACE2 SNPs (rs4240157, rs4646156, rs879922 and rs233575) were associated with higher LVMI. However, our results are in contrast to the study by Sheila K et al. [11] who reported that rs1978124 was correlated with higher LVMI and lower LVEF while rs4646188 was not. These observations suggest that rs4646188 and rs879922 may be genetic susceptibility markers of early T2D related left heart remodeling in Uygurs.
It is well known that the activation of RAAS not only plays an important role in the occurrence and development of diabetes mellitus but also runs through the whole diabetic related cardiovascular event chain (e.g., hypertension, dyslipidemia, ASCVD, MAU and left ventricular remodeling, etc.) [46]. ACE2, a homolog of ACE, is a monocarboxypeptidase that converts angiotensin II (Ang II) into angiotensin 1–7 (Ang 1–7) which, by virtue of its actions on the Mas receptor, opposes the molecular and cellular effects of Ang II, and exhibites notable cardiovascular protective effects [47]. Although the roles of ACE2 gene polymorphisms (mutations or variants) on diabetes and other associated cardiovascular complications were incompletely understood, it may be related to the cross-talk between ACE2/Ang-(1–7)/Mas axis and ACE/Ang II/AT1 axis [48]. ACE2 gene polymorphisms (e.g., rs2106809 [49], rs2074192 [48]) were associated with downregulation of circulating Ang-(1–7). The deletion of ACE2 in mice model was associated with increased plasma and tissue Ang II levels [50], led to impaired glucose homeostasis [51] and cardiovascular damage [50]. The molecular mechanism might be involved in posttranscriptional regulation via microRNA, because it has been reported that the changes of ACE2 expression was in protein level rather than mRNA level in diabetic mice [52], and microRNA might regulate RAAS activity via by altering the interaction between microRNA and mRNA of targeted gene [49] that need to be investigated further.
Some limitations should be mentioned. First, since our sample size is not large enough, further prospective large sample studies are needed to validate our findings. Secondly, the possibility of false-positive findings should be considered especially for secondary study based on our results.
Conclusion
ACE2 gene variants were associated with T2D and T2D with elevated blood pressure, dyslipidemia, carotid arteriosclerosis, MAU and left ventricular remodeling with characteristic of genetic pleiotropy in Uygurs. Our findings suggest that ACE2 SNPs rs879922 may be a common genetic loci and optimal genetic susceptibility marker for T2D and T2D related cardiovascular complications in Uygur. Our observations further support that the genetic predisposition of ACE2 SNPs associated with the risk of T2D and T2D related cardiovascular risk should need large-scale evaluation.
Additional file
Authors’ contributions
CL, literature search, study format, writing protocol, collecting data, processing data, data interpretation, analysing data, writing manuscript; YFL, literature search, study format, writing protocol, recruiting patients and data interpretation; TWG and YXL, carring out the molecular genetics; YS, AZ and HYZ, recruiting patients, following up patients, collecting data; FL and TM, recruiting patients and collecting data. All authors read and approved the final manuscript.
Acknowledgements
We thank Prof. Pingsheng Wu for excellent academic assistance, Prof. Fangyao Chen for statistical analysis assistance.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethics approval was obtained from the institutional review board (2014SYYLSZ-018).
Funding
This study was supported by grants from the Project of Natural Science Foundation of XUAR, China (NQ. 201318101-12) and the Science and Technology Planning Project of Guangdong Province, China (NQ. 2014A020212372).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACE2
angiotensin converting enzyme 2
- ACR
urinary albumin-to-creatinine ratio
- Alb
albumin
- ALD
aldosterone
- ALT
alanine aminotransferase
- Ang I/II
angiotensin I/II
- ASCVD
arteriosclerosis cardiovascular disease
- AST
aspartate aminotransferase
- BMI
body mass index
- BUN
blood urea nitrogen
- CAS
carotid arteriosclerosis stenosis
- CHOL
hypercholesterolemia
- Cr
creatinine
- DBP
diastolic blood pressure
- EH
essential hypertension
- FBG
fasting blood glucose
- HbA1C
glycosylated hemoglobin
- HDL-C
high-density lipoprotein cholesterol
- HsCRP
high-sensitivity C-reactive protein
- LDL-C
low-density lipoprotein cholesterol
- Lp(a)
lipoprotein A
- LVMI
left ventricular mass index
- LVEF
left ventricular ejection fraction
- MAU
microalbuminuria
- RAAS
renin–angiotensin–aldosterone system
- SBP
systolic blood pressure
- SNP
single nucleotide polymorphism
- T2D
type 2 diabetes mellitus
- TRIG
hypertriglyceridemia
- UA
blood uric acid
Contributor Information
Cheng Liu, Phone: (086)20-81048176, Email: eyliucheng@scut.edu.cn.
Yanfang Li, Email: liyanfang2016f@gmail.com.
Tianwang Guan, Email: tianwangguan001@gmail.com.
Yanxian Lai, Email: laiyanxian112@hotmail.com.
Yan Shen, Email: shenyan512n@hotmail.com.
Abudurexiti Zeyaweiding, Email: abudurexiti01@hotmail.com.
Haiyan Zhao, Email: zhaohaiyan76@hotmail.com.
Fang Li, Email: lifang512@tom.com.
Tutiguli Maimaiti, Email: tutiguli512@sina.com.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Huo X, Gao L, Guo L, Xu W, Wang W, Zhi X, Li L, Ren Y, Qi X, Sun Z, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4(2):115–124. doi: 10.1016/S2213-8587(15)00508-2. [DOI] [PubMed] [Google Scholar]
- 3.Huo L, Magliano DJ, Ranciere F, Harding JL, Nanayakkara N, Shaw JE, Carstensen B. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997–2011. Diabetologia. 2018;61(5):1055–1063. doi: 10.1007/s00125-018-4544-z. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi Y, Jiang Y, He J, Xu Y, Wang L, Xu M, Zhang M, Li Y, Wang T, Dai M, et al. Status of cardiovascular health in Chinese adults. J Am Coll Cardiol. 2015;65(10):1013–1025. doi: 10.1016/j.jacc.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Kotseva K, De Bacquer D, De Backer G, Ryden L, Jennings C, Gyberg V, Abreu A, Aguiar C, Conde AC, Davletov K, et al. Lifestyle and risk factor management in people at high risk of cardiovascular disease. A report from the European Society of Cardiology European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE) IV cross-sectional survey in 14 European regions. Eur J Prev Cardiol. 2016;23(18):2007–2018. doi: 10.1177/2047487316667784. [DOI] [PubMed] [Google Scholar]
- 7.Pazoki R, Dehghan A, Evangelou E, Warren H, Gao H, Caulfield M, Elliott P, Tzoulaki I. Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood pressure levels and cardiovascular events. Circulation. 2018;137(7):653–661. doi: 10.1161/CIRCULATIONAHA.117.030898. [DOI] [PubMed] [Google Scholar]
- 8.Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. 2018;67(1):3–11. doi: 10.2337/dbi17-0013. [DOI] [PubMed] [Google Scholar]
- 9.Frojdo S, Sjolind L, Parkkonen M, Makinen VP, Kilpikari R, Pettersson-Fernholm K, Forsblom C, Fagerudd J, Tikellis C, Cooper ME, et al. Polymorphisms in the gene encoding angiotensin I converting enzyme 2 and diabetic nephropathy. Diabetologia. 2005;48(11):2278–2281. doi: 10.1007/s00125-005-1955-4. [DOI] [PubMed] [Google Scholar]
- 10.Currie D, McKnight AJ, Patterson CC, Sadlier DM, Maxwell AP. Group UKWGS: investigation of ACE, ACE2 and AGTR1 genes for association with nephropathy in Type 1 diabetes mellitus. Diabetic Med. 2010;27(10):1188–1194. doi: 10.1111/j.1464-5491.2010.03097.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel SK, Wai B, Ord M, MacIsaac RJ, Grant S, Velkoska E, Panagiotopoulos S, Jerums G, Srivastava PM, Burrell LM. Association of ACE2 genetic variants with blood pressure, left ventricular mass, and cardiac function in Caucasians with type 2 diabetes. Am J Hypertens. 2012;25(2):216–222. doi: 10.1038/ajh.2011.188. [DOI] [PubMed] [Google Scholar]
- 12.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblit PD. Common medications used by patients with type 2 diabetes mellitus: what are their effects on the lipid profile? Cardiovasc Diabetol. 2016;15:95. doi: 10.1186/s12933-016-0412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YK, Hwang MY, Kim YJ, Moon S, Han S, Kim BJ. Evaluation of pleiotropic effects among common genetic loci identified for cardio-metabolic traits in a Korean population. Cardiovasc Diabetol. 2016;15:20. doi: 10.1186/s12933-016-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269–273. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meschia JF, Klaas JP, Brown RD, Jr, Brott TG. Evaluation and management of atherosclerotic carotid stenosis. Mayo Clin Proc. 2017;92(7):1144–1157. doi: 10.1016/j.mayocp.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 18.Bundock PC, Eliott FG, Ablett G, Benson AD, Casu RE, Aitken KS, Henry RJ. Targeted single nucleotide polymorphism (SNP) discovery in a highly polyploid plant species using 454 sequencing. Plant Biotechnol J. 2009;7(4):347–354. doi: 10.1111/j.1467-7652.2009.00401.x. [DOI] [PubMed] [Google Scholar]
- 19.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9:25. doi: 10.1186/s13098-017-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du GL, Su YX, Yao H, Zhu J, Ma Q, Tuerdi A, He XD, Wang L, Wang ZQ, Xiao S, et al. Metabolic risk factors of type 2 diabetes mellitus and correlated glycemic control/complications: a cross-sectional study between rural and urban uygur residents in Xinjiang Uygur Autonomous Region. PLoS ONE. 2016;11(9):e0162611. doi: 10.1371/journal.pone.0162611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181–187. doi: 10.2337/dc16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossman A, Grossman E. Blood pressure control in type 2 diabetic patients. Cardiovasc Diabetol. 2017;16(1):3. doi: 10.1186/s12933-016-0485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YX, Song L, Xing AJ, Gao M, Zhao HY, Li CH, Zhao HL, Chen SH, Lu CZ, Wu SL. Predictive value of cumulative blood pressure for all-cause mortality and cardiovascular events. Sci Rep. 2017;7:41969. doi: 10.1038/srep41969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bays HE, Jones PH, Orringer CE, Brown WV, Jacobson TA. National lipid association annual summary of clinical lipidology 2016. J Clin Lipidol. 2016;10(1 Suppl):S1–S43. doi: 10.1016/j.jacl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Bao Q, He B, Pan Y, Zhang R, Mao X, Tang Z, Qu L, Zhu C, Tian F, et al. Association of angiotensin I converting enzyme, angiotensin II type 1 receptor and angiotensin I converting enzyme 2 gene polymorphisms with the dyslipidemia in type 2 diabetic patients of Chinese Han origin. J Endocrinol Invest. 2012;35(4):378–383. doi: 10.3275/7797. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Chang PY, Zhang Y, Kizer JR, Best LG, Howard BV. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: the Strong Heart Study. Diabetes Care. 2017;40(4):529–537. doi: 10.2337/dc16-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolppanen AM, Pulkkinen L, Kuulasmaa T, Kolehmainen M, Schwab U, Lindstrom J, Tuomilehto J, Uusitupa M, Kuusisto J. The genetic variation in the tenomodulin gene is associated with serum total and LDL cholesterol in a body size-dependent manner. Int J Obes. 2008;32(12):1868–1872. doi: 10.1038/ijo.2008.217. [DOI] [PubMed] [Google Scholar]
- 30.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546–551. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papa G, Degano C, Iurato MP, Licciardello C, Maiorana R, Finocchiaro C. Macrovascular complication phenotypes in type 2 diabetic patients. Cardiovasc Diabetol. 2013;12:20. doi: 10.1186/1475-2840-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W, Huang W, Su S, Li B, Zhao W, Chen S, Gu D. Association study of ACE2 (angiotensin I-converting enzyme 2) gene polymorphisms with coronary heart disease and myocardial infarction in a Chinese Han population. Clin Sci. 2006;111(5):333–340. doi: 10.1042/CS20060020. [DOI] [PubMed] [Google Scholar]
- 33.Wu YH, Li JY, Wang C, Zhang LM, Qiao H. The ACE2 G8790A polymorphism: involvement in type 2 diabetes mellitus combined with cerebral stroke. J Clin Lab Anal. 2017 doi: 10.1002/jcla.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vangjeli C, Dicker P, Tregouet DA, Shields DC, Evans A, Stanton AV, Project M. A polymorphism in ACE2 is associated with a lower risk for fatal cardiovascular events in females: the MORGAM project. J Renin Angiotensin Aldosterone Syst. 2011;12(4):504–509. doi: 10.1177/1470320311405557. [DOI] [PubMed] [Google Scholar]
- 35.Beaney KE, Cooper JA, McLachlan S, Wannamethee SG, Jefferis BJ, Whincup P, Ben-Shlomo Y, Price JF, Kumari M, Wong A, et al. Variant rs10911021 that associates with coronary heart disease in type 2 diabetes, is associated with lower concentrations of circulating HDL cholesterol and large HDL particles but not with amino acids. Cardiovasc Diabetol. 2016;15(1):115. doi: 10.1186/s12933-016-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corella D, Asensio EM, Coltell O, Sorli JV, Estruch R, Martinez-Gonzalez MA, Salas-Salvado J, Castaner O, Aros F, Lapetra J, et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc Diabetol. 2016;15:4. doi: 10.1186/s12933-015-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1—executive summary. J Clin Lipidol. 2014;8(5):473–488. doi: 10.1016/j.jacl.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Zhu B, Zou S, Shi J. The association between ACE2 Gene polymorphism and the stroke recurrence in Chinese population. J Stroke Cerebrovasc Dis. 2018 doi: 10.1016/j.jstrokecerebrovasdis.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Sotoodehnia N, Li G, Johnson CO, Lemaitre RN, Rice KM, Rea TD, Siscovick DS. Genetic variation in angiotensin-converting enzyme-related pathways associated with sudden cardiac arrest risk. Heart Rhythm. 2009;6(9):1306–1314. doi: 10.1016/j.hrthm.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng N, Zhang Y, Ma J, Li H, Zhou F, Qu Y. Association of polymorphisms of angiotensin I converting enzyme 2 with retinopathy in type 2 diabetes mellitus among Chinese individuals. Eye. 2015;29(2):266–271. doi: 10.1038/eye.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843–2863. doi: 10.2337/dc14-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer BR, Jarvis MD, Pilbrow AP, Ellis KL, Frampton CM, Skelton L, Yandle TG, Doughty RN, Whalley GA, Ellis CJ, et al. Angiotensin-converting enzyme 2 A1075G polymorphism is associated with survival in an acute coronary syndromes cohort. Am Heart J. 2008;156(4):752–758. doi: 10.1016/j.ahj.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Thomas MC. Type 2 diabetes and heart failure: challenges and solutions. Curr Cardiol Rev. 2016;12(3):249–255. doi: 10.2174/1573403X12666160606120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Packer M. Heart failure: the most important, preventable, and treatable cardiovascular complication of type 2 diabetes. Diabetes Care. 2018;41(1):11–13. doi: 10.2337/dci17-0052. [DOI] [PubMed] [Google Scholar]
- 45.Lieb W, Graf J, Gotz A, Konig IR, Mayer B, Fischer M, Stritzke J, Hengstenberg C, Holmer SR, Doring A, et al. Association of angiotensin-converting enzyme 2 (ACE2) gene polymorphisms with parameters of left ventricular hypertrophy in men. Results of the MONICA Augsburg echocardiographic substudy. J Mol Med. 2006;84(1):88–96. doi: 10.1007/s00109-005-0718-5. [DOI] [PubMed] [Google Scholar]
- 46.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol. 2014;11(7):413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YY, Zhang P, Zhou XM, Liu D, Zhong JC, Zhang CJ, Jin LJ, Yu HM. Relationship between genetic variants of ACE2 gene and circulating levels of ACE2 and its metabolites. J Clin Pharm Ther. 2018;43(2):189–195. doi: 10.1111/jcpt.12625. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Chen Y, Zhang P, Zhong J, Jin L, Zhang C, Lin S, Wu S, Yu H. Association between circulating levels of ACE2-Ang-(1–7)-MAS axis and ACE2 gene polymorphisms in hypertensive patients. Medicine. 2016;95(24):e3876. doi: 10.1097/MD.0000000000003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47(4):718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 51.Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine. 2008;34(1–3):56–61. doi: 10.1007/s12020-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 52.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.